Abstract
Recent studies have elucidated that cell-based therapies are promising for cancer treatments. The human amniotic fluid stem (AFS) cells are advantageous cells for such therapeutic schemes that can be innately changed to express therapeutic proteins. HAFSCs display a natural tropism to cancer cells in vivo. They can be useful in cancer cells targeting. Moreover, they are easily available from surplus diagnostic samples during pregnancy and less ethical and legal concern are associated with the collection and application than other putative cells are subjected. This review will designate representatives of amniotic fluid and stem cell derived from amniotic fluid. For this propose, we collect state of human AFS cells data applicable in cancer therapy by dividing this approach into two main classes (nonengineered and engineered based approaches). Our study shows the advantage of AFS cells over other putative cells types in terms differentiation ability to a wide range of cells by potential and effective use in preclinical studies for a variety of diseases. This study has shown the elasticity of human AFS cells and their favorable potential as a multipotent cell source for regenerative stem cell therapy and capable of giving rise to multiple lineages including such as osteoblasts and adipocyte.
Introduction
Stem cells (SCs) have obtained a great deal of attention represent a potential and attractive cellular therapy for cancer diseases (Kim et al. Citation2010, Citation2011, Yi et al. Citation2011). A huge number of cancer therapy researches have been applied using adult stem cells, focusing on mesenchymal stem cells (MSCs) (natural tumor tropism MSCs) (Shinagawa et al. Citation2015). Few main sources of MSCs are available in the body such as bone marrow MSCs (BM-MSCs), peripheral blood, umbilical cord blood, cord tissue and adipose tissue (Nazari-Shafti et al. Citation2015). However, pluripotent embryonic stem cells (ESCs) have the potential to differentiate into a wide range of cell types, the drawbacks and limitations of adult MSCs are allowing them to present multipotency and capable of downstream generation of mesodermal cell types. This drawback could be interpreted to the low number of MSCs availability in related tissue (which declines with increasing age), slowly propagated in culture condition and a restricted differentiation potential (Nazari-Shafti et al. Citation2015, Murakami et al. Citation2015). In addition, less ethical and technical problems with the low risk of immunological rejection and teratoma formation and carcinoma development (Kern et al. Citation2006).
One of the main properties of MSCs is a low level of immune rejection for the reason that has the capability to escape from the natural immune system (Kovach et al. Citation2015). Amniotic fluid (AF) is a substitute source of ESCs that have promising clinical therapeutic applications. AF is commonly achieved in the second trimester during amniocentesis to identify any chromosomal malformations, abnormalities and also to determine the sex of the fetus (La Marca-Ghaemmaghami et al. Citation2015). In recent years, scientists have isolated and characterized AF derived stem cell populations that are highly multipotent, with the capability to differentiate into hematopoietic, chondrogenic, osteogenic, adipogenic, myogenic, endothelial, neural and lung cells, among other cell lineages (Loukogeorgakis and De Coppi Citation2016).
AF comprises of different cell types, deriving from embryonic and extra-embryonic tissues resembling closely to MSCs in typical characteristic and morphologically are close to fibroblast than having higher nuclear/cytoplasm ratio that is characteristic of pluripotent cells (Gosden Citation1983, Priest et al. Citation1978). The properties of these cells such as interacting with immune cells to modulate the immune response and the production of anti-inflammatory factors make perinatal stem cells an attractive alternative for cell therapy (Carlsson et al. Citation2015, Heldring et al. Citation2015). Consequently, the use of these stem cells for regeneration or replacement of damaged or diseased tissue such as bone defects, blood and immune system, neural degeneration, myocardial infarction, lung disease and diabetes would be valuable (Granero-Molto et al. Citation2008, Prentice Citation2006). It is well known that human embryonic stem cells are derivatives of the inner cell mass of fertilized embryos. These cells have two significant features: pluripotency and self-renewal (Xu et al. Citation2015) and in vitro, they can differentiate into cells of three germ layer. HESCs have many ethical problems and limitations in researches (Rezania Citation2015).
Amniotic-derived stem cells having unique characteristics such as (1) low immunogenicity because of the low expression level of major histocompatibility complex antigens, (2) low anti-inflammation when introduced to other bodies, (3) do not have any ethical objection, (4) their original sources including amniotic membrane and fluid are easily available and (5) a less restricted differentiation potential (hematopoietic, chondrogenic, osteogenic, adipogenic, myogenic, endothelial, neural and lung cells, among other cell lineages) (Gholizadeh-Ghalehaziz et al. Citation2015, Han et al. Citation2012). They have been most popular, because of this unique characteristic of human embryonic stem cells (Han et al. Citation2012) ().
Table 1. The biological properties of different stem cells.
This review will discuss the possible role of amniotic derived stem cells for various antitumor applications as new possibilities of human stem cells, by focusing on two approaches: Nonengineered and engineered stem cells strategies.
Development of AFSCs
The characterization of the composition of AFSCs and other cell populations surrounded by the amniotic fluid has been expansively studied (De Coppi et al. Citation2007a, Moorefield et al. Citation2010). Not amazingly, gestational age plays a significant role in the composition of cell populations derived from the AF (Perin et al. Citation2008). Therefore, when studying the therapeutic probability of AFSC, the probable changes in inherent characteristics of AFSCs, at their time of harvest, must be measured when assaying their regenerative or therapeutic potential (Da Sacco et al. Citation2010).
Gestationally older donor-derived AFSC could express lineage markers of terminally differentiated cell populations, which may be confirmed valuable for the replacement of specific injured populations or repopulation of adult lung tissue, while AFSCs derived from primitive uncommitted gestationally younger may be helpful for applications of de novo tissue engineering () (De Coppi et al. Citation2007a, Moorefield et al. Citation2010).
How to isolate and characterize AFSCs?
There are several isolation methods for AFSCs. These methods have indicated in previous studies and we collected useful isolation and characterization methods at previous study (Gholizadeh-Ghalehaziz et al. Citation2015).
One of the isolation and characterization techniques for AF stem cells, as shown by De Coppi et al. using a positive selection for cell membrane receptor c-kit (De Coppi et al. Citation2007a), which is specific for stem cell factor. The c-kit receptor and its ligand are also implicated in hematopoiesis and recognize a particular hematopoietic progenitor cell. The stem cell population is usually selected with the FACS (fluorescence-activated cell sorter) or MACS (magnetic-activated cell sorter) system only and 0.8–1% of the entire cell population expresses the surface marker.
