Abstract
Previously, we showed the rapid and eco-friendly synthesis of gold and silver nanoparticles within 3 and 45 min by fresh leaves extract of herbal medicinal plant Panax ginseng. In addition, we characterized the nanoparticles in terms of shape, size, morphology and stability by FE-TEM, EDX, elemental mapping, SEAD, XRD and particles size analysis. In addition of this, we showed their antimicrobial, anti-coagulant, and biofilm inhibition activity of nanoparticles. Continuing our previous study, here we highlight the further characterization and biomedical applications of P. ginseng leaf-mediated gold and silver nanoparticles. We characterized the nanoparticles further in terms of active functional group and capping layer, surface charge, and temperature stability. Based on these factors, we explored the nanoparticles for antioxidant efficacy, biocompatibility in HaCaT cells, 3T3-L1 pre-adipocytes cells, for anticancer efficacy in A549 lung cancer and B16BL6 skin melenoma cancer cell lines and for anti-inflammation efficacy in RAW 264.7 cell lines. Based on our findings, we suggest that the P. ginseng-mediated gold nanoparticles have high antioxidant activity and highly biocompatibility in HaCaT cells, 3T3-L1 pre-adipocytes cells, RAW 264.7 cells lines and could be considered for future drug delivery carriers. The silver nanoparticles also showed high potent antioxidant efficacy, additionally it showed high anticancer effect in A549 lung cancer and B16BL6 skin melenoma cancer cell lines as compared to precursor salts. Moreover, both gold and silver nanoparticles have anti-inflammatory efficacies in RAW 264.7 cells. Thus, the study may provide useful insights of P. ginseng leaves extract-mediated biocompatible gold and silver nanoparticles and improving their applicability in designing nanoparticles carrier systems for drug delivery applications.
Introduction
Ginseng means the essence of man and has been found as the most important pharmacological herbal medicinal plant. Panax ginseng was discovered over 5000 years ago in China and since then ginseng roots have been used as a highly valued medicinal herb in Traditional Chinese Medicine (Radad et al. Citation2006). P. ginseng resembling the “human-body” is a very slow-growing perennial herb of which root grows actively from the third year, and by the fourth year it increases in diameter, and then finally at sixth year its length reaches about 7–10 cm and breadth 3 cm (Hu Citation1977, Wen and Zimmer Citation1996). P. ginseng plant have many important active components including ginsenosides, sugar residues, flavonoids, proteins etc. among them the ginsenosides have been shown to be the major pharmacological ingredient (Kim et al. Citation2014). It has been proposed that the ginsenosides are the actual components that are responsible for pharmacological efficacies of ginseng plants and products. Even though, the ginseng roots are the active source for ginsenosides, however, leaves also contribute to ginsenosides production and most importantly easily available and in large amount as compared to roots (Wang et al. Citation2006). Thus, we considered fresh ginseng leaves for our study.
Recently, various important herbal medicinal plants and microorganisms have been reported for the green synthesis of metal nanoparticles, for instance; gold and silver nanoparticles (Jo et al. 2016; Singh et al. Citation2015a, Citation2015b, Citation2016d, Citation2016e, Citation2016f, Citation2016g). These green gold and silver nanoparticles are one of the central research focus in the field of bio-nanotechnology for medical applications due to their easy green synthesis, biocompatibility, nontoxic nature, and higher efficacy as compared to the one synthesized by the chemical and physical methodologies (Huang Citation2006, Kemp et al. Citation2009, Shah et al. Citation2014). Recently, gold and silver nanoparticles have come out with the advantage of photothermal and photoimaging properties, thus, are under the clinical trial for the treatment of various diseases, for instance, cancer.
