Abstract
Biosynthesis of silver nanoparticles (AgNPs) from Catharanthus roseus leaf extract was carried out, and their characterization, as well as antioxidant, antimicrobial, and wound-healing activities were evaluated. Color change, UV-vis spectrum, XRD, FTIR, and AFM assessments supported the biosynthesis and characterization of AgNPs. The synthesized AgNPs showed strong in vitro antioxidant and antimicrobial activities against various pathogens. The in vivo assessment of wound healing in AgNPs-treated mice revealed their effectiveness in closuring and reducing size of wounds. Such potent bioactivity may justify their biomedical use as antioxidant and antimicrobial agents for controlling various health-related diseases, particularly in wound healing.
Introduction
In recent years, nanoparticles have been addressed to play a vital role in medicine, science, and various biotechnological fields. They have various sizes, shapes, and high surface-to-volume ratios; therefore, nanoparticles are potentiated for various biomedical applications, such as target drug delivery, imaging and biosensor, diagnosis, and disease therapy (Wang and Wang Citation2014). Metal and metal oxide nanoparticles have been further explored, and their biological applications have remarkably been demonstrated; for instance, anticancer, antioxidant, anti-inflammatory, wound-healing, and antimicrobial activities (Palaniselvam et al. Citation2014, Schrofel et al. Citation2014, Singh et al. Citation2013).
Silver is used to treat infected wounds long before antibiotics were introduced in modern medicine (Singh et al. Citation2013). However, the use of silver is limited because of its high toxicity and the availability of antibiotics. Recently, silver nanoparticles (AgNPs) have attracted the scientific attention of researchers regarding their use as potential antimicrobial agents (Narayanan and Sakthivel Citation2011). They possess a wide range of applications in medicine; including pharmaceutical, cosmetics, and medical devices because of their broad bactericidal and fungicidal spectrum (Palaniselvam et al. Citation2014).
Green synthesis of nanoparticles is gaining interest worldwide because of its advantages, such as being ecofriendly, non-toxic, and economic, over chemical and traditional physical methods (Mohanpuria et al. Citation2008). Several biological methods have been used for nanoparticle production from organisms involving bacteria, fungi, and plant extracts (Aruna et al. Citation2014, Hemanth et al. Citation2010, Natarajan and Ramchandra Citation2010). AgNP synthesis from plant extracts has been developed because it is easily obtained and safe and has large variety of metabolites supporting silver ion reduction. A mechanism proposed that phytochemicals are directly involved in the alleviation of ions and realization of AgNPs (Jha et al. Citation2009).
Catharanthus roseus (Madagascar periwinkle or Sadabahar), which belongs to the plant family Apocynaceae, has been widely introduced as one of the important medicinal plants that is used to treat different diseases in folk medicine (Appidi et al. Citation2008, Singh et al. Citation2001). The leaves and roots of C. roseus are rich in phytochemicals; for instance alkaloids, which have been demonstrated to have anticancer and antihypertensive effects (Ei-Sayed and Verpoorte Citation2005).
The present study aimed to determine the biological synthesis of AgNPs produced from C. roseus leaf aqueous extract and characterize these nanoparticles using X-ray diffraction (XRD), Fourier transform infrared (FTIR), and atomic force microscopy (AFM). Moreover, antioxidant effect, wound-healing property, and antimicrobial activity of synthesized AgNPs against clinical isolates of bacteria (Escherichia coli, Staphylococcus aureus, Klebsiella pneumonia, Citrobacter koseri, Pseudomonas aeruginosa) and the fungus Candida albicans were evaluated.
Materials and methods
Materials
Brain heart agar (BHA), Sabouraud dextrose agar (SDA), silver nitrate (AgNO3), and 2,2-diphenyl-1-picrylhydrazyl (DPPH) were purchased from Sigma-Aldrich company (Germany).
Microorganisms
E. coli, S. aureus, K. pneumonia, C. koseri, P. aeruginosa, and C. albicans were used to investigate the antimicrobial potential of prepared AgNPs. They were identified and kindly supplied by Division of Biotechnology, Department of Applied Science, University of Technology, Baghdad, Iraq.