Using FACS sorting, it was indicated that AFSCs express some surface markers and transcription factors characteristic of embryonic stem cells such us SSEA-4, NANOG and Oct4, proving that they own some essential characteristics that embryonic stem cells also have, and signifying that these cells keep pluripotential ability (Pashaiasl et al. Citation2016, Todorov et al. Citation2015).
Additionally, they stained positively for a range of cell surface markers distinctive of mesenchymal and/or neural stem cells, including CD44 (hyaluronan receptor), CD73, CD29, CD90, and CD105. The AFSCs are negative for markers of the hematopoietic stem cells (CD34, CD133) and of hematopoietic lineage (CD45) (De Coppi et al. Citation2007a, Toselli et al. Citation2008, Maraldi et al. Citation2014).
The c-kit-positive cells are instantaneously cultured in a Petri dishes with no require of feeder layer in Chang Media, with 5% CO2 atmosphere at 37 °C. In one week, they maintain a round shape while afterward they can develop into elongated and believe a fibroblast-like morphology. After this time, if having 70–80% of confluency, they can be cultured for many population doublings (De Coppi et al. Citation2007a, Toselli et al. Citation2008, Maraldi et al. Citation2014).
Others isolation techniques are a single-stage method (for adhering AF cells) (Graham and Fauza Citation2007) and two-stage method (Tsai et al. Citation2004) (for nonadhering AF cells). Both of them after obtaining written consent, AF was centrifuged, the cell pellets are seeded in special cell culture media such as DMEM (high glucose DMEM) (Steigman and Fauza Citation2007) or M199 (In ‘t Anker et al. Citation2004, Bossolasco et al. Citation2006b) or Iscove’s modified Dulbecco’s medium (IMDM) (Cipriani et al. Citation2007) or alpha MEM with 20% of Chang medium (Chang B plus Chang C) this procedure for single-stage method was continued for 2–3 weeks and twice per week cells medium was changed (Chiavegato et al. Citation2007). In two-stage method, after 5 days of primary amniocytes culture, non-adhering amniotic fluid cells in the supernatant medium were collected (first stage), centrifuged, and then plated in special AFSCs medium (second stage) such as AmnioMAx II complete medium. As revealed, two-stage method is more advantages compared to other methods, these advantages were illustrated in our previous study (Gholizadeh-Ghalehaziz et al. Citation2015).
Differentiation abilities of AFSCs
Many researchers have established the existence Oct4+/c-Kit + AFSCs cells and have informed their possible to differentiate into hematopoietic, osteogenic, neurogenic, adipogenic, chondrogenic, hepatic, renal, and various other lineages.
Differentiation to adipose tissue
To facilitate stimulation of the c-kit-positive AFSCs to differentiate into adipocyte cells, they are cultured in DMEM low glucose medium with 1% penicillin/streptomycin, 10% FBS and a different adipogenic supplement such as 3-isobutyl-1-methylxanthine, dexamethasone, insulin and indomethacin (Wolbank et al. Citation2007).
Differentiation to neural cells
The first verification that amniotic fluid contained cells harboring the possible of neurogenic differentiation was provided in 2004 by Prusa et al (Prusa et al. Citation2004). Several evidences signify the existence of more than one stem cell type in AF. Some authors have publicized that the c-kit-negative hAFSC population better differentiates into the neuronal lineage. On the other hand, monoclonal c-Kit+/Oct4 + AFS cells have been informed to differentiate into the neurogenic lineage, as established by morphology changes, specific marker expression, and electro-physiological analysis (De Coppi et al. Citation2007a, Toselli et al. Citation2008, Maraldi et al. Citation2014).
Differentiation to chondrocyte cells
Kolambkar et al. indicate that AFSCs are capable of generating a cartilage-like matrix in both hydrogel and pellet cultures. They also found that chondrogenic differentiation of AFSCs is culture condition dependent and seems to be less robust than that of bone marrow MSCs in pellet culture at three weeks with TGF-b1 supplementation (Kolambkar et al. Citation2007). This study indicates that AFSCs have the ability to differentiate along the chondrogenic lineage, thus establishing the probability of using these cells for cartilage repair applications.
Differentiation to endothelial cells
In one study, Griffith et al. show the effect of hypoxic culture on the endothelial differentiation of human amniotic fluid-derived stem cells and the differentiation potential of AFSCs to Endothelial cells (Lloyd‐Griffith et al. Citation2015). The 14-day culture period caused the AFSCs in normoxia, intermittent hypoxia, and continuous hypoxia to adopt a similar, albeit much less mature, endothelial gene expression profile to human umbilical vein endothelial cells (HUVECs). This endothelial expression profile was visible in the form of increased CD31, VEGFR2, and vWF expression and decreased angiopoietin 1 expression in comparison with AFSCs in growth media.
Characteristics of amniotic fluid/membrane
The membranous sac that contains the fetus and amniotic fluid is the amnion (De Coppi et al. Citation2007a). One of the main role of this compartment during parturition is enhancing the biosynthesis of prostaglandins (necessary for the initiation and maintenance of uterine contraction) (Toda et al. Citation2007). The fetus can directly supply by diffusion from the amniotic fluid and underlining decidua (Dobreva et al. Citation2010).
The AF includes water, growth factors (GF), fetus urine, proteins, lactate, electrolytes composition, carbohydrates, lipids, amino acids, pyruvate, hormones, and enzymes (Underwood et al. Citation2005). Furthermore, fluid excretions from the embryo into the AF transfer a multiplicity of embryo cells, follow-on in a heterogeneous populace of cells derived from embryo respiratory, gastrointestinal, skin, and urinary tracts, and the amniotic cover (Parolini et al. Citation2009). As the fetus matures, the bulk and opus of the AF conversion extremely, and the supplement of cells distinguished in AF samples taken at altered gestational eternities differs significantly (Calvet et al. Citation2016). Human AF was made at 2 weeks after reproduction in the amniotic cavity of primary maturation. Through gestation, AF is concealed mostly as an outcome of active passage of Na+ and Cl−, which is supplemented by the passage of water over the chorio-AM and embryo’s skin, along with many of protein molecules (Prusa et al. Citation2004). The assembly of urine and respiratory fluid both donates to the bulk of AF. AF is significant to save the fetus, and it cares tissue growth. The human AF has been proposed as a source of stem cells (Kaviani et al. Citation2001).
