Abstract
Background: There is an urgent need for novel noninvasive prognostic molecular tumor marker for monitoring the recurrence of breast cancer. MicroRNA-21 (miR-21) play a crucial role in the progression and aggressiveness of breast cancer, but its prognostic significance for patients with breast cancer remains inconclusive. The aim of this meta-analysis is to summarize the role of circulating miR-21 as a molecular marker in patients with breast cancer.
Material and methods: Eligible studies were searched from the PubMed, EMBASE and Web of Science databases. The χ2 and I2 tests were used to evaluate heterogeneity between studies. The pooled hazard ratios (HR) with 95% confidence interval (CI) were calculated by a fixed-effects model, if no heterogeneity existed. If there was heterogeneity, a random-effects model was applied. The meta-analysis was conducted using the Review Manager 5 software.
Results: A total of 7 articles which included 1629 cases were selected for the meta-analysis. Elevated miR-21 expression was significantly predictive of poor overall survival (HR = 1.51, 95%CI 1.15–1.98, p = 0.003). The subgroup analysis consisted of in tissue sample (HR = 1.66, 95%CI 1.03–2.67, p = 0.04) and serum sample (HR = 1.73, 95%CI 1.22–2.46, p = 0.002). The association between miR-21 expression level and lymph node metastasis was statistically significant (OR = 2.36, 95%CI 1.04–4.78, p = 0.03).
Conclusion: Our findings suggest that the circulating miR-21 expression level can predict poor prognosis in patients with breast cancer.
Introduction
Breast cancer, the second largest cancer among women, is a heterogeneous and complex disease, which detail of its precise progression mechanisms is less understood (Hemmatzadeh et al. Citation2016). While approximately 20–30% of early stage breast cancer cases will eventually experience recurrence and develop distant metastasis, especially in Asian, there are many factors involved in breast cancer development and metastasis. Breast cancer is difficult to prognosis because of non-specific clinical presentations. Though current prognostic methods, such as imaging examination and biopsy, have significantly improved accuracy, these methods still have certain limitations as invasive and harmful procedure (Chen and Wang, Citation2014, Ozgun et al. Citation2013, Roa et al. Citation2010, Yan et al. Citation2016).
MicroRNA-21 (miR-21) have a good potential to serve as prognostic biomarkers for breast cancer (Lee et al. Citation2011, Li et al. Citation2016, Shen et al. Citation2015). MicroRNAs are endogenous, small non-coding RNA molecules, 18–25 nucleotides in length. Studies also demonstrate that miR-21 can interact with breast cancer suppressor genes (Hafez et al. Citation2012, Qian et al. Citation2009, Yan et al. Citation2011), including tropomyosin1 (TPM1), programmed cell death 4 (PDCD4), and may play a role in cancer invasion and metastasis (Chen et al. Citation2016, Frankel et al. Citation2008, Ivan et al. Citation2016, Marino et al. Citation2014). MicroRNA-21 (miR-21), on chromosome 17q21.3, is one of the most up-regulated microRNAs in cancer that silences multiple target genes involved in cancer-signaling pathways (Abdel-Hamid et al. Citation2015).
In this meta-analysis, we also discussed the possibility of circulating miR-21 as prognostic biomarker in patients with breast cancer.
Materials and methods
Search strategy
We searched online in PubMed, EMBASE and Web of Science databases until March 2016 to identify relevant studies. Two distinct sets of key words were used simultaneously, namely “circulating/serum/plasma/tissue miR-21 and breast cancer” and “miR-21 and breast cancer prognosis” with all possible combinations. The references of all the studies were manually searched for additional eligible studies. We also inspected review articles and bibliographies of other pertinent articles to find related articles.
Inclusion and exclusion criteria
Studies were considered eligible if they met the following criteria: (1) they studied the patients with breast cancer in females; (2) they measured the expression of miR-21 in plasma or serum; (3) they investigated the association between miR-21 expression levels and breast cancer survival.
The articles were excluded based on the following criteria: (1) reviews, conference abstracts, case reports, letters; (2) articles which do not offer enough data to calculate the HR about overall survival; (3) non-English articles; laboratory studies; (4) overlapping articles.
Publication quality assessment
We used the Newcastle–Ottawa Scale to assess the quality of the eligible studies based on three perspectives: selection, comparability and exposure using a stars rating system with scores ranging from zero (worst) to nine stars (best) (Stang Citation2010). A score of six stars or above was considered a high-quality study.