In the present study, we applied the P. ginseng leaf extract mediated gold and silver nanoparticles for in vitro studies in cancer and inflammatory cell lines. Cancer is a leading cause of death worldwide with the mortality rate continuing to rise. The world health organization (WHO) estimated that more than 16 million new cases are registered every year. The high mortality rate of cancer disease is due to the inefficiencies of early diagnosis and subsequent treatment by the available drugs. Here, we present the biocompatibility of P. ginseng leaf-mediated gold and silver nanoparticles in HaCaT cells and 3T3-L1 pre-adipocytes cells and anticancer effect in A549 lung cancer and B16BL6 skin melenoma cancer cell lines. Secondly, inflammation plays a critical role in various human diseases, including cancer, neurological disorders, metabolic syndrome, cardiovascular disease, inflammatory bowel disease, arthritis, and infectious diseases (Yamamoto and Gaynor Citation2004). It’s a complex biological process involving the response of several vascular tissues to injurious stimuli, such as damaged cells, irritants, and pathogens. In the pathogenesis of inflammation, the overexpression of different cytokines such as COX-2, inducible nitric oxide synthase (iNOS), PGE2, and TNF-a produced by activated macrophages plays an important role in the induction of acute and chronic inflammatory diseases (Guzik et al. Citation2003). Lipopolysaccharide (LPS), a well-known component of the cell wall of gram-negative bacteria, stimulates a number of signaling pathways, including NF-jB/IKK signaling in macrophages (Guha and Mackman Citation2001). NF-jB plays a significant role in the pathogenesis and regulation of inflammatory responses, it is a common target in the development of anti-inflammatory drugs. Many therapeutic agents have been studied for anti-inflammatory activity, but are associated with diverse side effects. Thus, the discovery of anti-inflammatory therapeutic agents with few side effects could be very beneficial. In the current study, we explored the P. ginseng leaves-mediated gold and silver nanoparticles for anti-inflammatory effect in RAW 264.7 cell lines.
As reported previously, the P. ginseng plants parts are capable of synthesizing gold and silver nanoparticles, for instance; P. ginseng root, leaves, red ginseng product, Chinese white ginseng powder, etc. (Singh et al. Citation2016a, Citation2016b, Citation2016c). However, among all the ginseng sources for synthesizing nanoparticles, we found that the fresh ginseng leaf-mediated synthesis is very rapid, facile, stable, economical, and results in nanoparticles with high pharmacological importance and effect. Thus, we consider P. ginseng leaf-mediated gold and silver nanoparticles for further in vitro trial for biocompatibility, anti-cancer, and anti-inflammatory efficacies. Collectively, our study provides P. ginseng leaves-mediated gold and silver nanoparticles characterization in terms of biological active groups, surface charge, temperature stability, and further applied for antioxidant efficacy, cell viability on normal cells lines (HaCaT skin and 3T3-L1 pre-adipocytes cell lines), anticancer efficacy on lung cancer (A549 lung cancer) and skin cancer cell lines (B16BL6 melenoma cancer), and anti-inflammation effect on RAW 264.7.
Materials and methods
Materials
All materials for nanoparticles synthesis and purifications were used as described previously by Singh et al. (2016a). The different cell lines were purchased from the Korean Cell Line Bank (Seoul, South Korea).
Characterizations of gold and silver nanoparticles
Fourier transform-infrared (FT-IR) spectroscopy analysis
For FT-IR measurements, air-dried gold and silver nanoparticle powder samples were scanned on a FT-IR spectrophotometer (PerkinElmer Inc., Waltham, MA) over the range of 4000?450 cm?1. FT-IR spectrometer was utilized to study the interactions between the functional groups present as a source of reducing, catalytic, and capping agents on the surface of synthesized gold and silver nanoparticles. The spectra recorded were plotted as transmittance (%) versus wavenumber (cm−1).
Zeta potential analysis
Zeta potential of gold and silver nanoparticles was measured in triplicate to study the surface charge of nanoparticles by dynamic light scattering (DLS), Photal Otsuka Electronics, Hirakata, Japan at 25 °C and at a 12 angle.
Thermogravimetric analysis
TGA analysis of the gold and silver nanoparticles in powered form was performed on a TGA machine (SDT Q600, TA Instruments, New Castle, DE). For TGA analysis, nanoparticles were placed in an alumina pan and heated from 20 to 700 °C at a ramping time of 10 °C/min (Patra and Baek Citation2015).
