Abstract
Low cost and eco-friendly green synthesis of silver nanoparticles (AgNPs) from silver nitrate (AgNO3) using Prunus japonica leaves extract as reducing agent by a simple method at room temperature. The biosynthesized nanoparticles (NPs) were characterized by UV-Vis, tunneling electron microscopy (HR-TEM), scanning electron microscopy (SEM) coupled with X-ray energy dispersive spectrophotometer (EDAX), X-ray diffraction (XRD) and Fourier transform infrared spectroscopy (FTIR). In UV-Vis spectroscopy results, the λmax was observed at 441 nm. The AgNPs synthesized were spherical, hexagonal, and irregular in shapes. The EDAX and XRD spectrum confirmed the presence of silver ions and crystalline nature of synthesized AgNPs. FTIR showed the functional groups such as C = O, N–H and C–N groups involved in the reduction of Ag+ to Ag. 2, 2-Diphenyl-1-picrylhydrazyl (DPPH) radical scavenging assay was performed and it showed the percentage inhibition in concentration-dependent manner. The synthesized AgNPs showed antibacterial activity against Escherichia coli, Proteus vulgaris, Staphylococcus aureus and Bacillus cereus to different extents and the higher activity was observed in Proteus vulgaris.
Introduction
The noble nanoparticles (NPs) have been widely used in various technological applications because of their unique properties. Among the noble metal NPs, silver nanoparticles (AgNPs) in particular are known for their versatile applications in medical industries. In the twenty-first century, nanotechnology is emerging as cutting edge technology and has incredible applications in physics, chemistry, biology, material science, and medicine. The major thrust has been developing new materials and examining their properties by tuning the particle size, shape, and distribution. Green nanotechnology is gaining importance due to the elimination of harmful reagents and provides effective synthesis of expected products in an economical manner (Kalimuthu et al. Citation2008, Roopan and Khan Citation2010, Roopan et al. Citation2012, Citation2013). Noble metal nanoparticles (MNPs) such as silver, gold, and platinum are widely applied in medicinal applications. There is a growing need to develop an environmentally friendly process for the synthesis of NPs that does not employ toxic chemicals (Bhainsa and D’Souza Citation2006, Kowshik et al. Citation2003, Rajakumar et al. Citation2012, Shahverdi et al. Citation2007). Generally, NPs are prepared by a variety of chemical and physical methods and it is not environmentally friendly.
Green synthesis of MNPs is an economic, eco-friendly, and simple method in the synthesis route (Raffi et al. Citation2007, Sondi and Salopek-Sondi Citation2004). A number of bio-molecules act as reducing and protecting agents in the green synthesis of MNPs. Green/biosynthesis of MNPs were performed using bacteria, fungi, and plant extracts (Kaviya et al. Citation2011, Kumar et al. Citation2012, Philip et al. Citation2011). Green synthesis appears to be a cost efficient alternative to conventional physical and chemical method of AgNPs synthesis and would be suitable for developing a biological process for large-scale production. Nowadays plant extracts act as reducing and capping agents for the synthesis of NPs, which is more advantageous than chemical, microbial synthesis (Ahmad et al. Citation2003, Husseiny et al. Citation2007, Smitha et al. Citation2009). Although metallic NPs synthesis using plant extracts has already been reported in various plants such as geranium (Shankar et al. Citation2003), neem (Shankar et al. Citation2004), Aloe vera (Chandran et al. Citation2006), Cinnamomum camphora (Huang et al. Citation2007), mushroom (Philip Citation2009), Magnolia kobus (Song et al. Citation2009), pear fruit and Mangifera indica (Ghodake et al. Citation2010), there is still a lot of attention paid to this field because of the diversity and the high potential of plants in producing NPs with different shapes. Among these NPs, AgNPs have attracted the attention of many researchers interested in the field due to their biological applications such as cancer therapy and imaging (Daniel and Astru Citation2004, Philip Citation2010).