Data extraction and conversion
Two investigators (WJL and SXJ) reviewed all eligible papers and extracted following information: (1) the first author name and year of publication; (2) the number of cases; (3) the study design; (4) the clinical parameters, including age, clinical stage, treatment and other clinicopathological features; (5) the markers detected for miR-21; controversial problems were arbitrated by the third investigator (WGN).
Statistical analysis
Pooled hazard ratio (HR) of miR-21 expression for OS was calculated (Pan et al. Citation2014). The heterogeneity was assessed by Chi-square-based Q statistical test. The I2 statistic to quantify the total variation proportion is ascribed to inter-study heterogeneity instead of sampling error and is measured from 0% to 100%. A p < 0.05 for the Q-test indicated significant heterogeneity among the studies. If high heterogeneity exists among studies, pooled effect was calculated using the random-effects model (DerSimonian–Laird method) (Wang et al. Citation2015). Publication bias was assessed using the funnel plot. All statistical data were computed using Review Manager 5 software (The Nordic Cochrane Center, The Cochrane Collaboration, Copenhagen, Denmark). All the p values were for a two-side test and considered statistically significant when p < 0.05.
Results
Studies characteristics and quality assessment
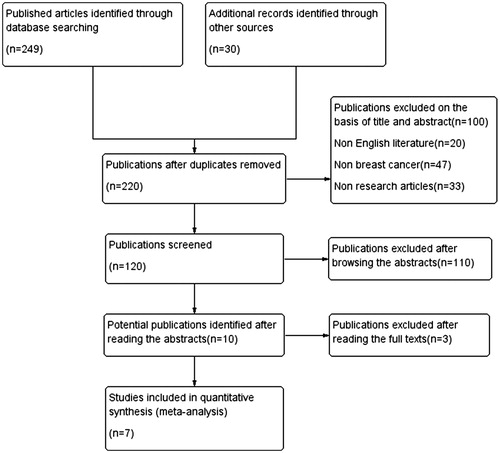
A flowchart demonstrating the publication search and selection process is shown in . Two hundred seventy-nine potentially eligible papers were retrieved in the initial search. According to the basis of title and abstract, 100 publications (20 non-English literature publications, 47 non-breast cancer and 33 non-research articles) were excluded. One hundred twenty full articles were captured, among which 110 were finally excluded due to the paucity of sufficient information on breast cancer and OS, DFS, or clinicopathological features. Three papers excluded after reading the abstracts for potential publications. Ultimately, our review enrolled a total of 7 studies.
The main characteristics of each enrolled publication are shown in . In total, 7 studies involving 1629 cases were enrolled in the present research. The papers included in our study were published between 2009 and 2016. Three papers enrolled less than 200 cases, and four papers enrolled more than 200 cases.
Table 1. Characteristics of all the studies included in the meta-analysis.
Each of the studies included in our review was evaluated in accordance with the NOS standard. A study with a quality value of 6 stars or more was of high quality. According to the NOS, all publications enrolled in our study were high-quality studies ().
Meta-analysis
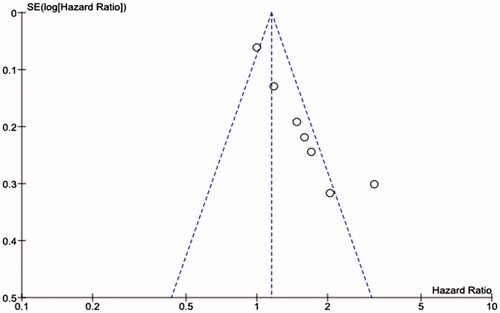
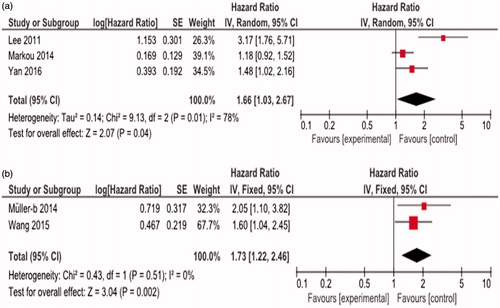
Elevated miR-21 expression was significantly predictive of poor OS (HR = 1.51, 95%CI 1.15–1.98, p = 0.003) (). We observed that the miR-21 expression was associated with poor OS both in tissue sample (HR = 1.66, 95%CI 1.03–2.67, p = 0.04) and serum sample (HR = 1.73, 95%CI 1.22–2.46, p = 0.002) ().
Figure 2. Forest plot of studies evaluating hazard ratio (HR) for association of miR-21 expression with overall survival (OS) in breast cancer patients.