Applications of gold and silver nanoparticles
In vitro DPPH radical scavenging assay of gold and silver nanoparticles
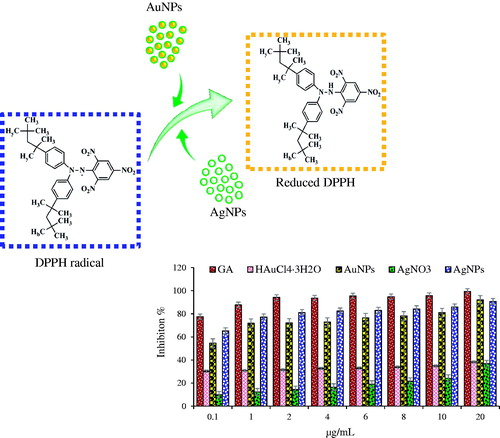
Antioxidant assay of biosynthesized nanoparticles were evaluated using a modified method of previously published DPPH scavenging assays (Ramamurthy et al. Citation2013). Nanoparticles solution of 20 μL was mixed with 180 μL of 1 mM methanolic DPPH solution. Different concentrations of nanoparticles ranging from 0.1, 1, 2, 4, 6, 8, 10 μg/mL were adopted. The reaction mixture was sonicated in dark condition at room temperature for 30 minutes, absorbance values were recorded at 517 nm using UV-Vis spectrophotometer (UV-Vis). The radical scavenging activity was expressed as percentage inhibition equation: [(Absorbance control – Absorbance sample)/Absorbance control] × 100, where Absorbance sample is the absorbance of the DPPH solution with nanoparticles and Absorbance control is the absorbance of the DPPH solution without nanoparticles. Aqueous methanolic solution (80%) was used as a reference. Gallic acid, a potent antioxidant, was used as positive control. All experiments were performed in triplicates and means with standard errors were calculated.
Cell viability and anti-cancer activity of nanoparticles
Cell viability assay
Cell viability was measured by a 3–(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H tetrazolium bromide (MTT) assay (Wang et al. Citation2016). HaCaT cells and 3T3-L1 pre-adipocytes, were seeded at a density of 1 × 104 cells per well in 96-well microtiter plates (Corning Costar, Lowell, NY) and incubated for 1 day. After confluency, the media was fully removed and gold and silver nanoparticles were added, respectively, to the wells at concentrations ranging between 0 and 200 μg/ml. After 1 day of treatment, 20 μl MTT solution (5 mg/ml) was added to the cells followed by incubation for 4 h. Dimethyl sulfoxide (DMSO) was added to each well to dissolve the generated formazan, and the absorbance was determined at 570 nm using a microplate reader (Bio-Tek Instruments Inc., Vinooski, VT). Cell viability was determined as a percentage of the control (untreated cells).
Anticancer efficacy
Anticancer efficacy on lung cancer (A549 lung cancer) and skin cancer cell lines (B16BL6 melenoma cancer – DMEM medium) were determined by basic MTT assay. Human lung cancer cells (A549) were culture in RPMI 1640 medium (GenDEPOT Inc., Barker, TX) while B16BL6 cell were cultured in DMEM (GenDEPOT Inc.), both medias were supplemented with 10% of fetal bovine serum (FBS), 100 IU/mL penicillin and 100 μg/mL streptomycin (Gibco-Brl. Gaithersburg, MD). Cells were kept at 37 °C in a humidified atmosphere containing 5% CO2. For cell viability was determined by MTT (3, 4, 5-dimethylthiazol-2-yl)-2–5-diphenyletrazolium bromide) assay. Cells were seeded in 96-well plates at a density of 1 × 105 cells/ml and incubated for 24 h. Further cells were treated with various concentrations of gold and silver nanoparticles for 24 h. After the incubation time, 10 μL of the MTT stock solution (5 mg/ml) was added to each well and incubated for 4 h. Then, the supernatants were removed and replaced with 100 μL of DMSO. The amount of formazan formed by viable cells was measured using multi-model plate reader (Bio-Tek Instruments, Winooski, VT) at a test wavelength of 570 nm with a reference wavelength of 630 nm.
Anti-inflammatory activity of nanoparticles
Cell culture
Anti-inflammatory effect of nanoparticles has been analyzed as reported previously by Ahn et al. (Citation2016). Murine macrophage cells line (RAW 264.7) were seeded at density of 5 × 103 cells/well at 96-microplate in RPMI-1640 medium containing 10% (v/v) fetal bovine serum (FBS), and 1% (V/V) P/S incubated at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air for 24 h.