Prunus japonica (also Cerasus japonica), also called Japanese bush cherry or Korean bush cherry, is a shrub species in the genus Prunus, which is widely cultivated for ornamental use. Its native range extends from Central China through to the Korean peninsula. Prunus japonica is an evergreen shrub growing to 1.5 m (5 ft). It produces flower in May, and the seeds ripen from July to September. The flowers are hermaphrodite (have both male and female organs) and are pollinated by insects. The kernels are aperient, carminative, demulcent, deobstruent, diuretic, hypotensive, laxative, lenitive, and ophthalmic. They are taken internally in the treatment of dry constipation, edema and insomnia following a trauma. The root is used in the treatment of constipation, children’s fever, pinworms, and teeth ailments. Although no specific mention has been seen for this species, all members of the genus contain amygdalin and prunasin, substances which break down in water to form hydrocyanic acid (cyanide or prussic acid). In small amounts this exceedingly poisonous compound stimulates respiration, improves digestion, and gives a sense of well-being (Bown Citation1995).
Materials and methods
All the reagents used for this reaction was of analytical grade and obtained from Sigma Aldrich (St. Louis, MO) without any further purifications. Silver nitrate (AgNO3) was purchased from Junsei Chemical Co. Ltd. (Tokyo, Japan). The bacterial cultures were obtained from National Collection of Industrial Microorganisms (NCIM) (Pune, India). Prunus japonica leaves were collected from Hanseo University campus and it was identified and authenticated by Biological sciences department, Hanseo University, South Korea.
Plant materials
Fifty grams of P. japonica leaves were collected in medicinal plant garden at Department of Biological Sciences, Hanseo University, South Korea. The leaves were thoroughly washed in running tap water for two times and finally with deionized water until complete removal of foreign matters. Then after, it was soaked in 100 ml of deionized water and macerated for 24 h. Finally, it was finely crushed in mortar and filtered using sterile gauge filter. The concentrated extract was diluted to 250 ml with deionized water and the solution was kept in refrigerator.
Synthesis of AgNPs
The AgNPs from P. japonica leaves extract was prepared by previously described method with slight modifications (Nazeruddin et al. Citation2014). Diluted extract (50 ml) was taken in burette and it was transferred drop wise to 100 ml of 0.1 M AgNO3 aqueous solution with constant magnetic stirring. As soon as the addition of extracts, color of the AgNO3 solution was altered to dark brown. After complete addition of extract, the precipitate was separated by high speed centrifugation at 7000 rpm. The separated solid mass was washed three times with ethanol to remove the alcohol soluble impurities. Then, it was dried in oven at 50 °C for 2–3 h. Finally, it was powdered in mortar and transferred to light-resistant containers for further characterization.
Characterization
To investigate the formation of AgNPs, the dried and powdered AgNPs was dispersed in deionized water and sonicated. Then, it was scanned in UV visible spectrophotometer (Shimadzu–1240, Tokyo, Japan) in the wavelength range of 300–800 nm. The synthesized AgNPs were characterized by FTIR (Fourier-transform infrared spectroscopy) (Nicolet-200, Thermo Fishers Scientifics, Madison, USA), using KBr pellets. The morphology and structure and elemental composition of AgNPs was measured by using SEM–EDAX (Scanning electron microscopy–Energy dispersive spectroscopy) (JEOL JSM 5600, Jeol USA, INC, MA, USA). The shape and particle size of the AgNPs that formed was observed using Hitachi-800 tunneling electron microscope (TEM). The AgNPs with extract was placed on the copper grid of TEM analyzer and allowed to dry at room temperature, and then placed in the sample holder of the TEM instrument and analyzed. XRD (X-ray diffraction) measurements were performed with diffraction angle between 10 and 90° (Rigaku Miniflex diffractometer, Rigaku America, TX, USA). Particle size of prepared AgNPs was determined using Scherrer’s equation (EquationEqation 1(1) ) (Zargar et al. Citation2014).
(1)
Where λ is the X-ray wavelength (1.5418 Å), β1/2 is the width of the XRD reference peak at half height and K is a shape factor, θ is diffraction angle.