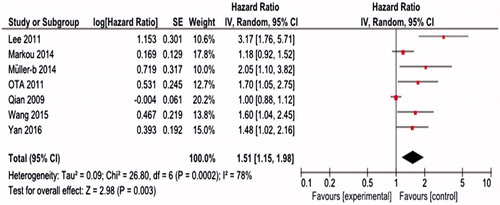
Figure 3. Subgroup analysis of relationship between elevated miR-21 expression and OS by sample type: (a) tissue; (b) serum.
There were 4 studies that reported correlations between miR-21 expression and some clinical characteristics (age, tumor size, lymph node metastasis, HER2/neu expression, Estrogen receptor status, Progesterone receptor status). The association between miR-21 expression level and lymph node metastasis was statistically significant (OR = 2.36, 95%CI 1.04–4.78, p = 0.03). However, no significant relationship between miR-21 expression level and age, tumor size was observed ().
Table 2. Correlation between high miR-21 expression and clinical characteristics.
Publication bias
Publication bias of the pooled studies was assessed by funnel plots. Visual inspection of the funnel plots was almost symmetric in studies reported OS (). Sensitivity analysis was performed by exclusion of the highest weighted study and there was no individual study that dominantly influenced overall HR. Additionally, no publication bias existed for studies regarding prognostic on patient with breast cancer and overall survival in this research.
Discussion
We show here that development of novel noninvasive prognostic biomarkers for monitoring the recurrence of breast cancer is critical for clinicians to tailor the treatment plan (De Mattos-Arruda et al. Citation2015, Wang et al. Citation2015). To the best of our knowledge, breast cancer is a malignant disease and is known to be quite complex and heterogeneous in development, progress and response to treatment. Biomarkers available such as ER, PR, HER2, could not reflect the whole prognostic significance for breast cancer patients (Muller et al. Citation2014, Sakurai et al. Citation2015). It is important to find out new novel noninvasive prognostic biomarker for patients with breast cancer (Pan et al. Citation2014). Several studies have shown that MicroRNA-21, known as a potential oncogenic role, is one of the most commonly observed aberrant miRNAs in a variety of cancer (Chen and Wang Citation2014, Lu et al. Citation2008, Motawi et al. Citation2016). For example, miR-21 was widely studied and was found aberrantly expressed in breast cancer. Our data show that miR-21 has not been reported to be associated with the prognosis of breast cancer, but it has been indicated as possible early detection marker or implicated in carcinogenesis process. Similarly, the prognostic ability was inconsistent or even contradictory in literature (Kumar et al. Citation2013, Markou et al. Citation2014, Ota et al. Citation2011, Petrovic Citation2016, Yan et al. Citation2008).
In this meta-analysis, the pooled HR from 1629 patients in 7 studies for OS was 1.51, (95%CI 1.15–1.98, p = 0.003), which was considered strongly predictive. We found strongly predictive value of miR-21 expression and survival outcome may be applied as a general prognostic marker of breast cancer. The studies detected the miR-21 expression with qRT-PCR method (Huang et al. Citation2009). Elevated expression of miR-21 in the serum also predicted poor prognosis as tissue sample, which may be favored in the clinical management of breast cancer (Rask et al. Citation2011). Therefore, larger confirmative studies of published data on circulation miR-21 hold a promise to generate better, reproducible prognostic signature for breast cancer.
The NOS scale was applied to assess the quality of the enrolled publications. Only high-quality studies (NOS scale ≥6 points) were included to avoid the potential impact of reports without sufficient information on the reliability of our meta-analysis.
The meta-analysis was performed on 7 studies evaluating hazard ratio (HR) for association of miR-21 expression with overall survival (OS) (Walter et al. Citation2011). As shown in , the pooled HR for OS was 1.51(95%CI 1.15–1.98, Z = 2.98; p = 0.003) with heterogeneity (I2 = 78%, p = 0.0002). In the case of heterogeneity, a random-effects model was used.
The presence of lymph node invasion is believed as an important prognostic marker in breast cancer patients that underwent breast operation. Our study found that lymph node metastasis occurred more frequently in patients with miR-21 high expression, which partially explains why miR-21 expression was a favorable prognostic predictor for breast cancer. Furthermore, the correlation between miR-21 expression and clinical characteristics was calculated. There was no significant association between high miR-21 expression and age, tumor size, while OR for lymph node metastasis, HER2/neu expression, Estrogen receptor, Progesterone receptor, were significant. Thus, elevated miR-21 expression was closely associated with poor prognosis clinical characteristics (Gao et al. Citation2016).