Cell viability assay
To determine the effects of gold and silver nanoparticles on cell viability, cells were treated with various concentrations of nanoparticles for 24 h. After 1-day treatment, cell viability was measured by MTT assay. 10 μl MTT solutions (5 mg/mL) were added to each well, and the plates were incubated for an additional 3–4 h, and the formazan formed was dissolved in DMSO; then absorbance of each well was recorded on a synergy 2 multi-mode microplate reader at 570 nm (BioteK, Vermont). The optical density of formazan formed in control (untreated) cells was taken as 100%.
Measurement of nitrite levels
RAW 264.7 cells were pretreated with gold and silver nanoparticles for 1 h and then stimulated with 1 μg/μl lipopolysaccharide (LPS) in the presence of the samples. Cells were incubated for 24 h, after that the Griess reagent was used to determine the nitrite levels in the medium. Briefly, 100 μl of supernatant was mixed with an equal volume of the Griess reagent. The resultant absorbance at 540 nm was measured using a microplate reader (Bio-Tek Instruments Inc.). A standard sodium nitrite curve was included for each experiment (Ahn et al. Citation2016).
Statistical analysis
The statistical significance of nanoparticles was determined using a student’s t-test. P < .05 was considered to be significant, and indicated in Figures with asterisks (*).
Results and discussion
Nanoparticles synthesis
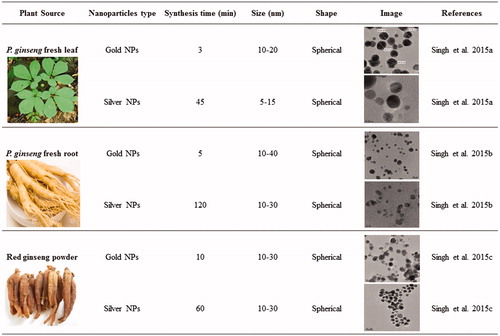
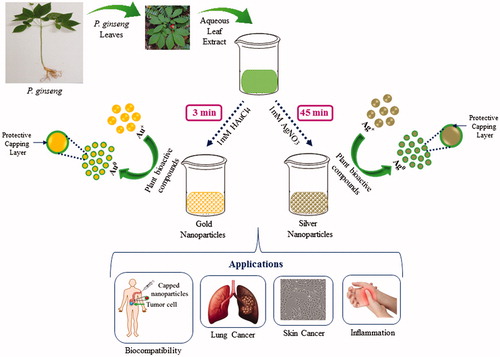
Previously, we showed gold and silver nanoparticles synthesis form different resources of P. ginseng including fresh leaves, fresh root, and red ginseng powder () (Singh et al. Citation2016a, Citation2016b, Citation2016c). Among these resources, the leaves came out as a potent resource for nanoparticles synthesis due to rapid and economical synthesis, which shows only 3 min for gold nanoparticles and 45 min for silver nanoparticles at 80 °C (Singh et al. Citation2016a). Thus, we considered the P. ginseng fresh leaves for our further studies. shows the schematic illustration of the P. ginseng leaf extract-mediated gold and silver nanoparticles rapid synthesis and their biomedical applications.