Antibacterial studies
Antibacterial activity was evaluated using agar well diffusion method. The antibacterial activity was measured using Gram-positive Staphylococcus aureus (NCIM 2079), Bacillus cereus (NCIM 2106) and Gram-negative Escherichia coli (NCIM 2931), Proteus vulgaris (NCIM 2813) pathogenic bacteria as per the previous method with slight modifications (Jagtap and Bapat Citation2013). Nutrient agar media was prepared and 30–35 ml was transferred in to sterile petri plates. After solidification of media, 100 μl of working stock culture (1 × 106 cells/ml) was spread with sterile cotton swab and holes were made with stainless steel cylinders. The different concentration of AgNPs (3.125, 6.25, 12.5, and 25 μg/ml) were filled and kept in refrigerator for diffusion for 30 minutes. The plates were then incubated at 37 °C for 24 h.
DPPH free radical scavenging assay
DPPH free radical scavenging assay was performed as per previous method with slight modifications (Choi et al. Citation2002). Different concentrations (12.5–200 μg/ml) of synthesized AgNPs and standard vitamin C were taken separately and mixed with 3 ml of 0.1 mM methanolic solution of DPPH and incubated in dark for 20 min. After incubation, absorbance of the solutions was measured using UV-visible spectrophotometer (Agile microplate reader, ACTGENE, INC, NJ, USA) at 517 nm against methanol as blank. The methanolic solution DPPH without sample was used as control and the percentage inhibition was calculated by the following formula:
Results and discussion
UV–vis spectroscopy analysis
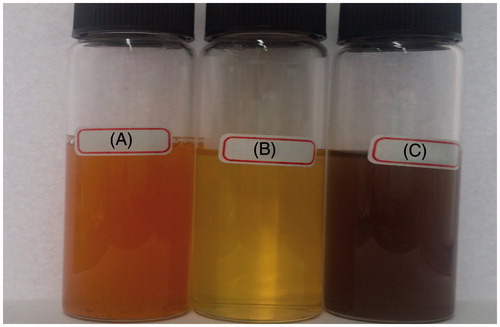
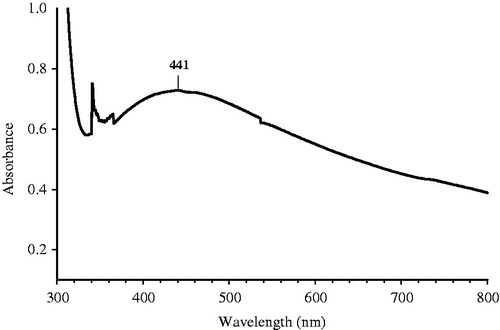
The colorless AgNO3 solution was turned to dark brownish black () indicated the formation of AgNPs. The dried powder dissolved in deionized water was exposed to UV visible region and the absorbance was recorded. Based on the surface plasmon resonance (SPR), the spherical AgNPs shows the characteristic SPR at the wavelength between the ranges of 400–450 nm. The shift of the λmax SPR from blue to red could be related to obtaining AgNPs at various sizes and shapes of the formed AgNPs (Kelly et al. Citation2003, Shameli et al. Citation2014, Stepanov Citation1997). shows the SPR of the sample solution at 441 nm, which strongly suggests that the AgNPs were spherical in shape. To study the stability of the nano particle synthesized they were subjected to screening under UV-vis spectral analysis in scanning mode at various intervals. The peak maxima for SPR were not shifted even after 24 h but the intensity was increased with increase in time.
XRD analysis
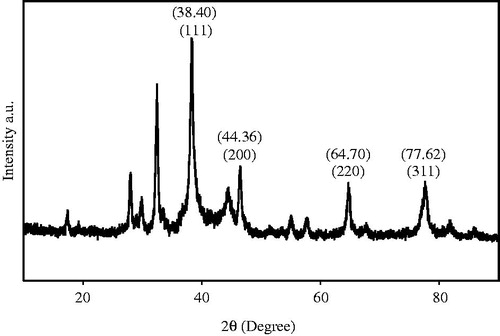
The crystalline nature of the synthesized AgNPs was confirmed by XRD analysis and it is shown in . The spectrum shows four distinct different peaks at 38.40, 44.36, 64.70, and 77.62 and its corresponding lattice plane value was indexed at (111), (200), (220) and (311) planes of face centered cubic nature (FCC), which correlate with that illustrated by Jeeva (Jeeva et al. Citation2014). The results obtained were in close agreement with the reference of FCC structure from joint committee of powder diffraction standard (JCPDS) Card No-04–0783. Average particle size of AgNPs prepared was calculated as 26 nm.