Experimental mice
For wound-healing experiment, 6- to 8-week-old male albino mice (20–22 g) were used. The animals were housed in sterile polypropylene cages and maintained at standard laboratory conditions (12 h of light–dark cycle, 25 ± 3 °C and 35–60% humidity), and had free access to food and water (ad libitum).
Preparation of leaves extract
Fresh leaves of C. roseus were collected from Al-Tarmyia (a region located 60 km northeast the capital Baghdad) and washed, air-dried, and ground to produce powder. Leaf extract was prepared via Soxhlet extraction method according to Sulaiman and co-workers (Sulaiman et al. Citation2013), where methanol was used as a solvent. After filtration of extract (Whatman filter paper No. 1), the solvent was evaporated in rotary evaporator, and the obtained extract was reconstituted to 10 mL with deionized distilled water and maintained at 4 °C for further experiments.
Synthesis of silver nanoparticles
An aqueous solution of 2 mM AgNO3 was prepared according to the method of Sulaiman and co-workers using distilled water at room temperature (Sulaiman et al. Citation2013). Briefly, the aqueous solution (90 mL) was mixed with 10 mL of leaf extract at 70 °C and stirred magnetically at 1000 rpm for 3 min. The bioreduced aqueous component was subjected for characterization of AgNPs.
Characterization of silver nanoparticles
The characterization of synthesized AgNPs was first monitored by measuring the UV-vis spectrum using Hitachi U-2910 Spectrophotometer (Tokyo, Japan). UV-vis spectroscopic analysis was performed via continuous scanning at the range of 280–760 nm. Then, XRD analysis was carried out, in which, purified AgNP solution drop-coated on glass was observed using XRD-6000 X-ray diffractometer (Shimadzu, Japan) operated at a voltage and current of 40 kV and 30 mA, respectively, with Cu Kα radiation in 2θ configurations. The size was calculated by using the following Scherrer formula was used: D = 0.94 λ/β Cos θ. FTIR analysis was carried out by using FTIR 8400S spectrometer in attenuated total reflection mode and spectral range of 4000–400 cm−1 with a resolution of 4 cm−1. Finally, the morphology of the synthesized AgNPs was determined via AFM analysis using AA 3000 Scanning Probe Microscope.
DPPH-free radical scavenging assay
The antioxidant activity of synthesized AgNPs was manifested through DPPH method. One milliliter of DPPH (0.1 mM; in ethanol) was added to the AgNPs. The reaction mixture was shaken and incubated in the dark place for 30 min. The absorbance was measured at 517 nm against a blank (ethanol). The reaction mixture with lower absorbance indicated a higher percentage of scavenging activity. The equation used for calculating DPPH-free radical scavenging activity is as follows.
where Ac and As are the peak intensity for control (DPPH) and test sample solvent, respectively.
Antimicrobial assessment of silver nanoparticles
The synthesized AgNPs were tested for antimicrobial activity via agar-well diffusion method. The pure cultures of pathogenic bacteria were sub-cultured on BHA. Each strain was swabbed uniformly onto individual plate using sterile cotton swabs. The discs (8 mm, diameter) containing the prepared nanoparticle solution were placed on agar medium and incubated at 37 °C for 24 h. For antifungal measurement, wells with a diameter of 8 mm were made on SDA plates by using the gel puncture method. Aliquot 50 μL of prepared nanoparticle solution were poured onto each well of all plates, and these plates were incubated at 30 °C for 48 h. The diameter of inhibition zone was measured in millimeter and recorded as mean ± SD of a triplicate assessment according to the standard protocol. The plant extract and silver nitrate were also tested in a similar manner. In addition, amoxicillin (30 μg mL−1) and fluconazole (5 μg mL−1) were used as a control for tested bacteria and C. albicans, respectively.
Excision wound model and AgNPs administration
The wound-healing experimental protocols were approved by the Biotechnology Division Committee, Applied Science Department, University of Technology, Baghdad, Iraq. Six mice were distributed into three groups (each of two animals): control (Group I), treated with plant extract only (Group II), and treated with AgNPs (Group III). For wound excision, the dorsal side hair of mice was shaved using sterile surgical blade, and ∼(2 × 2 cm2) full-thickness excision wound was done via removing a patch of skin under chloroform anaesthesia. The wound of control mice received 2 mL of normal saline, while wounds of treated mice received 2 mL of either plant extract or synthesized AgNP (2 mM) that was put in the dressing (size: 2 × 2 cm2) followed by topical application to the wound bed, once daily for a period of 12 days. Wound tissue contraction was monitored by tracking the wound healing at 0, 1, 4, 8, and 12 days. For a visual comparison, the wounds of control and treated mice were photographed on scheduled days, and images were inspected.