Most of amniotic cells in AF (between the first trimesters of pregnancy to the middle of the second trimester) are generated and drive from the fetus (Hoehn and Salk Citation1982), the yolk sac, amnion and placenta (Brace Citation1986). Generally, most of the AF is composed of fetal urine (Underwood et al. Citation2005), fetal respiratory (Duenhoelter and Pritchard Citation1976) and digestive tracts' cells (Minei and Suzuki Citation1976). These cells grow rapidly in routine culture (Li et al. Citation2015). Based on their morphological, biochemical, and growth characteristics of adherent AF cells, these cells can be classified into three groups: (1) AF-specific AF-type cells, (2) fibroblastic F-type cells, and (3) epithelioid E-type cells (Pipino and Pandolfi Citation2015). Both the AF-specific AF-type cells and the epithelioid E-type cells are found in the initial passages of cultivation. The AF-specific AF-type cells are likely derived from placental trophoblastic tissue and produce estrogen, chorionic gonadotropin, and progesterone, but the epithelioid E-type cells likely derived from the fetal skin (Pipino and Pandolfi Citation2015).
Characteristics of amniotic fluid stem cell
A wide variety of different cell types has found in AF which has properties that are mainly derived from fetal tissues (Gosden Citation1983). So, different types of stem cells can be obtained from the amniotic fluid such as amniotic membrane-derived mesenchymal (Jiao et al. Citation2012, Manuelpillai et al. Citation2010), amniotic membrane-derived epithelial (Manuelpillai et al. Citation2010, Marongiu et al. Citation2011), and AF stem cells.
The human amniotic membrane-derived mesenchymal stem cells (hAMSCs) are isolated from the amniotic membrane (Da Sacco et al. Citation2010, Dobreva et al. Citation2010, Jiao et al. Citation2012). They are positive for REX1, SOX2, NANOG, NESTIN (neural stem cell marker), and CF (stem cell factor, a ligand of c-kit) (Kobayashi et al. Citation2008, Kim et al. Citation2007). The hAMSCs have been shown to differentiate into different cell types such as chondrogenic, adipogenic, osteogenic, skeletal- and cardiomyogenic, hepatocyte-like cells, endothelial cells, and neuroglial cells.
On the other hand, human AF-derived stem cells (hAFSCs) comprises the developing embryo and during the gestation period, directly contacts with the amniotic membrane (De Coppi et al. Citation2007a, Phermthai et al. Citation2010). HAFSCs were scored positive for Oct4 (Prusa et al. Citation2003), CD73 (SH3/4), CD90, CD105 (SH2), and CD166, but negative for hematopoietic markers (Scherjon et al. Citation2003). The fibroblastic F-type cells are adherent cells and are characterized by rapid proliferation with phenotypes and multilineage differentiation similar to BMMSCs (Fauza Citation2004, Prusa and Hengstschlager Citation2002).
Flow cytometry has been extensively used for characterization of the adherent human AF cells expanded in culture. Human AFSCs can express some markers similar to BMMSCs, such as human leukocyte antigen class I (not human leukocyte antigen class II) CD166, CD105 (endoglin), CD90, CD73, CD44 (hyaluronan receptor) and CD29 (Bossolasco et al. Citation2006a, Roubelakis et al. Citation2007, Tsai et al. Citation2004). Because of owing differentiation into a variety of cell types including chondrogenic, osteogenic and adipogenic lineages, human AFSCs are multipotent cells (Bossolasco et al. Citation2006a, Kim et al. Citation2007, Tsai et al. Citation2004, Sessarego et al., Citation2008), and are usually termed human AF mammalian stem cells (hAF-mSCs) (Bossolasco et al., Citation2006a, Kim et al., Citation2007, Tsai et al., Citation2004, Sessarego et al., Citation2008).
Some of the markers that simultaneously express in hAFSCs (specific markers of human embryonic cells) associated with pluripotency are (1) stage-specific embryonicantigen-4 (SSEA-4), (2) NANOG protein (responsible for pluripotency), and (3) Oct4 (an embryonic SC marker) (Prusa et al. Citation2003, Pashaiasl et al. Citation2016, Tsai et al. Citation2004).
Generally, hAFSCs exhibit, under specific culture conditions, the ability to differentiate into hepatogenic, myogenic and neuronal cell lineages and express genes characteristic of ectodermal, mesodermal and endodermal germ layers (Bossolasco et al., Citation2006a, De Coppi et al. Citation2007b, Prusa et al. Citation2003). HAFSCs, as well as embryonic SCs and cancer cells, also exhibit a high level of telomerase activity, which protects theme against senescence by inhibiting the progressive shortening of chromosomal telomeres. Compared with BMMSCs, hAF-MSCs have a great proliferative capacity, which because of significantly greater telomere length (Sessarego et al. Citation2008). Owing a proliferative rate in culture greater than adult MSCs, hAFSCs have a high expansion rate in vitro.
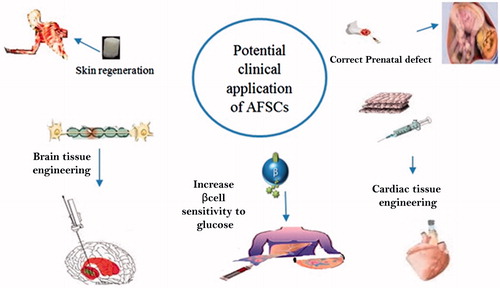
The hAFSCs do not induce teratoma transformation similar to embryonic SCs or do not undergo a neoplastic transformation in vitro (Sessarego et al. Citation2008), but they have a marked high proliferative and differentiation capacity. Because hAFSCs are not tumorigenic in vivo, so these can be used in eventual clinical applications for regenerative medicine because injections of hAF-MSCs into immunodeficient animals do not induce tumor formation (Sessarego et al. Citation2008). Using of AFSCs are free of ethical constraints, and without injury to the fetus, they can be readily isolated during amniocentesis, thus shown great promising in treatment of diseases including cancer (Bitsika et al. Citation2011, Li et al. Citation2015), and other diseases such as kidney (Hauser et al. Citation2010, Perin et al. Citation2010), Neural disorders (Rosser et al. Citation2007), cardiac disease (Bollini et al. Citation2011a, Citation2011b), intestinal disorders (Zani et al. Citation2013), lung diseases (Joo et al. Citation2012, Li et al. Citation2016), or dermal and embryo disorders (Shaw et al. Citation2011, Skardal et al. Citation2012) and some applications of human amniotic membrane-derived mesenchymal stem cell (Jiao et al. Citation2012, Zhang et al. Citation2011) and human amniotic membrane-derived epithelial stem cell (Luo et al. Citation2011, Manuelpillai et al. Citation2010, Marongiu et al. Citation2011) were shown in .