SEM, EDAX, and TEM analysis
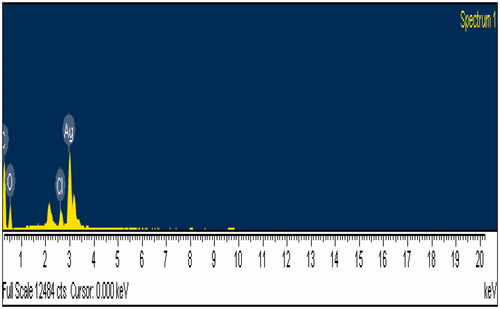
SEM image of the AgNPs was obtained and it reveals the cluster of spherical bead-like structure with nonuniform distribution (). It can be observed that larger particles of AgNPs are formed due to aggregation of NPs which might be induced by the evaporation of solvent during sample preparation. This could have contributed for the variation in particle size. Our results have good correlation with Nazeruddin’s report (Nazeruddin et al. Citation2014). The elemental composition of the powdered samples was determined and it is show in , which revealed the strong signal in silver region and confirmed the formation of AgNPs. Metallic silver nanocrystals generally show typical optical absorption peak approximately at 2.999 keV due to SPR. The spectral signals for carbon and oxygen was also observed, this is due to the extracellular organic materials (from plant extract) that were adsorbed on the surface or in the vicinity of the metallic NPs. depicts the results of HR-TEM analysis obtained for AgNPs that shows the spherical morphology with an average particle size of 24 nm (total number of particle taken into account for average particle size calculation was 200). The particle size found here was equivalent to the size calculated using XRD analysis.
FTIR analysis
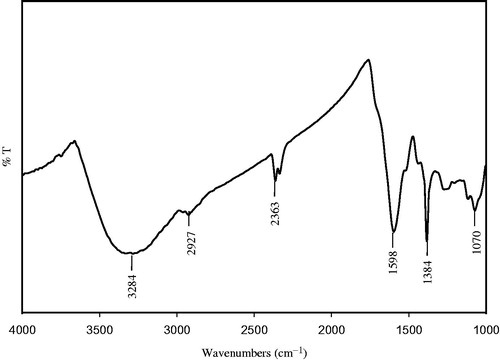
shows the FTIR spectroscopic analysis of synthesized AgNPs and the spectrum shows absorption bands at 3284 cm−1, 2927 cm−1, 2363 cm−1, 1598 cm−1 1384 cm−1 and 1070 cm−1. The band 3284 cm−1 corresponds to amide N–H stretching and 2927 cm−1 relevant to alkane C–H stretching vibrations of methyl, methylene, and methoxy groups. The peak observed at 2363 cm−1 may be due to C–H stretching of methylene group. The peak observed at 1598 cm− 1 corresponds to amide C = O stretching vibrations arising due to carbonyl stretch in proteins. The band at 1384 cm−1 corresponds to the presence of stretching vibrations of alcohol, ethers, esters, carboxylic acids, and amino acids (Sadeghi and Gholamhoseinpoor Citation2015) and the peak in 1070 cm−1 can be allocated to the stretching vibration of the C–OH bond from proteins in the plant extract (Kumar et al. Citation2014). The functional groups such as C = O, N–H, and C–H groups present in sample might be responsible for bioreduction of Ag+ to AgNPs. The observed peaks considered as major functional groups in different chemical classes such as polysaccharides, flavonoids, triterpenoids, and polyphenols (Nabiken et al. Citation2010).
Antibacterial activity
The antibacterial activity of synthesized AgNPs was evaluated by agar well diffusion method. and portrayed the results of antimicrobial activity that showed a dose-dependent activity for the prepared AgNPs. Gram-negative bacteria, P. vulgaris showed the highest zone of inhibition (18.83 mm) compared to others. In our results, the Gram-positive bacteria showed lower zone of inhibition while Gram-negative bacteria showed better results. In Gram-positive bacteria the rigid thicker peptidoglycan layer may be a reason for less zone of inhibition and it prevent the entering of nanoscaled particles in to the cell wall (Jayachandra reddy et al. Citation2014). The higher bacterial activity in Gram-negative bacteria was due to much thinner peptidoglycan layer and it easily enters in to the cell wall to denature or kill bacteria. It is very important that the binding of particles to the bacteria depends on the interaction of the surface area available. Based on this concept, smaller particles have larger surface area for interaction and will have stronger bactericidal effect than larger particles (Song et al. Citation2006).