Statistical analysis
The data analyses were evaluated using ANOVA test with SPSS/14 computer software (SPSS Inc., Chicago, IL). Results were presented as the mean ± SD and statistical significance levels were set at P ≤ .05.
Results and discussions
Characterization of silver nanoparticles
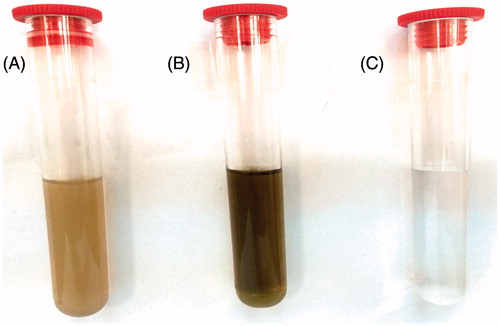
In present work, AgNPs were synthesized from C. roseus leaf extract. In general, AgNPs produced brown solution in water (). The observed color change (brownish), when the plant extract was mixed with AgNO3 solution, suggested the reduction of Ag+ to nanoparticles. The metal nanoparticles have free electrons, which provide the surface plasmon resonance (SPR) absorption band (Narayanan et al. Citation2012). This specific color alteration was caused by the excitation of SPR in the metal nanoparticles. The color change of the leaf extract was observed when the extract was incubated with the AgNO3 solution.
Figure 1. Photograph showing color changing (A) aqueous leaf extract of Catharanthus roseus (B) changing color from yellowish to reddish brown after adding 2 mM AgNO3 and exposing to heat at 70 °C for 3 min. (C) 2 mM AgNO3 only.
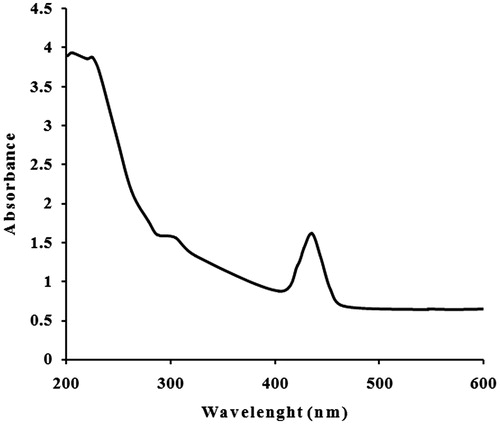
The absorption spectrum of the incubated solution at different wavelengths ranging within 200–600 nm revealed a peak at 425 nm (). AgNPs prepared from other plant extracts showed SPR peak at ∼420 nm (Logeswari et al. Citation2013, Maria et al. Citation2015, Mulvaney Citation1996). The frequency and width of the SPR absorption band based on the shape and size of the metal nanoparticles, dielectric constant of the metal itself (the composition of the particles) and the dielectric constant of surrounding medium. Also, a single SPR band reveals to the spherical shape, while two or more SPR bands correspond to the anisotropic particles (Udayasoorian et al. Citation2011). UV-vis spectroscopy technique could be used to examine shape- and size-controlled nanoparticles in aqueous suspensions (Wiley et al. Citation2006). Thus, the UV-vis results indicated that C. roseus leaf extract can possibly reduce Ag to AgNPs, and such finding was further inspected to confirm the synthesis of AgNPs from C. roseus.
