Abstract
We assessed the effect of purmorphamine along with collagen/hydroxyapatite scaffold in inducing osteogenesis of human endometrial stem cells (hEnSCs). The adhesion, viability, proliferation, and differentiation of cells on scaffold were assayed with SEM, MTT, real time-PCR, and ALP assay, respectively. The results were shown good integration of cells with scaffold. Also, qRT-PCR of differentiated cells shows that osteoblast cell markers are expressed after 21d in 2D and scaffold groups while in the scaffold group the expression of these markers were higher than the 2D group. Based on our findings, collagen/hydroxyapatite scaffold with PMA has the potential role in osteogenic differentiation of hEnSCs.
Introduction
The prevalent therapeutic strategies are incapable of reconstructing all the tissues and parts damaged by periodontal diseases (Pihlstrom et al. Citation2005). For example, different therapeutic techniques have been designed to be able to make up for osseous tissue destroyed through disease. These approaches induce osteogenesis at the damaged site, such as allogenic and autogenic material, and xenografts and alloplastics (Bartold et al. Citation2006, Narayanan and Bartold Citation1996). Although regenerative medicine have achieved clinical success, but these improvements are minimum and greatly vary in many cases. The success of such techniques has been limited by insufficient osteogenesis, bone degeneration, and the limited amount of graft removable from the donor site (Hoveizi et al. Citation2015; Pontoriero and Lindhe Citation1995). Tissue engineering is an alternative solution to the low efficiency of prevalent therapies in regeneration of live structures and functional bone (Srisuwan et al. Citation2006). The three main components of tissue engineering are a group of multi-potential progenitor cells, signaling molecules or morphogenic signal inducers and a scaffold of extracellular matrix (Nakashima and Reddi Citation2003). Not only does the scaffold act as a vehicle for transferring cells to the regeneration site but it also plays the main role in connecting, protecting, and keeping cells in the defect site (Nakashima and Reddi Citation2003). It also determines the morphologic characteristics of the cells and provides oxygen and nutrients to them (Nakashima and Reddi Citation2003). One of the most vital components of tissue engineering is to select an appropriate population of stem cells. The most important goal of stem cell research is to isolate adult human stem cells of high quality from easily accessible resources for the purpose of tissue regeneration (Hynes and Danijella Citation2000). Based on earlier studies; mesenchymal stem cells (MSCs) can differentiate into osteoblasts and, therefore, be considered a source for osseous engineering (Gronthos et al. Citation2003). To this day, most studies have been performed on bone marrow stem cells. However, the small numbers of stem cells derived from this source, as well as the impure populations derived, and their loss of differentiation potential at higher ages, have directed attention toward other resources like fat and endometrial tissue (Azami et al. Citation2013, Ebrahimi-Barough et al. Citation2015, Gargett and Masuda Citation2010, Wolff et al. Citation2011). The presence of stem cell markers – particularly in different layers of the uterus – has been proved using various stem cell markers, particularly Oct-4, CD146, and CD133. Moreover, the angiogenic potential of these cells has also been proved with the help of the CD31 antibody. The transformation of these cells into osteoblastic cells has been investigated to keep in mind the ease of access to human endometrial stem cells (hEnSCs), their angiogenic potential and non-carcinogenic nature (Gargett et al. Citation2009, Gargett and Masuda Citation2010, Wolff et al. Citation2011). The addition of exogenic factors helps to induce differentiation, but their use alone is not sufficient (Aravamudhan et al. Citation2013). Small molecules are an inseparable component of molecular activities in living creature’s bodies, which are directly produced in the uncoded genome through enzymatic activity (Wu et al. Citation2002). Their rapid dissemination potential through biologic membranes as a result of their small size (most small-molecules are smaller than 800 Da), the low cost, and the simplicity of producing them in comparison with their protein counterparts has led to their application in research (Wu et al. Citation2004). The molecular effect of 2,6,9-trisubstituted purine or purmorphamine (PMA) has been mentioned as a sonic hedgehog agonist (Shh) in medical chemistry (Wu et al. Citation2002). According to the literature, PMA can induce osteogenesis by activating the hedgehog signaling pathway (Wu et al. Citation2004). Activation of the hedgehog signaling pathway by PMA leads to a stepwise induction of alkaline phosphatase activity accelerating factor (the protein effective in osteogenesis). PMA increases alkaline phosphatase activity and accelerates the formation of bone-like structures in stem cell-derived osteoblasts (Beloti et al. Citation2005). Considering the bioavailability of these small molecules compared to differentiating factors, they may be used as a substitute for the latter. The goal of the current study is to determine the hEnSCs differentiation potential into osteoblasts and osteoid formation on a collagen/hydroxyapatite scaffold, in the presence of PMA.