Table 2. Some examples of the therapeutic potential of amniotic stem cells.
HAFSCs and cancer
When human amniotic stem cells are cocultured with human tumor cells, the viability of cancer cells can be decreased by the presence of human amniotic stem cells expressing cytotoxic factors (such as tumor necrosis factor-a, interferon, transforming growth factor-b or ILs) (Kang et al., Citation2012a).
Cancer treatment using cell-based therapies has been supported in recent studies. One of promising cell type for such therapeutic approach is the mesenchymal stem cells (MSCs) derived from amniotic fluid that display a distinctive tropism to solid tumors in vivo, innately modified to express therapeutic proteins and can be easily propagated in culture. Generally, two main approaches are for cell-based cancer therapy using amniotic stem cells: Non-engineered or engineered stem cells strategies (Kang et al., Citation2012a).
Nonengineered stem cells strategies
In nonengineered stem cell displacement approaches, amnion-derived stem cells efficiently goal cancer and inhibited the tumor progression by stating cytotoxic cytokines or cancer conqueror gene (Kang et al. Citation2012a). Also, they furthermore have a possible as unique delivery vehicles transporting remedy genes to the tumor development places in gene-focused enzyme/prodrug amalgamation therapy. Now, the cured human AF is broadly used as a biomaterial for clinical treatment (Grskovic et al. Citation2011).
However, extracted AFSCs to be used in the cell therapy essentials to be sensibly preferred to balance usefulness and care for a specific tumor type. MSCs from AM and AF likely one of the tumor cell progress suppressor or a novel transfer vehicle for antitumor outcomes (Lai Citation2010). They prevent propagation of cancer cell lines of the hematopoietic and nonhematopoietic source by promoting cell cycle capture or prompt C6 glioma apoptosis in vivo over the Bcl-2/caspase pathways. The two basic stem cells also are proficient of self-renewal and can produce segregated posterities for organ progress as well (Méndez-Ferrer et al. Citation2010). They are deliberated as a probable source for renewing treatment and tissue spare after disease. They are in an intermediary phase among pluripotent embryonic stem cells and extraction-limited adult stem cells (Takahashi et al. Citation2007).
In the absence of normal autologous cells, multipotent stem cells including the hAFSCs may be useful as a promising and secure source of flexible cells for bladder tissue engineering and regeneration applications (De Coppi et al. Citation2007a). Chung and Koh (Citation2013) investigate the role of FGF10 as a lead induction factor for stem cell differentiation bladder cancer lines toward urothelial cells. As the aim of directed induce to differentiation into urothelial cells, hAFSCs were co-cultured with immortalized bladder cancer lines. Cocultured stem cells began to express urothelial markers such as uroplakin II, III and cytokeratin 8. Collectively, this report recommends that differentiation of human amniotic stem cell into urothelial cells lineage can stimulate by paracrine FGF10 signaling. So using of hAFSCs in the presence of FGF10 leads to bladder regeneration and therapeutic application for bladder transplantation (Chung and Koh Citation2013).
Scientists detected the expressions of cytokines or tumoricidal factors in amniotic-derived stem cells. HAFS cells rapid both embryonic and adult stem cell markers and can be prompted to separate into cell forms derived from diverse germ layers, comprising cells of osteogenic, myogenic, neuronal, adipogenic, endothelial, and hepatic lineages (Eberli and Atala Citation2006). It has newly been conveyed that hAFS cells can form duct-like linkages and neuron-like cells, but there is slight evidence on their influence to wound remedial. Flow cytometry showed that hAFS cells prompt the embryonic stem cell markers Oct-4, hTERT, SSEA-1, SSEA-4, and CD117 but not SSEA-3. These cells also prompt mesenchymal stem cell markers CD29, CD44, CD73, CD90, and CD105, which are markers of the hematopoietic lineage but are adverse for CD45(Schiller and D’ippolito, Citation2014). HAFS cells also express both CD34 and CD133, markers of hematopoietic stem cells, proposing that hAFS cells have the features of embryonic stem cells (Spinelli et al. Citation2013, Zhou et al. Citation2014). In addition, hAFS cells revelation little immunogenicity.
Engineered stem cells strategies
In one study scientists (Bitsika et al. Citation2011), investigate the AF-MSCs tropism and the potential to deliver interferon beta (IFNβ) to the bladder tumor model (region of neoplasia). They show the prolonged survival of mice in the presence of AF-MSC-IFN-β and significant inhibition of tumor growth. Generally, the results of this study have shown the great potential of AF-MSCs as anti-cancer vehicles, which specifically target the tumor site. This vehicle has high proliferation rate and expansion efficiency in culture.
In recent study (Kang et al. Citation2012b), scientists used hAFSCs as tools for targeted delivery of therapeutic suicide genes to breast cancer cells, which produce AF2.CD-TK cells so as to express two suicide genes encoding herpes simplex virus thymidine kinase (HSV-TK) cytosine deaminase (CD) and that convert nontoxic prodrugs, mono-phosphorylate ganciclovir (GCV-MP) and 5-fluorocytosine (5-FC), into cytotoxic metabolites, triphosphate ganciclovir (GCV-TP) and 5-fluorouracil (5-FU), respectively. Cell viability in vitro assay has revealed that, AF2.CD-TK cells in the presence of the GCV or 5-FC prodrugs or a combination of these two reagents, AF2.CD-TK cells inhibit the growth of MDA-MB-231 human breast cancer. Collectively, the results of this study present the AF2.CD-TK cells as excellent vehicles which can be used as a novel therapeutic cell-based gene-directed prodrug system to selectively target breast malignancies.
Studies have been considered the tumorigenic phenotype of aggressive cancer cells suppression using human embryonic stem cell microenvironment. LM Postovit et al (Citation2008) tested the possibility of cancer cells react to regulatory signals monitoring the Nodal signaling pathway. Metastatic tumor cells cannot express the inhibitor to Nodal, Lefty, which allow overexpressing of this embryonic morphogen in an unregulated manner. Exposure of the tumor cells to a hESC microenvironment containing Lefty results in a dramatic down-regulation in their Nodal expression as well as a reduction in clonogenicity and tumorigenesis (associated with the secretion of Lefty, exclusive to hESCs) and an increase in apoptosis. This tumor-suppressive effects hESC (neutralizing the expression of Nodal in aggressive tumor cells) introduce promising therapeutic modalities for cancer treatment.