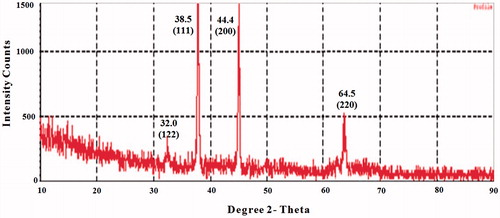
The XRD pattern of AgNPs produced from the leaf extract was further illustrated and confirmed by the characteristic peaks observed in the XRD spectra (). Four Bragg’s reflection patterns at 2θ, namely, 32.00°, 38.50°, 44.40°, 64.50°, and in the entire spectrum of value ranging within 10–90, were interpreted from XRD. These patterns corresponding to (122), (111), (200), and (220), respectively. The set of lattice planes were observed and compared with the reference values of Joint Committee on Powder Diffraction Standards (JCPDS: 89-3722) and further on the basis that they can be indexed as face-centered-cubic structure of silver with a diameter around of 20 nm. Therefore, the XRD pattern clearly revealed that AgNPs are crystalline in nature (Shameli et al. Citation2011). The sharp peaks clearly indicate the cubic crystalline nature of the synthesized AgNPs which is in nanoscale and agreement with report of Bhakya and co-workers (Bhakya et al. Citation2016). Hence, C. roseus leaf extract is promising target in the development of AgNPs.
Figure 3. XRD pattern of silver nanoparticles formed after reaction with Catharanthus roseus leaf extract.
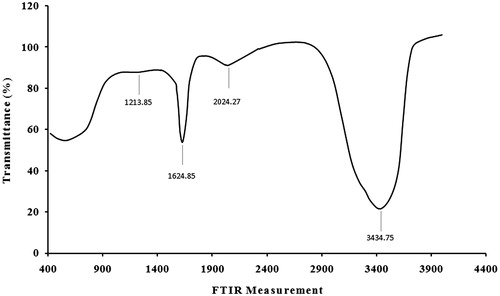
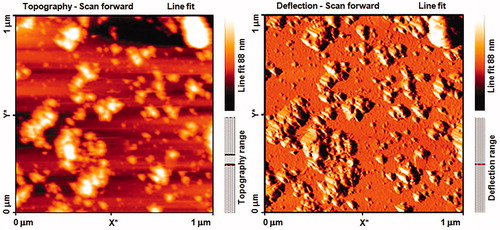
FTIR spectroscopic studies were conducted to investigate the plausible mechanism behind the formation of these AgNPs and provide information regarding the functional groups. The representative spectra of AgNPs are shown in . The FTIR signals of AgNPs were observed at 3434, 2024, 1624, and 1213. The absorption peak at 1213 and 1624 was attributed to the C–O and N–H stretching, which was possibly caused by the presence of carboxylic acid and amide groups, respectively. The strong absorption peaks at 3434 were caused by the N–H bond for amine groups, which were used for the stabilization of AgNPs. However, the presence of the amide group characteristic proteins/enzymes is responsible for the reduction of AgNO3 to Ag (Mohamed et al. Citation2014). AFM was used to observe the sample’s surface morphology and roughness. shows crystalline particles with grains sized 10–88 nm in diameter with mean size of about 49 nm. A few aggregations of AgNPs are also observed in some places, indicating agglomeration after several weeks of AgNPs preparation. Such finding confirmed the crystalline structure obtained by XRD assay. However, FESEM technique is required in a further analysis to visualize the size and shape of the synthesized AgNPs.
In vitro antioxidant activity of silver nanoparticles
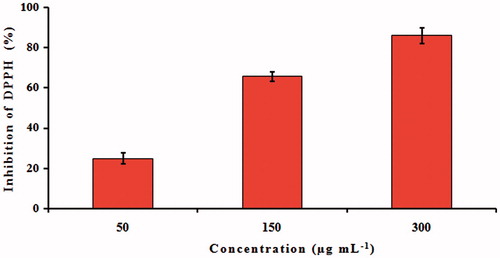
The antioxidant activity of bioconjugated AgNPs was evaluated using DPPH scavenging assay. DPPH is a stable compound and accepts hydrogen or electron from AgNPs. The color is changed from purple to yellow after reduction, which can be quantified by its decreased absorbance at wavelength of 517 nm. shows the dose-dependent increase in the inhibition percentage of synthesized AgNPs at 50, 150, and 300 μg mL−1. The 300 μg mL−1 concentration exhibited a higher inhibition (82%) compared to that of the other two concentrations. The disappearance of purple color when synthesized AgNPs were added might be caused by the presence of antioxidant in the medium. The antioxidant property of AgNPs could be explained to functional groups adhered to them which were produced from the C. roseus leaf extract.
Figure 6. DPPH-free radical scavenging activity of silver nanoparticles formed after reaction with Catharanthus roseus leaf extract. Results are expressed as percentage decrement of absorbance at 517 nm with respect to control. Each value represents the mean ± SD of three experiments.