Materials and methods
Scaffold preparation and fabrication
To prepare hydroxyapatite, solutions containing calcium and phosphorus – separately – were used, along with calcium nitrate tetrahydrate [Ca (NO3)2.4H2O] and diammonium phosphate [(NH4)2HPO4]. Distilled water was used to prepare water mixtures of collagen and hydroxyapatite. Freeze-drying technique was used for the fabrication of scaffolds. Since the goal was to build a 3D scaffold, the layers prepared were cut into smaller pieces and attached to each other using 10% collagen solution. The samples were inserted in glutaraldehyde (C5H8O2) solution to increase the strength of the composite scaffold.
Cell isolation and differentiation on 2D and 3D cultures
Isolation and culture of human EnSCs from reproductive aged women (mean age 31 years) were accomplished using the methods from our previous studies (Ebrahimi-Barough et al. Citation2013). A written informed consent form was obtained from each donor (according to instruction of Tehran University of Medical Sciences Research assistant) describes the procedures and aims of the study and sets the standards of quality and safety for the donation, testing, processing, storage, and distribution of human tissues and cells. Briefly, the biopsy tissue was washed in Hanks media, minced and treated with collagenase I (1 mg/ml, Gibco, Waltham, MA). Following tissue digestion, epithelial and stromal cells were separated using filtration with 70 and 40 μm cell strainers. The cells were then centrifuged and the isolated cells were cultured in DMEM/F12 medium containing 10% fetal bovine serum (FBS), 1% antibioticpenicillin/streptomycin, and 1% glutamine and then incubated at 37 °C with 5% CO2. Experimental groups were the cells that cultured on tissue culture plate as the 2D group and the cells that cultured on scaffolds as the 3D group. For cell seeding on the cylindrical collagen/hydroxyapatite scaffold (collagen/HA), scaffolds were cut into 8 mm diameter. They were then washed with PBS and placed in 24-well chambers. The scaffolds were sterilized by exposing the 24-well plates to an ultraviolet hood for 1 h. Then they were reversed and put under the UV hood for another hour to sterilize all their surfaces. At the seeding stage 2.2 × 106 hEnSCs derived from the third passage were diluted in the 500 μl of DMEM + FBS 10% culture solution and injected onto the collagen/HA scaffold. After cell seeding on the scaffolds, the scaffolds containing cells were again divided into three groups: in the first group (negative control) only DMEM + FBS 10% were added and in the second group (positive control) DMEM + FBS 10% + dexamethasone 10 nM + ascorbic acid 50 μg/ml + β glycerol phosphate 10 mM were added. The third group (experimental group): in addition to DMEM + FBS 10%, 3 μM of PMA was added. All the groups were transferred to the incubator and incubated for 21 d. The culture medium was changed twice a week to provide the cells with a new medium for growth and nutrition.
Assessment of cell attachment on scaffolds
Scanning electron microscope (SEM) analysis was used for investigation of collagen/HA scaffolds morphology. SEM of hEnSCs cultured on scaffolds for 5 d and the cell-containing scaffold was washed with PBS twice. Then, Karnovsky fixative (containing 2% formaldehyde and 2.5% glutaraldehyde pH 7.4) was added to the scaffolds and the scaffolds were kept in the fixative for 90 min. The scaffolds were placed in ethanol of various concentrations – ranging from lower to higher i.e. 30%, 50%, 75%, and 95% – for 5 min each time. Eventually, the scaffolds were immersed in 100% alcohol for 10 min. They were then completely dried under the hood and prepared for SEM imaging analysis. The samples containing fixed cells were assessed under the electron microscope and images with 20, 50, and 100 μm magnification were prepared.