Figure 8. Antibacterial activity of green synthesized AgNPs (a = No drug, b = 3.125, c = 6.25, d = 12.5, and e = 25 μg/ml) using P. japonica extract.
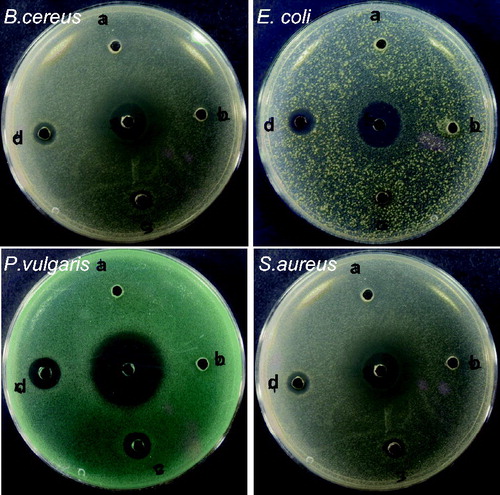
Table 1. Antibacterial activity of synthesized AgNPs.
DPPH free radical scavenging assay
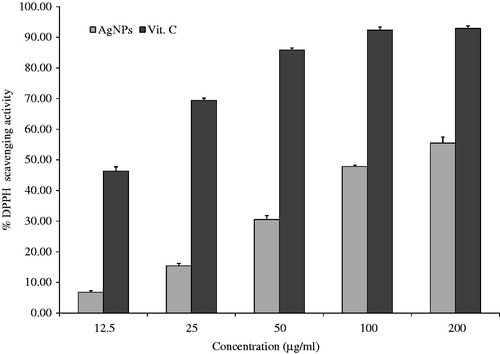
The results on the effect of different concentrations of AgNPs on DPPH radical scavenging activity is shown in . DPPH was a stable compound and accepts hydrogen or electrons from silver NPs. The results of DPPH radical scavenging activity tends to be increased with increase in the concentration of AgNPs. The maximum percentage inhibition observed in AgNPs was 55% and it was lower than that of standard vitamin C of 200 μg/ml (93%). Similar observations with enhanced DPPH scavenging activity by AgNPs in Piper longum fruit (Jayachandra reddy et al. Citation2014) have been reported.
Conclusion
In our study, the biosynthesis of AgNPs using Prunus japonica leaf extract has been reported. It is easy, economic, rapid, and eco-friendly way to synthesize AgNPs and capable of synthesizing within few hours at room temperature. This method produced fairly poly-dispersed spherical AgNPs with well-defined dimensions. In this method there is no external capping agent employed. As evidenced from FTIR, this high activity of the NPs ascribed to the relatively high levels of polysaccharides, flavonoids, triterpenoids, and polyphenols act as reducing agent. The synthesized AgNPs were found to have crystalline structure with FCC geometry as studied by XRD method. From the results of DPPH free radical scavenging assay, the synthesized AgNPs exhibited antioxidant property in tested concentrations to different extents. The synthesized NPs exhibited significant antibacterial activity against both Gram-Spositive (S. aureus, B. cereus) and Gram-negative bacteria (E. coli, P. vulgaris). But, stronger inhibition was observed in Gram-negative bacteria compared to Gram-positive bacteria. In conclusion, this type of study may also useful for preparing nanomedicines and targeted drug delivery in near future.
Disclosure statement
The authors have no declaration of interest, and all the authors are responsible for the entire content of this research paper.
Funding
The authors gratefully acknowledge the financial support received from Hanseo University Intramural Research Grant 2014, and we extend our thanks to the National Research Foundation of Korea (NRF), funded by the Ministry of Science, ICT, and Future Planning (Grant No. 2014–004694), for their partial financial support.
References
- Ahmad A, Mukherjee P, Senapati S, Mandal D, Islam Khan M, Kumar R, Sastry M. 2003. Extracellular biosynthesis of silver nanoparticles using the fungus Fusarium oxysporum. Colloids Surf B Biointerfaces. 28:313–318.