Several limitations should be considered in interpreting our study findings. At the same time, this meta-analysis was limited in some ways. First, the study covered by our meta-analysis was confined only to articles published in English, which probably brought about extra bias. Second, the credibility of HR calculated from data or extracted from survival curves might be less than of direct analysis of variance.
Overall, this meta-analysis assessed the circulating miR-21 might serve as a novel prognostic biomarker and the signature may be developed into a noninvasive blood test for predict poor prognosis in patients with breast cancer. Due to some limitations of this study, further high-quality studies were warranted to confirm our analysis.
Disclosure statement
The authors declared that they had no conflict of interest.
References
- Abdel-Hamid NR, Mohammed EA, Abbas AH, Badr FM. 2015. MicroRNA-21 expression in primary breast cancer tissue among egyptian female patients and its correlation with chromosome 17 aneusomy. Mol Diagn Ther. 19:365–373.
- Chen H, Liu H, Zou H, Chen R, Dou Y, Sheng S, et al. 2016. Evaluation of plasma miR-21 and miR-152 as diagnostic biomarkers for common types of human cancers. J Cancer. 7:490–499.
- Chen J, Wang X. 2014. MicroRNA-21 in breast cancer: diagnostic and prognostic potential. Clin Transl Oncol. 16:225–233.
- De Mattos-Arruda L, Bottai G, Nuciforo PG, Di Tommaso L, Giovannetti E, Peg V, et al. 2015. MicroRNA-21 links epithelial-to-mesenchymal transition and inflammatory signals to confer resistance to neoadjuvant trastuzumab and chemotherapy in HER2-positive breast cancer patients. Oncotarget. 6:37269–37280.
- Frankel LB, Christoffersen NR, Jacobsen A, Lindow M, Krogh A, Lund AH. 2008. Programmed cell death 4 (PDCD4) is an important functional target of the microRNA miR-21 in breast cancer cells. J Biol Chem. 283:1026–1033.
- Gao Y, Cai Q, Huang Y, Li S, Yang H, Sun L, Chen K, Wang Y. 2016. MicroRNA-21 as a potential diagnostic biomarker for breast cancer patients: a pooled analysis of individual studies. Oncotarget. 7:34498–34506.
- Hafez MM, Hassan ZK, Zekri AR, Gaber AA, Al Rejaie SS, Sayed-Ahmed MM, Al Shabanah O. 2012. MicroRNAs and metastasis-related gene expression in Egyptian breast cancer patients. Asian Pac J Cancer Prev. 13:591–598.
- Hemmatzadeh M, Mohammadi H, Jadidi-Niaragh F, Asghari F, Yousefi M. 2016. The role of oncomirs in the pathogenesis and treatment of breast cancer. Biomed Pharmacother. 78:129–139. Epub 2016/02/24.
- Huang GL, Zhang XH, Guo GL, Huang KT, Yang KY, Shen X, You J, Hu XQ. 2009. Clinical significance of miR-21 expression in breast cancer: SYBR-Green I-based real-time RT-PCR study of invasive ductal carcinoma. Oncol Rep. 21:673–679.
- Ivan C, Grazia CM, Ada D, Pietro R, Saveria A, Paola A, et al. 2016. Androgens downregulate miR-21 expression in breast cancer cells underlining the protective role of androgen receptor. Oncotarget. 7:12651–12661.
- Kumar S, Keerthana R, Pazhanimuthu A, Perumal P. 2013. Overexpression of circulating miRNA-21 and miRNA-146a in plasma samples of breast cancer patients. Indian J Biochem Biophys. 50:210–214.
- Lee JA, Lee HY, Lee ES, Kim I, Bae JW. 2011. Prognostic implications of microRNA-21 overexpression in invasive ductal carcinomas of the breast. J Breast Cancer. 14:269–275.
- Li S, Yang X, Yang J, Zhen J, Zhang D. 2016. Serum microRNA-21 as a potential diagnostic biomarker for breast cancer: a systematic review and meta-analysis. Clin Exp Med. 16:29–35.
- Lu Z, Liu M, Stribinskis V, Klinge CM, Ramos KS, Colburn NH, Li Y. 2008. MicroRNA-21 promotes cell transformation by targeting the programmed cell death 4 gene. Oncogene. 27:4373–4379.
- Marino AL, Evangelista AF, Vieira RA, Macedo T, Kerr LM, Abrahao-Machado LF, et al. 2014. MicroRNA expression as risk biomarker of breast cancer metastasis: a pilot retrospective case-cohort study. BMC Cancer. 14:739.