HESC differentiation can prevent by overexpression of Nodal, so inhibit of Nodal signaling in metastatic melanoma cells may decrease colony formation in soft agar and a significant tumor formation repeal in an orthotopic mouse model (Topczewska et al. Citation2006, Vallier et al. Citation2007). Accordingly, studies have been shown embryonic microenvironments can inhibit the tumorigenicity of a variety of cancer cell lines (Topczewska et al. Citation2006, Vallier et al. Citation2007, Hendrix et al. Citation2007).
For example, in one study, the embryonic microenvironment of mouse reprogram teratocarcinoma cells to a nontumorigenic phenotype, which has the possibility of differentiating into healthy tissues (Hendrix et al. Citation2007). Definitely, even though still present after a 3 months period of examination, melanoma cells implanted into zebrafish embryos lay latent and were incapable of forming tumors (Lee et al. Citation2005). Amusingly, this experience is unique to the embryonic zebrafish microenvironment, as human melanoma cells transplanted into zebrafish 2 days after fertilization form tumors and even provoke angiogenesis (Haldi et al. Citation2006).
Suicide genes (effectively converts nontoxic prodrugs into their highly cytotoxic forms) can be effectively used for cancer gene therapies using stem cells. As this aim, stem cells has been used as suicide gene transfer vehicles for tumors which known as gene-directed enzyme/prodrug combination (GEPC) therapy, such as carboxylesterase, cytosine deaminase (CD) and/or herpes simplex virus thymidine kinase by adenovirus, retrovirus or lentivirus (Aghi et al. Citation1998, Anderson et al. Citation2000).
Collectively, hAFSC gradually became a hot topic in human research direction for disease treatment, because of their reduced immunogenicity, their plasticity, and their tumor tropism apart from the tumor size, source, and location. Li et al. (Citation2015) detect high motility of hAFSC to migrate to ovarian cancer site in nude mice model, but did not have the tumorigenicity. The results of this study enhance the potential of AFMSCs as a drug carrier in human cell-based therapy ().
Conclusions
Stem cells have capable of self-renewal and can create differentiated progenies for the development of an organ, so have the therapeutic potential for regenerative medicine and tissue replacement after injury or disease and for treating human diseases including cancers (Kang et al. Citation2012a).
Stem cells derived from human amniotic membrane/fluid have a high proliferative potential, express Oct4 and NANOG mRNA (that is specific to pluripotent stem cells) (Prusa et al. Citation2003) and was scored positive for mesenchymal markers, such as CD73 (SH3/4), CD105 (SH2), CD90, and CD166, but negative for hematopoietic markers (Scherjon et al. Citation2003). AF-derived stem cells are more advantageous than adult stem cells and are known for having unique characteristics, such as nontumorigenic and cause low immunogenicity and anti-inflammation, can be isolated noninvasively in large scales without the ethical reservations associated with embryo research and a less restricted differentiation potential, as well as they are in an intermediate stage between pluripotent ESCs and lineage-restricted adult stem cells, expressing the transcription factor Oct4 and NANOG that has an important role in maintaining pluripotency and self-renewal (Kang et al. Citation2012a, Pashaiasl et al. Citation2016).
Several studies have proved that AF-derived stem cells suggest a new tool in the stem cell therapy, as they can efficiently target the tumor site and reduce tumor burden (Bitsika et al. Citation2011, Li et al. Citation2015). Natural tumor tropism of this cells and their low immunogenicity presents AF-derived stem cells as promising therapeutic tools in cancer gene therapy. Collectively, the results of all studies discussed previously shown that human AF-derived stem cells may present promising their antitumor effects via tumor tropism and can be a novel approach to selectively target human cancers. This result may suitable evidence for forthcoming clinical applications of hAFS cells. We consider that hAFS cells can expose a new area of stem cell study and offer a new source of germ cells in cancer therapy. There are required further studies to investigate the most precise mechanism by which amniotic fluid-derived stem cells exert their anticancer effect on cancer cells.
Acknowledgements
The authors thank the Department of Molecular Medicine, Faculty of Advanced Medical Sciences, Tabriz University of Medical Science and Women’s Reproductive Health Research Center Al-Zahra Hospital, Tabriz University of Medical Sciences for all support provided (Study ID: 93/4-10/2).
Disclosure statement
The authors declare no conflicts of interest. The authors alone are responsible for the content and writing of the manuscript.
References
- Aghi M, Cho TC, Chiocca EA, Kramm CM, Breakefield XO. 1998. Synergistic anticancer effects of ganciclovir/thymidine kinase and 5-fluorocytosine/cytosine deaminase gene therapies. J Natl Cancer Inst 90:370–380.
- Anderson LM, Krotz S, Weitzman SA, Thimmapaya B. 2000. Breast cancer-specific expression of the Candida albicans cytosine deaminase gene using a transcriptional targeting approach. Cancer Gene Ther 7:845–852.
- Bitsika V, Roubelakis MG, Zagoura D, Trohatou O, Makridakis M, Pappa KI, et al. 2011. Human amniotic fluid-derived mesenchymal stem cells as therapeutic vehicles: a novel approach for the treatment of bladder cancer. Stem Cells Dev 21:1097–1111.
- Bollini S, Cheung KK, Riegler J, Dong X, Smart N, Ghionzoli M, et al. 2011a. Amniotic fluid stem cells are cardioprotective following acute myocardial infarction. Stem Cells Dev 20:1985–1994.
- Bollini S, Pozzobon M, Nobles M, Riegler J, Dong X, Piccoli M, et al. 2011b. In vitro and in vivo cardiomyogenic differentiation of amniotic fluid stem cells. Stem Cell Rev Rep 7:364–380.
- Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Buiatiotis S, et al. 2006a. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res 16:329–336.
- Bossolasco P, Montemurro T, Cova L, Zangrossi S, Calzarossa C, Buiatiotis S, et al. 2006b. Molecular and phenotypic characterization of human amniotic fluid cells and their differentiation potential. Cell Res 16:329–336.
- Brace RA. 1986. Amniotic fluid volume and its relationship to fetal fluid balance: review of experimental data. Semin Perinatol 10:103–112.
- Calvet G, Aguiar RS, Melo AS, Sampaio SA, De Filippis I, Fabri A, et al. 2016. Detection and sequencing of Zika virus from amniotic fluid of fetuses with microcephaly in Brazil: a case study. Lancet Infect Dis 16:653–660.
- Carlsson PO, Korsgren O, Le Blanc K. 2015. Mesenchymal stromal cells to halt the progression of type 1 diabetes? Curr Diab Rep 15:46. doi: 10.1007/s11892-015-0616-3.