Cell proliferation and viability assay
To evaluation of cell attachment and proliferation, 4′,6-diamidino-2-phenylindole (DAPI) staining and MTT assay were done. DAPI staining was used in days 1 and 7 cell seeding on scaffolds. The scaffolds with seeded cells were washed with PBS. Then they were fixed in PFA for 20 min. After staining the scaffolds with DAPI they were examined under the microscope for the presence of cells. The 3-(4,5-dimethylthiazoyl-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was used to investigation of cell metabolic activity of cultured cells on scaffolds. Cells were seeded at a density of 104 cells per 4 mm diameter of scaffold and tissue culture plate (2D) in 96-well plates and incubated for 1, 3, 5, and 7 d. Then, 100 μl of MTT solution (0.5 mg/ml) was added to each well, and the plates were incubated at 37 °C for 4 h. during this time light yellow MTT transforms into dark blue formazan by the activity of a mitochondrial dehydrogenase. After removing of medium, dimethyl sulfoxide (DMSO) was added to each well to dissolve the formazan crystals. After removing of scaffolds from plates, the absorbance of each well was measured at 570 nm using an ELIZA reader (Expert 96, Asys Hitch, Ec Austria). The experiment was repeated three times as triplicate, the results of which are presented as means ± SD.
RNA extraction and quantitative real-time PCR
For comparison of gene expression between the groups, cells were collected using 0.25% Trypsin/EDTA after 21 d post-induction. Then, total RNAas extracted using the RNeasy Plus Mini Kit (Qiagen, Valencia, CA) and treated with DNase I (Ambion, Austin, TX). Then, RNA (2 μg) was reverse transcribed using the TaqMan Reverse Transcription Kit (Applied Bio Systems, Carlsbad, CA). Real-time PCR used specific primers (), a 96-well optical reaction plate, and the 7500 Real-Time PCR System (Applied Biosystems, Lincoln, CA). In each PCR reaction, 10 μl SYBRRGreen PCR Master Mix (Applied Biosystems, Lincoln, CA) was mixed with 30 ng of cDNA and 0.5 μM from each primer in a total volume of 20 μM. The comparative Ct method, 2 − DDCt, was used for relative gene expression analysis. All Ct values calculated from the target genes were normalized to GAPDH and calibrated using calculation from the undifferentiated human EnSCs as control group for analysis. PCR reactions were performed on a mastercycler gradient machine (Eppendorf, Hamburg, Germany). Initial denaturation at 95 °C for 30 s, annealing at 60 °C for 30s, extension for 35 s at 72 °C followed by 46 cycles. Three different mRNA samples were assessed in duplicate for each gene of interest.
Table 1. Primers used for real time RT-PCR.
Alkaline phosphatase assessment
Alkaline phosphatase assay was conducted to comparing the common osteogenic media and PMA inducer factor in osteogenesis of hEnSCs. Media were decanted after exposing seeded cells on 2D group with induction differentiation media with common osteogenic media and PMA at days 7, 14, and 21 culture, cell layers washed with PBS and then removed with a cell scraper. Cells were centrifuged, washed, and cell lysates were prepared by vortexing in 500 μl deionized water plus 25 μl 1% Triton X-100 and homogenized via sonification. After that, the total protein content of cells was determined using a commercially available kit (Micro/Macro BCA; Pierce Chemical Co., Rockford, IL). In addition, the ALP activity was measured by commercial kinetic kit (Pars Azmun, Tehran, Iran) based on the conversion of p-nitrophenylphosphate to p-nitrophenol and phosphate at 37 °C and pH 9.8. The absorbance change was monitored by using spectrophotometrically at 405 nm at 37 °C temperature. ALP levels were normalized to the total protein content of cells at the end of the experiment.
Statistical tests
Statistical analysis was conducted using SPSS software (SPSS Inc., Chicago, IL) and the application of t-test and one-way ANOVA. Mean (SD) was used to describe the data and P < .05 was considered significant.
Results
Human EnSC characteristics
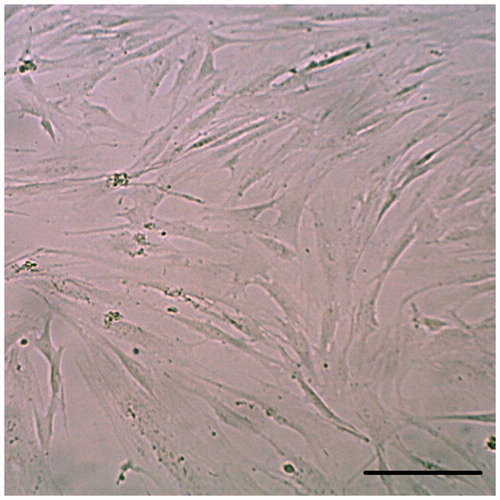
hEnSCs easily adhere to the bottom of the flask and can be collected. Twenty-four hours after cell culture, mesenchymal stem cells were observed at the bottom of the flask. Approximately 10 d later, the cells grew into numerous colonies that could be cultured. After the three passages, the hENSCs became homogenous and were visible under reverse microscopy in the form of elongated or sickle shapes (). According to the previous published data, the cells were positive for CD146 (97%), CD44(65%), CD105(79%), and CD90(80%) and negative for CD34, CD31, and CD133 (Ebrahimi-Barough et al. Citation2013).