- Markou A, Yousef GM, Stathopoulos E, Georgoulias V, Lianidou E. 2014. Prognostic significance of metastasis-related microRNAs in early breast cancer patients with a long follow-up. Clin Chem. 60:197–205.
- Motawi TM, Sadik NA, Shaker OG, El Masry MR, Mohareb F. 2016. Study of microRNAs-21/221 as potential breast cancer biomarkers in Egyptian women. Gene. 590:210–219.
- Muller V, Gade S, Steinbach B, Loibl S, von Minckwitz G, Untch M, et al. 2014. Changes in serum levels of miR-21, miR-210, and miR-373 in HER2-positive breast cancer patients undergoing neoadjuvant therapy: a translational research project within the Geparquinto trial. Breast Cancer Res Treat. 147:61–68.
- Ota D, Mimori K, Yokobori T, Iwatsuki M, Kataoka A, Masuda N, et al. 2011. Identification of recurrence-related microRNAs in the bone marrow of breast cancer patients. Int J Oncol. 38:955–962.
- Ozgun A, Karagoz B, Bilgi O, Tuncel T, Baloglu H, Kandemir EG. 2013. MicroRNA-21 as an indicator of aggressive phenotype in breast cancer. Onkologie. 36:115–118.
- Pan F, Mao H, Deng L, Li G, Geng P. 2014. Prognostic and clinicopathological significance of microRNA-21 overexpression in breast cancer: a meta-analysis. Int J Clin Exp Pathol. 7:5622–5633. Epub 2014/10/23.
- Petrovic N. 2016. miR-21 might be involved in breast cancer promotion and invasion rather than in initial events of breast cancer development. Mol Diagn Ther. 20:97–110.
- Qian B, Katsaros D, Lu L, Preti M, Durando A, Arisio R, Mu L, Yu H. 2009. High miR-21 expression in breast cancer associated with poor disease-free survival in early stage disease and high TGF-beta1. Breast Cancer Res Treat. 117:131–140.
- Rask L, Balslev E, Jorgensen S, Eriksen J, Flyger H, Moller S, et al. 2011. High expression of miR-21 in tumor stroma correlates with increased cancer cell proliferation in human breast cancer. APMIS 119:663–673.
- Roa W, Brunet B, Guo L, Amanie J, Fairchild A, Gabos Z, et al. 2010. Identification of a new microRNA expression profile as a potential cancer screening tool. Clin Invest Med. 33:E124.
- Sakurai M, Masuda M, Miki Y, Hirakawa H, Suzuki T, Sasano H. 2015. Correlation of miRNA expression profiling in surgical pathology materials, with Ki-67, HER2, ER and PR in breast cancer patients. Int J Biol Markers. 30:e190–e199.
- Shen L, Wan Z, Ma Y, Wu L, Liu F, Zang H, Xin S. 2015. The clinical utility of microRNA-21 as novel biomarker for diagnosing human cancers. Tumour Biol. 36:1993–2005.
- Stang A. 2010. Critical evaluation of the Newcastle–Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 25:603–605.
- Walter BA, Gomez-Macias G, Valera VA, Sobel M, Merino MJ. 2011. miR-21 expression in pregnancy-associated breast cancer: a possible marker of poor prognosis. J Cancer. 2:67–75.
- Wang G, Wang L, Sun S, Wu J, Wang Q. 2015. Quantitative measurement of serum microRNA-21 expression in relation to breast cancer metastasis in Chinese females. Ann Lab Med. 35:226–232.
- Wang Y, Zhang Y, Pan C, Ma F, Zhang S. 2015. Prediction of poor prognosis in breast cancer patients based on microRNA-21 expression: a meta-analysis. PLoS One. 10:e0118647.
- Yan LX, Huang XF, Shao Q, Huang MY, Deng L, Wu QL, Zeng YX, Shao JY. 2008. MicroRNA miR-21 overexpression in human breast cancer is associated with advanced clinical stage, lymph node metastasis and patient poor prognosis. RNA. 14:2348–2360.
- Yan LX, Liu YH, Xiang JW, Wu QN, Xu LB, Luo XL, et al. 2016. PIK3R1 targeting by miR-21 suppresses tumor cell migration and invasion by reducing PI3K/AKT signaling and reversing EMT, and predicts clinical outcome of breast cancer. Int J Oncol. 48:471–484. Epub 2015/12/18.
- Yan LX, Wu QN, Zhang Y, Li YY, Liao DZ, Hou JH, et al. 2011. Knockdown of miR-21 in human breast cancer cell lines inhibits proliferation, in vitro migration and in vivo tumor growth. Breast Cancer Res. 13:R2.