- Chiavegato A, Bollini S, Pozzobon M, Callegari A, Gasparotto L, Taiani J, et al. 2007. Human amniotic fluid-derived stem cells are rejected after transplantation in the myocardium of normal, ischemic, immuno-suppressed or immuno-deficient rat. J Mol Cell Cardiol 42:746–759.
- Chung SS, Koh CJ. 2013. Bladder cancer cell in co-culture induces human stem cell differentiation to urothelial cells through paracrine FGF10 signaling. In Vitro Cell Dev Biol-Anim 49:746–751.
- Cipriani S, Bonini D, Marchina E, Balgkouranidou I, Caimi L, Grassi Zucconi G, Barlati S. 2007. Mesenchymal cells from human amniotic fluid survive and migrate after transplantation into adult rat brain. Cell Biol Int 31: 845–850.
- Da Sacco S, Sedrakyan S, Boldrin F, Giuliani S, Parnigotto P, Habibian R, et al. 2010. Human amniotic fluid as a potential new source of organ specific precursor cells for future regenerative medicine applications. J Urol 183: 1193–1200.
- De Coppi P, Bartsch G, Siddiqui MM, Xu T, Santos CC, Perin L, et al. 2007a. Isolation of amniotic stem cell lines with potential for therapy. Nat Biotechnol 25:100–106.
- De Coppi P, Callegari A, Chiavegato A, Gasparotto L, Piccoli M, Taiani J, et al. 2007b. Amniotic fluid and bone marrow derived mesenchymal stem cells can be converted to smooth muscle cells in the cryo-injured rat bladder and prevent compensatory hypertrophy of surviving smooth muscle cells. J Urol 177: 369–376.
- Dobreva MP, Pereira P, Deprest J, Zwijsen A. 2010. On the origin of amniotic stem cells: of mice and men. Int J Dev Biol 54:761–777.
- Duenhoelter JH, Pritchard JA. 1976. Fetal respiration: quantitative measurements of amnionic fluid inspired near term by human and rhesus fetuses. Am J Obstet Gynecol 125:306–309.
- Eberli D, Atala A. 2006. Tissue engineering using adult stem cells. Method Enzymol 420: 287–302.
- Fauza D. 2004. Amniotic fluid and placental stem cells. Best Pract Res Clin Obstet Gynaecol 18:877–891.
- Gholizadeh-Ghalehaziz S, Farahzadi R, Fathi E, Pashaiasl M. 2015. A mini overview of isolation, characterization and application of amniotic fluid stem cells. Int J Stem Cells 8:115–120.
- Gosden CM. 1983. Amniotic fluid cell types and culture. Br Med Bull 39:348–354.
- Graham CD, Fauza DO. 2007. Isolation of mesenchymal stem cells from amniotic fluid and placenta. Curr Protoc Stem Cell Biol 1E. 2.1–1E. 2.14.
- Granero-Molto F, Weis JA, Longobardi L, Spagnoli A. 2008. Role of mesenchymal stem cells in regenerative medicine: application to bone and cartilage repair. Expert Opin Biol Ther 8:255–268.
- Grskovic M, Javaherian A, Strulovici B, Daley GQ. 2011. Induced pluripotent stem cells-opportunities for disease modelling and drug discovery. Nat Rev Drug Discov 10:915–929.
- Haldi M, Ton C, Seng WL, McGrath P. 2006. Human melanoma cells transplanted into zebrafish proliferate, migrate, produce melanin, form masses and stimulate angiogenesis in zebrafish. Angiogenesis 9:139–151.
- Han Y, Tao R, Sun T, Chai J, Xu G, Liu J. 2012. Advances and opportunities for stem cell research in skin tissue engineering. Eur Rev Med Pharmacol Sci 16:1873–1877.
- Hauser PV, De Fazio R, Bruno S, Sdei S, Grange C, Bussolati B, Benedetto C, Camussi G. 2010. Stem cells derived from human amniotic fluid contribute to acute kidney injury recovery. Am J Pathol 177:2011–2021.
- Heldring N, Mäger I, Wood MJ, Le Blanc K, Andaloussi SE. 2015. Therapeutic potential of multipotent mesenchymal stromal cells and their extracellular vesicles. Hum Gene Ther 26:506–517.
- Hendrix MJ, Seftor EA, Seftor RE, Kasemeier-Kulesa J, Kulesa PM, Postovit LM. 2007. Reprogramming metastatic tumour cells with embryonic microenvironments. Nat Rev Cancer 7:246–255.
- Hoehn H, Salk D. 1982. Morphological and biochemical heterogeneity of amniotic fluid cells in culture. Methods Cell Biol 26:11–34.
- In ‘T Anker PS, Scherjon SA, Kleijburg-Van Der Keur C, De Groot-Swings GM, Claas FH, Fibbe WE, Kanhai HH. 2004. Isolation of mesenchymal stem cells of fetal or maternal origin from human placenta. Stem Cells 22:1338–1345.
- Jiao H, Guan F, Yang B, Li J, Song L, Hu X, Du Y. 2012. Human amniotic membrane derived-mesenchymal stem cells induce C6 glioma apoptosis in vivo through the Bcl-2/caspase pathways. Mol Biol Rep 39:467–473.
- Joo S, Ko IK, Atala A, Yoo JJ, Lee SJ. 2012. Amniotic fluid-derived stem cells in regenerative medicine research. Arch Pharmacal Res 35:271–280.
- Kang N, Hwang K, Kim S, Kim Y, Hyun S, Jeung E, Choi K. 2012a. Potential antitumor therapeutic strategies of human amniotic membrane and amniotic fluid-derived stem cells. Cancer Gene Ther 19:517–522.
- Kang N, Hwang K, Yi B, Lee H, Jeung E, Kim S, Choi K. 2012b. Human amniotic fluid-derived stem cells expressing cytosine deaminase and thymidine kinase inhibits the growth of breast cancer cells in cellular and xenograft mouse models. Cancer Gene Ther 19:412–419.
- Kaviani A, Perry TE, Dzakovic A, Jennings RW, Ziegler MM, Fauza DO. 2001. The amniotic fluid as a source of cells for fetal tissue engineering. J Pediatr Surg 36:1662–1665.
- Kern S, Eichler H, Stoeve J, Klüter H, Bieback K. 2006. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 24:1294–1301.
- Kim J, Lee Y, Kim H, Hwang K, Kwon H, Kim S, et al. 2007. Human amniotic fluid-derived stem cells have characteristics of multipotent stem cells. Cell Prolif 40:75–90.