Superoxide anions are free radicals generated by the transfer of one electron and play a vital role in the formation of other reactive oxygen species (ROS), such as hydrogen peroxide, hydroxyl radical, or singlet oxygen within living systems (Stief Citation2003). Antioxidants are beneficial for the management of many deleterious diseases because of their scavenging ability. Antioxidants can also react with nitric oxide to form peroxynitrite, which can generate toxic radicals, such as the hydroxyl radical (Halliwell Citation1997). Although the mechanism of action in a more detailed level needs more advanced experimental proofs, AgNPs are promising as a potential biological label.
Antimicrobial assay of silver nanoparticles
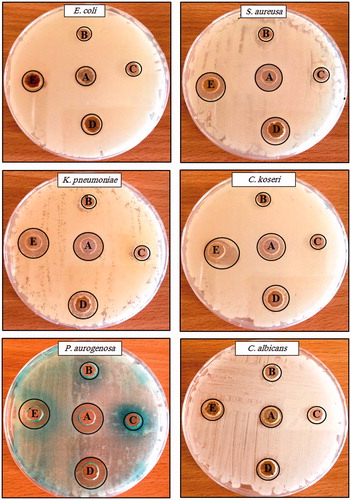
The synthesized AgNPs manifested good antibacterial property against gram-negative bacteria, such as E. coli, C. koseri, K. pneumonia, P. aeruginosa, and S. aureus. They also showed antifungal activity against C. albicans. AgNPs exhibited more significant effect than the use of plant extract or silver nitrate only (). Researchers suggested different mechanism(s) of the AgNPs action onto bacteria. Yliniemi and Vahvaselka (Yliniemi and Vahvaselka Citation2008) demonstrated the AgNPs’ bacterial cell membrane permeability and respiration function, which lead to cell death. The smaller size of AgNPs provides a large surface area that is available for interaction, which would provide more considerable effect than the larger Ag+ (Rai et al. Citation2009).
Figure 7. Antimicrobial activity assay of silver nanoparticles against different pathogens by the well diffusion method. (A) Amoxicillin/or fluconazole (B) Catharanthus roseus leaf extract, (C) silver nitrate, (D) synthesized silver nanoparticles at 100 μg mL−1, and (E) synthesized silver nanoparticles at 200 μg mL−1. Antibiotic amoxicillin at concentration 30 μg mL−1 was used as a control for all tested bacteria while, fluconazole at concentration 5 μg mL−1 was used as a control for Candida albicans.
In addition, AgNPs do not only attach to the bacterial cell surface but also enter inside the bacteria, which results in a disruption of adenosine triphosphate (ATP) production and DNA replication, generation of ROS and direct damage to cell structures (Sahayaraj and Rajesh Citation2011). Furthermore, the bactericidal effect of silver might be attributed to the inactivation of phosphomannose isomerase, which catalyzes the conversion of mannose-6-phosphate to fructose-6-phosphate. The latter is an important intermediate of glycolysis, which is the most common pathway in bacteria for sugar catabolism (Beddy et al. Citation2004).
Wound-healing activity of silver nanoparticles
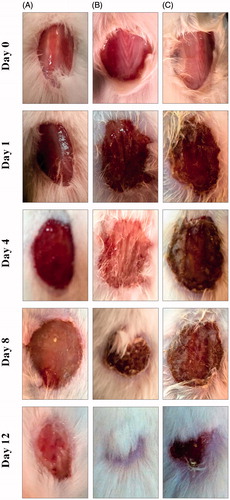
The wound-healing activity of synthesized AgNPs was proven in male albino mice using an excision wound model. As shown in , AgNP-treated animals showed better wound-healing activity compared with that of either negative- or positive-control groups. AgNP-treated wounds exhibited no evidence of microbial contamination, bleeding or pus formation during treatment, whereas control wounds revealed notable inflammation. On the 4th day onwards, AgNP-treated group recorded distinguished wound closure and reduced wound size, which were enhanced during the remaining days of treatment compared to controls. At the end of the experiment, the AgNP-treated wound showed approximately 98% of closure, whereas control wound appeared approximately 85% of closure (). The reduced wound size and enhanced wound closure might be due to the bactericidal effects of AgNPs against bacterial contamination in the wound area, which can consequently restore tissue integrity and often results in a satisfactory repair of damaged sites (Chatterjee et al. Citation2014).