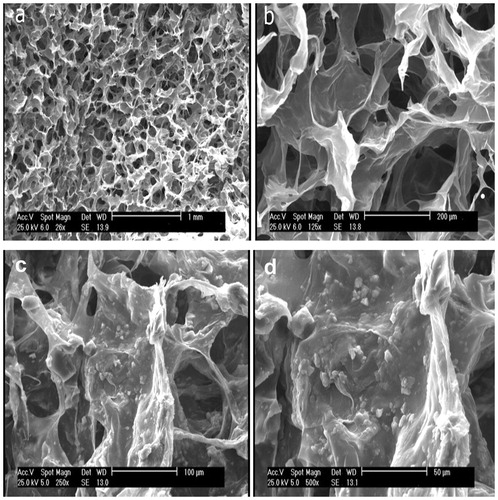
Fabrication of collagen/hydroxyapatite scaffold and cell attachment
In this study, collagen/HA scaffold was used because this is attractive for biomedical applications research due to its biocompatibility and biodegradability. Freeze-drying technique was used to fabricate this scaffold. Cell attachment and interaction between cells and scaffolds were assessed by cellular adhesion and expansion in the SEM analysis after 5 d of cell seeding on scaffold (). These results showed that collagen/HA can be potential scaffold for differentiation of hEnSCs into osteoblast-like cells.
Proliferation and viability assessment
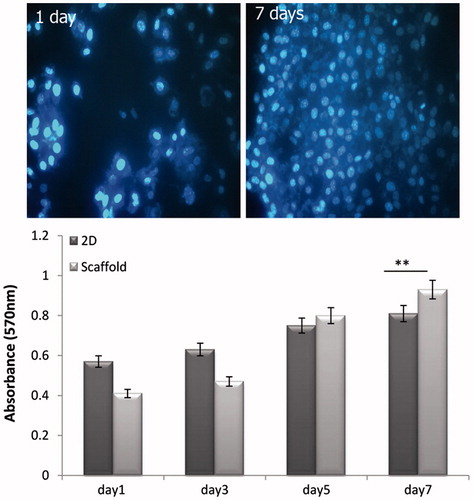
The cells seeded onto the scaffold were examined after 1 and 7 d after cell seeding on scaffold. DAPI staining figures showed the good attachment of cells onto the scaffolds.
MTT assay was performed to investigate the viability of hEnSCs cultured on collagen/HA scaffolds at 1, 3, 5, and 7 d. As shown in , until day 3, the cells cultured on 2D culture showed slightly higher viability than cells cultured on the collagen/HA scaffold. However, in days 5 and 7, the viability of cultured cells on scaffold enhanced in compared with cells in the 2D group (). These results obviously confirmed the biocompatibility of collagen/HA scaffold for viability and proliferation of hEnSCs.
Osteoblast cell differentiation of hEnSCs on collagen/hydroxyapatite scaffold
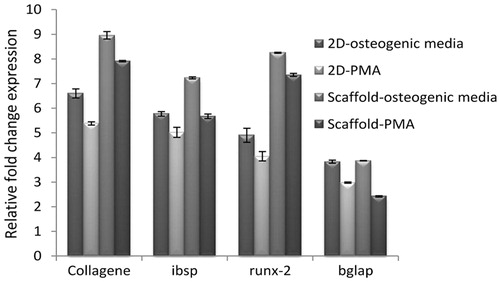
Quantitative RT-PCR was done for 2D and 3D groups with different induction media on day 21 post-induction. The mRNA expression of collagen I (COL-I), osteocalcin (OC), bone Bone sialoprotein (BSP), and Runt-related transcription factor 2 (RUNX-2) by hEnSCs-derived osteoblast cells cultured in a 2D culture and scaffold in a medium enriched with osteogenic inducers and PMA was measured. The expression of OC in osteoblast-like cells in the 2D osteogenic culture medium was 3.83 ± 0.06 and was 2.98 ± 0.02 in the PMA-containing culture medium. OC gene expression in the scaffold placed in osteogenic medium was 3.87 ± 0.0 and that of the PMA-containing scaffold was 2.47 ± 0.02. The higher values belonged to the cells cultured in the 2D medium in both the PMA and control groups. Upon comparing the control and PMA groups, the cells cultured under the influence of the osteoinductive factor exhibited higher levels of OC gene expression (P < .01). Statistically, however, it may be proven that the cells cultured under PMA were able to genetically express OC and differentiate into osteoblasts. BSP gene expression under the influence of PMA was significant and its osteoinductive potential was comparable with that of the control group. The highest BSP expression was observed in the control group cells seeded on scaffold (7.23 ± 0.04). The gene expression of BSP in the hEnSCs cultured on scaffold in