- KIM KY, Kim SU, Leung PC, Jeung EB, Choi KC. 2010. Influence of the prodrugs 5-fluorocytosine and CPT-11 on ovarian cancer cells using genetically engineered stem cells: tumor-tropic potential and inhibition of ovarian cancer cell growth. Cancer Sci 101:955–962.
- Kim SU, Jeung EB, Kim YB, Cho MH, Choi KC. 2011. Potential tumor-tropic effect of genetically engineered stem cells expressing suicide enzymes to selectively target invasive cancer in animal models. Anticancer Res 31:1249–1258.
- Kobayashi M, Yakuwa T, Sasaki K, Sato K, Kikuchi A, Kamo I, Yokoyama Y, Sakuragawa N. 2008. Multilineage potential of side population cells from human amnion mesenchymal layer. Cell Transplant 17:291–301.
- Kolambkar YM, Peister A, Soker S, Atala A, Guldberg RE. 2007. Chondrogenic differentiation of amniotic fluid-derived stem cells. J Mol Histol 38:405–413.
- Kovach TK, Dighe AS, Lobo PI, Cui Q. 2015. Interactions between MSCs and immune cells: implications for bone healing. J Immunol Res 2015:752510. doi: 10.1155/2015/752510.
- La Marca-Ghaemmaghami P, Dainese SM, La Marca R, Zimmermann R, Ehlert U. 2015. The acute autonomic stress response and amniotic fluid glucocorticoids in second-trimester pregnant women. Psychosom Med 77:41–49.
- Lai E. 2010. Regenerative medicine at early echelons: changing medical care & outcomes. DTIC Document.
- Lee LM, Seftor EA, Bonde G, Cornell RA, Hendrix MJ. 2005. The fate of human malignant melanoma cells transplanted into zebrafish embryos: assessment of migration and cell division in the absence of tumor formation. Dev Dynam 233:1560–1570.
- Li L, Li S, Cai T, Wang H, Xie X, Liu Z, Zhang Y. 2016. The targeted inhibitory effects of human amniotic fluid stem cells carrying CXCR4 promoter and DAL-1 on non-small cell lung carcinoma growth. Gene Ther 23:214–222.
- Li L, Wang D, Zhou J, Cheng Y, Liang T, Zhang G. 2015. Characteristics of human amniotic fluid mesenchymal stem cells and their tropism to human ovarian cancer. PLoS One 10:e0123350. doi: 10.1371/journal.pone.0123350.
- Lloyd‐Griffith C, Duffy GP, O’Brien FJ. 2015. Investigating the effect of hypoxic culture on the endothelial differentiation of human amniotic fluid‐derived stem cells. J Anat 227:767–780.
- Loukogeorgakis SP, De Coppi P. 2016. Stem cells from amniotic fluid–Potential for regenerative medicine. Best Pract Res Clin Obstet Gynaecol 31:45–57.
- Luo H, Huang X, Huang F, Liu X. 2011. Preliminary study on transdifferentiation of human amniotic epithelial cells and its intrasplenic transplantation. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 25:144–148.
- Manuelpillai U, Tchongue J, Lourensz D, Vaghjiani V, Samuel CS, Liu A, Williams ED, Sievert W. 2010. Transplantation of human amnion epithelial cells reduces hepatic fibrosis in immunocompetent CCl4-treated mice. Cell Transplant 19:1157–1168.
- Maraldi T, Bertoni L, Riccio M, Zavatti M, Carnevale G, Resca E, et al. 2014. Human amniotic fluid stem cells: neural differentiation in vitro and in vivo. Cell Tissue Res 357:1–13.
- Marongiu F, Gramignoli R, Dorko K, Miki T, Ranade AR, Paola Serra M, et al. 2011. Hepatic differentiation of amniotic epithelial cells. Hepatology 53:1719–1729.
- Méndez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, et al. 2010. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466:829–834.
- Minei LJ, Suzuki K. 1976. Role of fetal deglutition and micturition in the production and turnover of amniotic fluid in the monkey. Obstet Gynecol 48:177–181.
- Moorefield EC, Delo DM, De Coppi P, Atala A. 2010. Amniotic fluid and placental stem cells. Pluripotent Stem Cells. New York: Nova Science. pp. 113–135.
- Murakami M, Hayashi Y, Iohara K, Osako Y, Hirose Y, Nakashima M. 2015. Trophic effects and regenerative potential of mobilized mesenchymal stem cells from bone marrow and adipose tissue as alternative cell sources for pulp/dentin regeneration. Cell Transplant 24:1753–1765.
- Nazari-Shafti TZ, Bruno IG, Martinez RF, Coleman ME, Alt EU, McClure SR. 2015. High yield recovery of equine mesenchymal stem cells from umbilical cord matrix/Wharton’s jelly using a semi-automated process. Methods Mol Biol 1235:131–146.
- Parolini O, Soncini M, Evangelista M, Schmidt D. 2009. Amniotic membrane and amniotic fluid-derived cells: potential tools for regenerative medicine? Regen Med 4:275–291.
- Pashaiasl M, Khodadadi K, Kayvanjoo AH, Pashaei-Asl R, Ebrahimie E, Ebrahimi M. 2016. Unravelling evolution of Nanog, the key transcription factor involved in self-renewal of undifferentiated embryonic stem cells, by pattern recognition in nucleotide and tandem repeats characteristics. Gene 578:194–204.
- Perin L, Sedrakyan S, Da Sacco S, De Filippo R. 2008. Characterization of human amniotic fluid stem cells and their pluripotential capability. Methods Cell Biol 86:85–99.
- Perin L, Sedrakyan S, Giuliani S, Da Sacco S, Carraro G, Shiri L, et al. 2010. Protective effect of human amniotic fluid stem cells in an immunodeficient mouse model of acute tubular necrosis. PLoS One 5:e9357.
- Phermthai T, Odglun Y, Julavijitphong S, Titapant V, Chuenwattana P, Vantanasiri C, Pattanapanyasat K. 2010. A novel method to derive amniotic fluid stem cells for therapeutic purposes. BMC Cell Biol 11:79.
- Pipino C, Pandolfi A. 2015. Osteogenic differentiation of amniotic fluid mesenchymal stromal cells and their bone regeneration potential. World J Stem Cells 7:681.
- Postovit LM, Margaryan NV, Seftor EA, Kirschmann DA, Lipavsky A, Wheaton WW, et al. 2008. Human embryonic stem cell microenvironment suppresses the tumorigenic phenotype of aggressive cancer cells. Proc Natl Acad Sci U.S.A. 105:4329–4334.