Figure 8. Photographs of wounds from animals elucidation on different days of (A) negative control, (B) silver nanoparticles, and (C) Catharanthus roseus leaf extract-treated mice.
Tian et al. demonstrated the potential role of AgNPs on wound healing in an animal model and executed that rapid healing and better cosmetic appearance occur in a dose-dependent manner (Beddy et al. Citation2004). Furthermore, AgNPs showed positive effects through their antimicrobial potentials, reduction in wound inflammation through decreasing lymphocyte and mast cell infiltration, and amendment of fibrogenic cytokines (Tian et al. Citation2007). Similarly, Lee et al. investigated the effect of AgNPs in dermal contraction and epidermal reepithelialization during wound healing and suggested that AgNPs could increase the rate of wound closure. This property was interpreted through the promotion of reproduction and migration of keratinocytes (Lee et al. Citation2010). Additionally, AgNPs could boost the differentiation of fibroblasts into myofibroblasts, thereby inducing wound contraction (Gunasekaran et al. Citation2012).
Conclusion
C. roseus leaf extract-synthesized AgNPs exhibited a strong antimicrobial activity against several pathogens that were tested in present study. The tailored AgNPs prompted wound-healing potential in albino male mice using wound closure assay. The AgNPs also enhanced the wound-healing activity in mice by inhibiting the pathogenic bacterial growth in the wound area. On the basis of our previous and current findings, it is possible to conclude that the biosynthesized AgNPs could be considered as cost-effective, antioxidant, and effective therapeutic agent for controlling bacterial and fungal growth.
Disclosure statement
The authors declare that there are no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Appidi JR, Grierson DS, Afolayan AJ. 2008. Ethnobotanical study of plants used for the treatment of diarrhoea in the Eastern Cape, South Africa. Pak J Biol Sci. 11:1961–1963.
- Aruna A, Nandhini R, Karthikeyan V, Bose P. 2014. Synthesis and characterization of silver nanoparticles of insulin plant (costuspictus D. Don) leaves. Asian J Biomed Pharm Sci. 4:1–6.
- Beddy D, Watson RW, Fitzpatrick JM, O’Connell PR. 2004. Increased vascular endothelial growth factor production in fibroblasts isolated from strictures in patients with Crohn’s disease. Br J Surg. 91:72–77.
- Bhakya S, Muthukrishnan S, Sukumaran M, Muthukumar M. 2016. Biogenic synthesis of silver nanoparticles and their antioxidant and antibacterial activity. Appl Nanosci. 6:755–766.
- Chatterjee AK, Chakraborty R, Basu T. 2014. Mechanism of antibacterial activity of copper nanoparticles. Nanotechnology. 25:135101.
- Ei-Sayed M, Verpoorte R. 2005. Methyljasmonate accelerates catabolism of monoterpenoid indole alkaloids in Catharanthus roseus during leaf processing. Fitoterapia. 76:83–90.
- Gunasekaran T, Nigusse T, Dhanaraju MD. 2012. Silver nanoparticles as real topical bullets for wound healing. J Am Coll Clin Wound Spec. 3:82–96.
- Halliwell B. 1997. Antioxidants and human disease: a general introduction. Nutr Rev. 55:44–49.
- Hemanth NKS, Karthik L, Bhaskara RKV. 2010. Extracellular biosynthesis of silver nanoparticles using the filamentous fungus Penicillium sp. Arch Appl Sci Res. 2:161–167.
- Jha A, Prasad K, Kulkarni AR. 2009. Plant system: nature’s nanofactory. Colloids Surf B Biointerfaces. 73:219–223.
- Lee PY, Ho CM, Lui VCH. 2010. Silver nanoparticles mediate differential responses in keratinocytes and fibroblasts during skin wound healing. Chem Med Chem. 5:468–475.
- Logeswari P, Silambarasan S, Abraham J. 2013. Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Scientia Iranica F. 20:1049–1054.
- Maria BS, Devadiga A, Kodialbail VS, Saidutta MB. 2015. Synthesis of silver nanoparticles using medicinal Zizyphus xylopyrus bark extract. Appl Nanosci. 5:755–762.