the presence of PMA was 5.67 ± 0.04 units. BSP expression in both groups was equivalent and was 5.77 ± 0.09 in the control group. In the qRT-PCR analysis, collagen-I (COL-I) expression in the control group and PMA-on-scaffold was higher than its expression in the 2D culture medium. In the osteogenic medium, it was 8.96 ± 0.15 and in the PMA medium it was 7.91 ± 0.02. Meanwhile, these values were 6.60 ± 0.18 and 5.38 ± 0.06, in the 2D culture medium. Similar to COL-I, RUNX-2 expression was higher in the cells cultured on the scaffold in the control and PMA groups as compared to the 2D medium. RUNX-2 expression was 8.25 ± 0.02 and 7.35 ± 0.06 in the osteogenic and PMA media, respectively. The proximity of these two values, similar to the other genes assessed, indicates the osteoinductive potential of PMA on hEnSCs ().
Figure 4. Relative gene expression using real-time PCR for expression osteoblast-like cells markers. Quantitative real-time PCR detected the expression of osteoblast specific mRNAs, COL-I, Osteocalcin (Bglap), bone sialoprotein (ibsp), and Runx-2 after 21 d of induction. GAPDH is used as a housekeeping gene control. All groups were statistically significant with each other P < .01 (n = 3 biological samples, mean ± SD).
Alkaline phosphatase assay
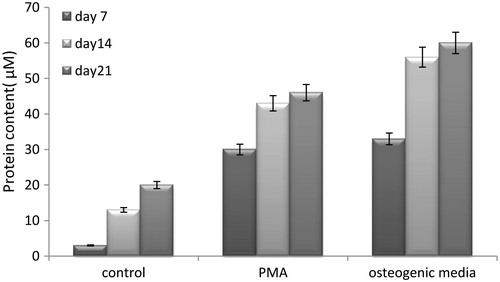
The ALP secreted by osteoblast-like cells derived from hEnSCs was measured in the three following groups on days 7, 14, and 21 of the hEnSC cultured on the 2D group with induction media containing common osteogenic media and PMA (experimental groups) and without induction media (negative control group). Based on the ELISA assay results, the mean ± SD ALP secreted by the negative control group was 5.33 ± 1.93 μM mg/ml, which was the lowest when compared with those secreted by the common osteogenic media (53.33 ± 12.80 μM mg/ml) and PMA groups (44.11 ± 9.13 μM mg/ml), indicating undifferentiating of the osteoblastic stem cells in the group without the inductive factor. Upon comparing the control and PMA groups, a high level of ALP secretion from days 7 to 21 was observed in the control group, which indicates the differentiation of osteoblastic cells. The ALP secretion in the PMA group was 33.66 ± 4.72 μM mg/ml on day 7, and increased to 47.33 ± 4.04 μM mg/ml on day 14, indicating the effect of PMA in inducing osteogenic differentiation (). The results show that osteogenic potential of PMA approximately is similar to common osteogenic media and it can be used for inductive factor for differentiation of hEnSCs into osteoblast-like cells.
Figure 5. Alkaline phosphatase production of cells in three groups includes the hEnSC cultured on the 2D group with induction media containing common osteogenic media and PMA (experimental groups) and without induction media (control group) on days 7, 14, and 21. All groups were statistically significant with control group P < .001.