- Bhainsa KC, D’Souza SF. 2006. Extracellular biosynthesis of silver nanoparticles using the fungus Aspergillus fumigatus. Colloids Surf B Biointerfaces. 47:160–164.
- Bown D. 1995. Encyclopaedia of Herbs and Their Uses. London: DK publishing.
- Chandran SP, Chaudhary M, Pasricha R, Ahmad A, Sastry M. 2006. Synthesis of gold nanotriangles and silver nanoparticles using Aloe vera plant extract. Biotechnol Prog. 22:577–583.
- Choi CW, Kim SC, Hwang SS, Choi BK, Ahn HJ, Lee MY, Park SH, Kim SK. 2002. Antioxidant activity and free radical scavenging capacity between Korean medicinal plants and flavonoids by assay guided comparison. Plant Sci. 163:1161–1168.
- Daniel MC, Astru D. 2004. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 104:293–346.
- Ghodake GS, Deshpande NG, Lee YP, Jin ES. 2010. Pear fruit extract-assisted room-temperature biosynthesis of gold nanoplates. Colloids Surf B Biointerfaces. 75:584–589.
- Huang J, Li Q, Sun D, Lu Y, Su Y, Yang X, et al. 2007. Biosynthesis of silver and gold nanoparticles by novel sundried Cinnamomum camphora leaf. Nanotechnology. 18:105104–105115.
- Husseiny MI, Abd El-Aziz M, Badr Y, Mahmoud MA. 2007. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A Mol Biomol Spectrosc. 67:1003–1006.
- Jagtap UB, Bapat VA. 2013. Green synthesis of silver nanoparticles using Artocarpus heterophyllus Lam. seed extract and its antibacterial activity. Ind Crops Prod. 46:132–137.
- Jayachandra Reddy N, Nagoor Vali D, Rani M, Sudha Rani S. 2014. Evaluation of antioxidant, antibacterial and cytotoxic effects of green synthesized silver nanoparticles by Piper longum fruit. Mat Sci Eng C. 34:115–122.
- Jeeva K, Thiyagarajan M, Elangovan V, Geetha N, Venkatachalam P. 2014. Caesalpinia coriaria leaf extracts mediated biosynthesis of metallic silver nanoparticles and their antibacterial activity against clinically isolated pathogens. Ind Crops Prod. 52:714–720.
- Kalimuthu K, Suresh Babu R, Venkataraman D, Bilal M, Gurunathan S. 2008. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 65:150–153.
- Kaviya S, Santhanalakshmi J, Viswanathan B, Muthumary J, Srinivasanc K. 2011. Biosynthesis of silver nanoparticles using citrus sinensis peel extract and its antibacterial activity. Spectrochim Acta A Mol Biomol Spectrosc. 79:594–598.
- Kelly KL, Coronado E, Zhao LL, Schatz GC. 2003. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric Environment. J Phys Chem B. 107:668–677.
- Kowshik M, Ashtaputre S, Kharazi S, Vogel W, Urban J, Kulkarni SK, Paknikar KM. 2003. Extracellular synthesis of silver nanoparticles by a silver-tolerant yeast strain MKY3. Nanotechnology. 14:95–100.
- Kumar B, Smita K, Cumbal L, Debut A. 2014. Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts. Saudi J Biol Sci. 21:605–609.
- Kumar R, Roopan SM, Prabhakarn A, Khanna VG, Chakroborty S. 2012. Agricultural waste Annona squamosa peel extract: biosynthesis of silver nanoparticles. Spectrochim Acta A 90:173–176.
- Nabiken A, Kathiresan K, Anburaj, Nabeel MA. 2010. Synthesis of antimicrobial silver nanoparticles by callus and leaf extracts from saltmarsh plant, Sesuvium portulacastrum L. Colloids Surf B Biointerface. 79:488–493.
- Nazeruddin GM, Prasada NR, Prasad SR, Shaikh YI, Waghmare SR, Adhyapak P. 2014. Coriandrum sativum seed extract assisted in situ green synthesis of silver nanoparticle and its anti-microbial activity. Ind Crops Prod. 60:212–216.