- Prentice DA. 2006. Current science of regenerative medicine with stem cells. J Investig Med 54:33–37.
- Priest R, Marimuthu K, Priest J. 1978. Origin of cells in human amniotic fluid cultures: ultrastructural features. Lab Invest 39:106–109.
- Prusa AR, Hengstschlager M. 2002. Amniotic fluid cells and human stem cell research: a new connection. Med. Sci. Monit 8:RA253–RA257.
- Prusa AR, Marton E, Rosner M, Bettelheim D, Lubec G, Pollack A, Bernaschek G, Hengstschläger M. 2004. Neurogenic cells in human amniotic fluid. Am J Obstet Gynecol 191:309–314.
- Prusa AR, Marton E, Rosner M, Bernaschek G, Hengstschläger M. 2003. Oct-4-expressing cells in human amniotic fluid: a new source for stem cell research? Human Reprod 18:1489–1493.
- Rezania A. 2015. Differentiation of human embryonic stem cells. US Patent 20,150,191,700.
- Rosser AE, Zietlow R, Dunnett SB. 2007. Stem cell transplantation for neurodegenerative diseases. Curr Opin Neurol 20:688–692.
- Roubelakis MG, Pappa KI, Bitsika V, Zagoura D, Vlahou A, Papadaki HA, Antsaklis A, Anagnou NP. 2007. Molecular and proteomic characterization of human mesenchymal stem cells derived from amniotic fluid: comparison to bone marrow mesenchymal stem cells. Stem Cells Dev 16:931–952.
- Scherjon SA, Kleijburg-Van Der Keur C, Noort WA, Claas FH, Willemze R, Fibbe WE, Kanhai HH. 2003. Amniotic fluid as a novel source of mesenchymal stem cells for therapeutic transplantation. Blood 102:1548–1549.
- Schiller PC, D’Ippolito G. 2014. Non-expanded post-natal multilineage-inducible cells. Google Patents.
- Sessarego N, Parodi A, Podestà M, Benvenuto F, Mogni M, Raviolo V, et al. 2008. Multipotent mesenchymal stromal cells from amniotic fluid: solid perspectives for clinical application. Haematologica 93:339–346.
- Shaw SWS, David AL, De Coppi P. 2011. Clinical applications of prenatal and postnatal therapy using stem cells retrieved from amniotic fluid. Curr Opin Obstet Gynecol 23:109–116.
- Shinagawa K, Kitadai Y, Yuge R, Onoyama M, Tanaka S, Yasui W, Chayama K. 2015. Treatment with regorafenib inhibits the tumor-promoting effect of bone marrow-derived mesenchymal stem cells in an orthotopic nude mice model of colon cancer. Cancer Res 75:5068–5068.
- Skardal A, Mack D, Kapetanovic E, Atala A, Jackson JD, Yoo J, Soker S. 2012. Bioprinted amniotic fluid-derived stem cells accelerate healing of large skin wounds. Stem Cells Transl Med 1:792.
- Spinelli V, Guillot PV, De Coppi P. 2013. Induced pluripotent stem (iPS) cells from human fetal stem cells (hFSCs). Organogenesis 9:101–110.
- Steigman SA, Fauza DO. 2007. Isolation of mesenchymal stem cells from amniotic fluid and placenta. Curr Protoc Stem Cell Biol. doi: 10.1002/9780470151808.sc01e02s1.
- Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S. 2007. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131:861–872.
- Toda A, Okabe M, Yoshida T, Nikaido T. 2007. The potential of amniotic membrane/amnion-derived cells for regeneration of various tissues. J Pharmacol Sci 105:215–228.
- Todorov P, Petrova N, Mihova A, Guenova M, Arabadzhiev B, Hristova E. 2015. The female age influences the expression of pluripotent stem cells markers in human ovarian cells. C R Acad Bulg Sci 68:65–70.
- Topczewska JM, Postovit LM, Margaryan NV, Sam A, Hess AR, Wheaton WW, et al. 2006. Embryonic and tumorigenic pathways converge via Nodal signaling: role in melanoma aggressiveness. Nature Med 12:925–932.
- Toselli M, Cerbai E, Rossi F, Cattaneo E. 2008. Do amniotic fluid-derived stem cells differentiate into neurons in vitro? Nat Biotechnol 26:269–270.
- Tsai MS, Lee JL, Chang YJ, Hwang SM. 2004. Isolation of human multipotent mesenchymal stem cells from second‐trimester amniotic fluid using a novel two‐stage culture protocol. Human Reprod 19:1450–1456.
- Underwood MA, Gilbert WM, Sherman MP. 2005. Amniotic fluid: not just fetal urine anymore. J Perinatol 25:341–348.
- Vallier L, Alexander M, Pedersen R. 2007. Conditional gene expression in human embryonic stem cells. Stem Cells 25:1490–1497.
- Wolbank S, Peterbauer A, Fahrner M, Hennerbichler S, Van Griensven M, Stadler G, Redl H, Gabriel C. 2007. Dose-dependent immunomodulatory effect of human stem cells from amniotic membrane: a comparison with human mesenchymal stem cells from adipose tissue. Tissue Eng 13:1173–1183.
- Xu B, Ji X, Chen X, Yao M, Han X, Chen M, Tang W, Xia Y. 2015. Effect of perfluorooctane sulfonate on pluripotency and differentiation factors in mouse embryoid bodies. Toxicology 328:160–167.
- Yi BR, Kang NH, Hwang KA, Kim SU, Jeung EB, Kim YB, Heo GJ, Choi KC. 2011. Genetically engineered stem cells expressing cytosine deaminase and interferon-β migrate to human lung cancer cells and have potentially therapeutic anti-tumor effects. Int J Oncol 39:833–839.
- Zani A, Cananzi M, Fascetti-Leon F, Lauriti G, Smith VV, Bollini S, et al. 2013. Amniotic fluid stem cells improve survival and enhance repair of damaged intestine in necrotising enterocolitis via a COX-2 dependent mechanism. Gut 63:300–309.
- Zhang D, Jiang M, Miao D. 2011. Transplanted human amniotic membrane-derived mesenchymal stem cells ameliorate carbon tetrachloride-induced liver cirrhosis in mouse. PLoS One 6:e16789.
- Zhou J, Wang D, Liang T, Guo Q, Zhang G. 2014. Amniotic fluid-derived mesenchymal stem cells: characteristics and therapeutic applications. Arch Gynecol Obstet 290:223–231.