Discussion
In this study, we assessed the osteoblastic differentiation potential of human endometrial stem cells (hEnSCs) seeded on a collagen/HA scaffold treated with PMA and compared the latter’s osteoinductive potential to that of an osteogenic culture medium. To prove the efficiency of PMA the expression of osteoblastic genes (BSP, COL-I, RUNX-2, and OC) and the amount of ALP secretion were compared in the cells treated with PMA with those in the positive osteogenic control group. During the third passage of cellular culture, the hEnSCs acquired fibroblastlike morphology. The results of molecular analyses of hEnSCs from earlier studies show the expression of the markers CD44, CD90, CD105, and CD146, and lack of expression of CD31, CD34, and CD113. CD146 has been expressed by hEnSCs and is the marker specific to these cells (Ebrahimi-Barough et al. Citation2013, Shamosi et al. Citation2016). Here we used the DAPI and SEM tests to examine the results of cell morphology analyses under the microscope, and used RT-PCR analysis to examine gene expression. ELISA results demonstrated ALP secretion and RT-PCR analysis demonstrated the expression of genes associated with osteogenic activity of cells cultured on collagen/HA scaffold under the effect of PMA. To our knowledge, this study is the first of its kind to investigate the effect of the small molecule PMA on hEnSCs seeded on a collagen/HA scaffold. Cells fit for tissue engineering should be easily culturable and be able to connect to the medium. MSCs are the most appropriate cells for osseous tissue engineering (Wolff et al. Citation2011). EnSCs are derived from endometrial biopsies and are a new species of MSCs (Gargett et al. 2009). Ever since Chan reported the existence of EnSCs in 2004, many have focused on hEnSC studies (Chan et al. Citation2004). Unlike embryonic stem cells hEnSCs are not tumorigenic and can go through 40 passages (Gargett et al. Citation2012). Moreover, bone marrow and fat MSCs require local anesthesia.
However, the endometrium is the only source of human MSCs that does not require anesthesia from which MSCs can easily be harvested through the cervix by an endometrial biopsy (Rodriguesa et al. Citation2003, Wang et al. Citation2012). Past research has shown that hEnSCs are capable of differentiating into a variety of cells such as neurons, oligodendrocytes, adipocytes, and osteoblasts (Ebrahimi-Barough et al. Citation2013). Henceforth, we also used hEnSCs for osseous tissue engineering in our study.
In this study, we presented the hybrid collagen/HA composite scaffolds as scaffolds appropriate for generation of new osseous tissue, as they are biologically adaptive and possess a good 3D matrix for adhesion and expansion of human osteoblasts (Wei and Peter Citation2004). Collagen I is an osteoinductive and bioactive factor and HA is an osteoconductive factor, which make a suitable context for osteoblast growth. HA’s affinity toward proteins changes its surface interactions and morphology, and transforms it into a better substance for adsorption into the scaffold (Parenteau-Bareil et al. Citation2010). Collagen is a protein that is distributed throughout the body tissues, and among them, the most important characteristics are its biodegradability, biocompatibility, bioavailability, and high adaptability. However, we will not achieve desirable results for reconstruction of bony and hard tissue defects if we use collagen alone, so we need to combine it with a structure of higher stiffness (Gleeson et al. Citation2010). Moreover, the adhesive nature of human osteoblasts to HA particles and collagen fibers allows the cells to expand well on the scaffold surface (Gleeson et al. Citation2010). Gleeson created a highly porous scaffold of appropriate bioactivity and stiffness by adding HA to the collagen scaffold.
Faghihi’s study focused on the appropriate and effective dose of PMA for osteogenic induction (Faghihi et al. Citation2013). In this and other studies, doses of 1, 2, 3, and 5 μM of this small-molecule were compared and eventually the most appropriate dose for osteogenic induction was arrived at 3 μM. Hence, we too used this dose for PMA in our study (Faghihi et al. Citation2014). Based on our findings, the addition of PMA to the 2D culture medium leads to increased secretion of ALP from osteoblast-like cells, which was considerably higher than that in the negative control group on days 7, 14, and 21. However, it was slightly lower in the osteogenic control group, but was significantly effective in inducing ALP secretion. In Faghihi’s study, the significant rise of ALP was more pronounced on the 14th than the 21st day. In our study, the ALP rise was greater on the 21st day, so much so that the ALP secretion rate almost reached the osteogenic medium’s ALP secretion rate. However, this rise was not very different when compared with days 14 and 21, and was significantly higher only on the 7th day. During bone completion, a sequence of gene expressions of RUNX-2, BSP, COL-I, and OC take place. Similar to Arpornmaeklong’s study, we examined the expression of these genes to prove osteoblastic differentiation. According to Arpornmaeklong’s study, bone mineralization had taken place on the collagen scaffold without creating a teratoma, and that the hEnSCs seeded on the defect were capable of bone regeneration. Furthermore, COL-I expression incrementally increased in hEnSCs when compared with hBMSCs from day 3 to day 21. We too observed the gradual increase in COL-I expression by hEnSCs; and this increase was observed in both the 2D culture medium and the PMA-treated scaffold, likening to osteogenic conditions (Oliveira et al. Citation2012). Faghihi too assessed the expression of the BSP gene to prove the osteoblastic differentiation of hBMSCs (Baht et al. Citation2008). In the aforementioned study, BSP gene expression did not statistically differ in the cells cultured in the PMA-treated medium from those cultured in the osteogenic medium.