- Philip D. 2009. Biosynthesis of Au, Ag and Au-Ag nanoparticles using edible mushroom extract. Spectrochim Acta A Mol Biomol Spectrosc. 73:374–381.
- Philip D. 2010. Rapid green synthesis of spherical gold nanoparticles using Mangifera indica leaf. Spectrochim Acta A Mol Biomol Spectrosc. 77:807–810.
- Philip D, Unni C, Aswathy Aromal S, Vidhu VK. 2011. Murraya Koenigii leaf-assisted rapid green synthesis of silver and gold nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 78:899–904.
- Raffi M, Rumaiz AK, Hasan MM, Shah SI. 2007. Studies of the growth parameters of silver nanoparticle synthesis by inert gas condensation. J Mater Res. 22:3378–3384.
- Rajakumar G, Abdul Rahuman A, Mohana Roopan S, Gopiesh Khanna V, Elango G, Kamaraj C, Abduz Zahir A, Velayutham K. 2012. Fungus-mediated biosynthesis and characterization of TiO2 nanoparticles and their activity against pathogenic bacteria. Spectrochim Acta A. 91:23–29.
- Roopan SM, Bharathi A, Prabhakarn A, Abdul Rahuman A, Velayutham K, Rajakumar G, et al. 2012. Efficient phyto-synthesis and structural characterization of rutile TiO2 nanoparticles using Annona squamosa peel extract. Spectrochim Acta A. 98:86–90.
- Roopan SM, Khan FRN. 2010. ZnO nanoparticles in the synthesis of AB ring core of camptothecin. Chem Pap. 64:812–817.
- Roopan SM, Rohit, Madhumitha G, Abdul Rahuman A, Kamaraj C, Bharathi A, Surendraa TV. 2013. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crops Prod. 43:631–635.
- Sadeghi Babak F, Gholamhoseinpoor A. 2015. A study on the stability and green synthesis of silver nanoparticles using Ziziphora tenuior (Zt) extract at room temperature. Spectrochim Acta A Mol Biomol Spectrosc. 134:310–315.
- Shahverdi AR, Minacian S, Shahverdi HR, Jamalifar H, Nohi AA. 2007. Rapid synthesis of silver nanoparticles using culture supernatants of Enterobacteria: a novel biological approach. Proc Biochem. 42:919–923.
- Shameli K, Ahmad MB, Shabanzadeh P, Al-Mulla EAJ, Zamanian A, Abdul-lahi Y, et al. 2014. Effect of Curcuma longa tuber powder extract on size of silver nanoparticles prepared by green method. Res Chem Intermed. 40:1313–1325.
- Shankar SS, Ahmad A, Pasricha R, Sastry M. 2003. Bioreduction of chloroaurate ions by geranium leaves and its endophytic fungus yields gold nanoparticles of different shapes. J Mater Chem. 13:1822–1826.
- Shankar SS, Rai A, Ahmad A, Sastry M. 2004. Rapid synthesis of Au, Ag, and bimetallic Au core-Ag shell nanoparticles using Neem (Azadirachta indica) leaf broth. J Colloid Interface Sci. 275:496–502.
- Smitha SL, Philip D, Gopchandran KG. 2009. Green synthesis of gold nanoparticles using Cinnamomum zeylanicum leaf broth. Spectrochim Acta A Mol Biomol Spectrosc. 74:735–739.
- Sondi I, Salopek-Sondi B. 2004. Silver nanoparticles as antimicrobial agent: a case study on E. coli as a model for Gram-negative bacteria. J Colloid Interface Sci. 275:177–182.
- Song HY, Ko KK, Oh IH, Lee BT. 2006. Fabrication of silver nanoparticles and their antimicrobial mechanisms. Eur Cells Mater. 11:58.
- Song JY, Jang HK, Kim BS. 2009. Biological synthesis of gold nanoparticles using Magnolia kobus and Diopyros kaki leaf extracts. Proc Biochem. 44:1133–1138.
- Stepanov AL. 1997. Optical properties of metal nanoparticles synthesized in a polymer by ion implantation: a review. Tech Phys. 49:143–153.
- Zargar M, Shameli K, Najafi GR, Farahani F. 2014. Plant mediated green biosynthesis of silver nanoparticles using Vitex negundo L. extract. J Ind Eng Chem. 20:4169–4175.