- Mohamed NH, Ismail MA, Abdel-Mageed WM, Shoreit AAM. 2014. Antimicrobial activity of latex silver nanoparticles using Calotropis procera. Asian Pac J Trop Biomed. 4:876–883.
- Mohanpuria P, Rana NK, Yadav SK. 2008. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 10:507–517.
- Mulvaney P. 1996. Surface plasmon spectroscopy of nanosized metal particles. Langmuir. 12:788–800.
- Narayanan KB, Sakthivel N. 2011. Green synthesis of biogenic metal nanoparticles by terrestrial and aquatic phototrophic and heterotrophic eukaryotes and biocompatible agents. Adv J Colloid Interface Sci. 169:59–79.
- Narayanan S, Sathy BN, Mony U, Koyakutty M, Nair SV, Menon D. 2012. Biocompatible magnetite/gold nanohybrid contrast agents via green chemistry for MRI and CT bioimaging. ACS Appl Mater Interfaces. 4:251–260.
- Natarajan KSS, Ramchandra M. 2010. Microbial production of silver nanparticles. Dig J Nanomater Bios. 5:135–140.
- Palaniselvam K, Mashitah MY, Gaanty PM, Natanamurugaraj G. 2014. Biosynthesis of metallic nanoparticles using plant derivatives and their new avenues in pharmacological applications – an updated report. Saudi Pharm J. 24:473–484.
- Rai M, Yadav A, Gade A. 2009. Silver nanoparticles as a new generation of antimicrobials. Biotechnol Adv. 27:76.
- Sahayaraj K, Rajesh S. 2011. Bionanoparticles: synthesis and antimicrobial applications. In: Méndez-Vilas A, Ed. Science against Microbial Pathogens: Communicating Current Research and Technological Advances. Badajoz: Formatex Research Center, pp. 228–244.
- Schrofel A, Kratosova G, Safarik I, Safarikova M, Raska I, ShorL M. 2014. Applications of biosynthesized metallic nanoparticles – a review. Acta Biomaterialia. 10:4023–4042.
- Shameli K, Ahmad MB, Zargar M, Yunus WM, Ibrahim NA. 2011. Fabrication of silver nanoparticles doped in the zeolite framework and antibacterial activity. Int J Nanomedicine. 6:331–341.
- Singh S, Saikia JP, Buragohain AK. 2013. A novel ‘green’ synthesis of colloidal silver nanoparticles (SNP) using Dillenia indica fruit extract. Colloids Surf B Biointerfaces. 102:83–85.
- Singh SN, Vats P, Suri Shyam R, Kunria MM, Ranganathan S, et al. 2001. Effect of an antidiabetic extract of Catharanthus roseus on enzymic activities in streptozotocin induced diabetic rats. J Ethnopharmacol. 76:269–277.
- Stief TW. 2003. The physiology and pharmacology of singlet oxygen. Med Hypotheses. 60:567–572.
- Sulaiman GM, Mohammad AAW, Abdul-wahed H, Ismail MM. 2013. Biosynthesis, antimicrobial and cytotoxic effects of silver nanoparticles using Rosmarinus officinalis extract. Dig J Nanomater Bios. 8:273–280.
- Tian J, Wong KK, Ho CM, Lok CN, Yu WY, Che CM, Chiu JF, Tam PK. 2007. Topical delivery of silver nanoparticles promotes wound healing. Chem Med Chem. 2:129–136.
- Udayasoorian C, Vinoth Kumar K, Jayabalarishnan RM. 2011. Extracellular synthesis of silver nanoparticles using leaf extract of Cassia auriculata. Dig J Nanomater Bios. 6:279–283.
- Wang EC, Wang AZ. 2014. Nanoparticles and their applications in cell and molecular biology. Integr Biol (Camb). 6:9–26.
- Wiley BJ, Im SH, Li ZY, McLellanm J, Siekkinen A, Xia Y. 2006. Maneuvering the surface plasmon resonance of silver nanostructures through shape-controlled synthesis. J Phys Chem B. 110:15666–15675.
- Yliniemi K, Vahvaselka M. 2008. Antimicrobial activity of colloidal silver nanoparticles prepared by sol-gel method. Chemistry. 18:199.