However, our study has shown otherwise, i.e. BSP gene expression in the PMA-treated medium was considerable and its osteogenic induction potential was similar to that of the control group.
Based on our findings, BSP & COL-I gene expression increase along with the osteogenic differentiation of EnSCs under the effect of PMA, whereas other studies including that of Faghihi did not show this up-regulating effect of PMA (Faghihi et al. Citation2013). Here we observed increased expression of the RUNX-2 gene in both the positive control and the PMA-treated group. This finding supports the hypothesis that PMA has osteoblastic gene induction potential and that it has been acknowledged as an osteoinductive factor. ALP increase in both control and PMA-treated groups extended up to day 21 of the culture. Even at this stage, the ALP level was considerably higher in the control group than in the PMA-treated group (Arpornmaeklong et al. Citation2010, Campos et al. Citation2013). This finding echoes with that of Campos, where increased ALP activity was reported in 2D cultures containing osteoprogenitor cells’ osteogenic inductor.
Conclusions
According to our findings, as a hedgehog signaling pathway agonist, purmorphamine affects the osteogenic differentiation of hEnSCs seeded on a collagen/HA scaffold. The effect of PMA is smaller than the effect of the osteogenic inductor; nevertheless it considerably affects osteogenic differentiation. PMA as small molecule with osteogenic differentiation potential can be used along with scaffolds for bone tissue engineering.
Acknowledgement
The authors thank Tehran University of Medical Sciences for this research.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Aravamudhan A, Ramos DM, Nip J, Subramanian A, James R, Harmon MD. 2013. Osteoinductive small molecules: growth factor alternatives for bone tissue engineering. Curr Pharm Des. 19:3420–3428.
- Arpornmaeklong P, Wang Z, Pressler MJ, Brown SE, Krebsbach PH. 2010. Expansion and characterization of human embryonic stem cell-derived osteoblast-like cells. Cell Reprogram. 12:377–389.
- Azami M, Ai J, Ebrahimi-Barough S, Farokhi M, Fard SE. 2013. In vitro evaluation of biomimetic nanocomposite scaffold using endometrial stem cell derived osteoblast-like cells. Tissue Cell. 45:328–337.
- Baht GS, Hunter GK, Goldberg HA. 2008. Bone sialoprotein-collagen interaction promotes hydroxyapatite nucleation. Matrix Biol. 27:600–608.
- Bartold PM, Xiao Y, Lyngstaadas SP, Paine ML, Snead ML. 2006. Principles and applications of cell delivery systems for periodontal regeneration. Periodontology. 2000 41:123–135.
- Beloti MM, Bellesini LS, Rosa AL. 2005. Purmorphamine enhances osteogenic activity of human osteoblasts derived from bone marrow mesenchymal cells. Cell Biol Int. 29:537–541.
- Campos D, Soares G, Anselme K. 2013. Role of culture conditions on in vitro transformation and cellular colonization of biomimetic HA-Col scaffolds. Biomaterials. 3:e24922.
- Chan RW, Schwab KE, Gargett CE. 2004. Clonogenicity of human endometrial epithelial and stromal cells. Biol Reprod. 70:1738–1750.
- Ebrahimi-Barough S, Hoveizi E, Norouzi Javidan A, Ai J. 2015. Investigating the neuroglial differentiation effect of neuroblastoma conditioned medium in human endometrial stem cells cultured on 3D nanofibrous scaffold. J Biomed Mater Res a. 103:2621–2627.
- Ebrahimi-Barough S, Kouchesfahani HM, Ai J, Massumi M. 2013. Differentiation of human endometrial stromal cells into oligodendrocyte progenitor cells (OPCs). J Mol Neurosci. 51:265–273.
- Faghihi F, Eslaminejad MB, Nekookar A, Najar M, Salekdeh GH. 2013. The effect of purmorphamine and sirolimus on osteogenic differentiation of human bone marrow-derived mesenchymal stem cells. Biomed Pharmacother. 67:31–38.
- Faghihi F, Papadimitropoulos A, Martin I. 2014. Effect of purmorphamine on osteogenic differentiation of human mesenchymal stem cells in a three-dimensional dynamic culture system. Biomedicine* 157:1–12.
- Hoveizi E, Tavakol S, Ebrahimi-Barough S. 2015. Neuroprotective effect of transplanted neural precursors embedded on PLA/CS scaffold in an animal model of multiple sclerosis. Mol Neurobiol. 51:1334–1342.
- Hynes K, Danijella M. 2000. Clinical utility of stem cells for periodontal regeneration. Periodontology. 2000 59:203–227.
- Gargett CE, Schwab KE, Zillwood RM, Nguyen HP, Wu D. 2009. Isolation and culture of epithelial progenitors and mesenchymal stem cells from human endometrium. Biol Reprod. 80:1136–1145.
- Gargett C, Masuda H. 2010. Adult stem cells in the endometrium. Mol Hum Reprod. 16:818–834.
- Gargett CE, Nguyen HP, Ye L. 2012. Endometrial regeneration and endometrial stem/progenitor cells. Rev Endocr Metab Disord. 13:235–251.
- Gleeson JP, Plunkett NA, O’Brien FJ. 2010. Addition of hydroxyl apatite improves stiffness, inteconnectivity and osteogenic potential of highly porous collagen-based scaffold for bone. Eur Cell Mater. 20:218–230.
- Gronthos S, Zannettino AC, Hay SJ, Shi S, Graves SE, Kortesidis A, Simmons PJ. 2003. Molecular and cellular characterization of highly purified stromal stem cells derived from human bone marrow. J Cell Sci. 116:1827–1835.
- Nakashima M, Reddi AH. 2003. The application of bone morphogenetic proteins to dental tissue engineering. Nat Biotechnol. 21:1025–1032.
- Narayanan AS, Bartold PM. 1996. Biochemistry of periodontal connective tissues and their regeneration: a current perspective. Connect Tissue Res. 34:191–201.
- Oliveira FS, Bellesini LS, Herrero CF, Beloti MM, Rosa A. 2012. Hedgehog signaling and osteoblast gene expression are regulated by purmorphamine in human mesenchymal stem cells. J Cell Biochem. 113:204–208.
- Parenteau-Bareil R, Gauvin R, Berthod F. 2010. Collagen-based biomaterials for tissue engineering applications. Materials. 3:1863–1887.
- Pihlstrom BL, Michalowicz BS, Johnson NW. 2005. Periodontal diseases. Lancet. 366:1809–1820.
- Pontoriero R, Lindhe J. 1995. Guided tissue regeneration in the treatment of degree III furcation defects in maxillary molars. J Clin Periodontol. 22:810–812.
- Rodriguesa CVM, Serricellab P, Linharesc ABR, Guerdesd RM, Borojevicb R, Rossie MA, Duartec MEL, Farina M. 2003. Characterization of a bovine collagen–hydroxyapatite composite scaffold for bone tissue engineering. Biomaterials. 24:4987–4997.
- Shamosi A, Mehrabani D, Azami M, Ebrahimi-Barough S, Siavashi V, Ghanbari H, et al. 2016. Differentiation of human endometrial stem cells into endothelial-like cells on gelatin/chitosan/bioglass nanofibrous scaffolds. Artif Cells Nanomed Biotechnol. 16:1–11.
- Srisuwan T, Tilkorn DJ, Wilson JL, Morrison WA, Messer HM, Thompson EW, Abberton KM. 2006. Molecular aspects of tissue engineering in the dental field. Periodontology. 2000 41:88–108.
- Wang H, Jin P, Sabatino M, Ren J, Civini S, Bogin V, Ichim TE, Stroncek DF. 2012. Comparison of endometrial regenerative cells and bone marrow stromal cells. J Transl Med. 10:207–213.
- Wei G, Peter X. 2004. Structure and properties of nano-hydroxyapatite/polymer composite scaffolds for bone tissue engineering. Biomaterials. 25:4749–4757.
- Wolff EF, Gao XB, Yao KV, Andrews ZB, Du H, Elsworth JD, Taylor HS. 2011. Endometrial stem cell transplantation restores dopamine production in a Parkinson's disease model. J Cell Mol Med. 15:747–755.
- Wu X, Ding S, Ding Q, Gray NS, Schultz PG. 2002. A small molecule with osteogenesis-inducing activity in multipotent mesenchymal progenitor Cells. J Am Chem Soc. 124:14520–14521.
- Wu X, Walker J, Zhang J, Ding S, Schultz PG. 2004. Purmorphamine induces osteogenesis by activation of the hedgehog signaling pathway. Chem Biol. 11:1229–1238.