Characterization of gold and silver nanoparticles
Fourier transform-infrared (FT-IR) spectroscopy profile analysis
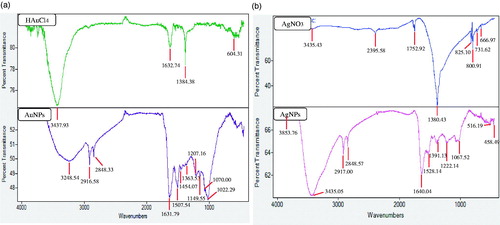
Fourier Transform-Infrared (FT-IR) analysis was conducted to identify the organic molecules responsible for the bio-reduction of metal ions into metal nanoparticles and protein interactions excreted by the plant extract. FT-IR spectrum of nanoparticles was compared against FT-IR spectrum of precursor salts as positive control (). The results revealed intense band at 3248.54, 2916.58, 2848.33, 1631.79, 1507.54, 1454.07, 1363.53, 1207.16, 1149.55, 1070.0, and 1022.29 for gold nanoparticles and at 3853.76, 3435.05, 2917.00, 2848.57, 1640.04, 1528.14, 1391.13, 1222.14, 1067.52, 516.19, and 458.49 cm−1. Bands in the range of 3500–3000 cm−1 represent –N–H and OH–O stretching. The peaks at 2916.68 and 2848.33 cm−1 could be attributed to –CH2 asymmetric stretching. The band at 1631.79 cm−1 could be equated to an amide 1 bond of the polypeptides. Peaks ranging from 1650 to 1450 cm−1 indicate the presence of an aromatic ring and elongation of the aromatic C = C bonds. The bands between 1100 and 1010 cm−1 in the spectrum were assigned to C–O and C–H chemical bonds. The deformation vibration of the C–C bonds in the phenolic groups creates peaks between 1500 and 1400 cm−1 (Debnath et al. Citation2016). Following it, the silver nanoparticles FTIR peaks indicated that the proteins and other aromatic organic molecules from P. ginseng leaves extract played major role in the synthesis and stabilization of nanoparticles. In the case of gold nanoparticles, the obtained peaks suggested that phenolic compounds from P. ginseng leaves extract could mainly be responsible for the gold nanoparticles synthesis. Proteins and amino acid residues are reported to be responsible for providing stability to the nanoparticles from plant extracts. Following, phenolic compounds from P. ginseng leaves extract are predicted to form a coating around the nanoparticles, thereby contributing to their stability (Logeswari et al. Citation2013). Previously, studies have reported that gold nanoparticles from plant extracts contain strong characteristic peaks of primary amines and carbonyl stretch in the amide linkages, which suggest that free amino groups due to amino acid residues and surface-bound proteins are incumbent for the formation of capping layers on the nanoparticles and preventing their agglomeration. Further, the nanoparticles from plant extract possess characteristic binding affinity to thiols, amines, and amides, which are present in amino acid residues. Thus, the active groups surrounding the gold and silver nanoparticles from P. ginseng leaves extract play a major role in providing stability, additionally, it may help in the binding of some active drug or biomolecules due to free surface groups, which further, could be helpful for drug or biomolecules delivery. Similar characteristic peaks of FT-IR spectra were also observed in the previously published reports (Abbai et al. Citation2016).
Zeta potential analysis
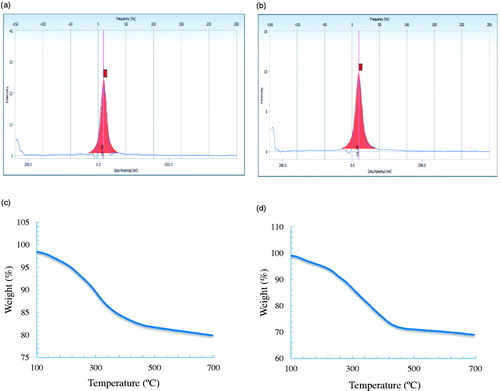
The Zeta potential measurements revealed relative colloidal stability with negatively surface net charge value of −16.0 and −19.3 mV on the surface of gold and silver nanoparticles, respectively ().
Thermogravimetric analysis
The TGA spectrum of the gold and silver nanoparticles showed significant weight loss of nanoparticles when heated from 20 °C to 700 °C (), indicating that, at higher temperature, the organic compounds from P. ginseng leaf extract, which acted as the capping agent and surrounds the nanoparticles were completely degraded (Patra et al. Citation2015).
Applications of gold and silver nanoparticles
In vitro DPPH radical scavenging activity of nanoparticles
Numerous studies have been carried out to show the antioxidant effect of nanoparticles, hence, we aimed to study the capability of ginseng leaf-mediated nanoparticles as antioxidant agents. The antioxidant activity of nanoparticles was evaluated in vitro according to the previous methods (Abbai et al. Citation2016). DPPH reducing ability of nanoparticles was assessed by evaluating the color change spectrophotometrically from purple to yellow at 517 nm. DPPH is composed of stable free-radical molecules and is readily reduced by accepting hydrogen or electron from nanoparticles. The results of the findings are summarized in . The results on the effect of different concentrations of nanoparticles reveal that the synthesized gold and silver nanoparticles possess free radical scavenging activity. We observed that the scavenging activities increased in a dose-dependent manner and were significantly higher than those exhibited by their precursor salts. The increased free radical scavenging activity of gold and silver nanoparticles can be attributed to the antioxidant activity of ginseng leaf (Kharat and Mendhulkar Citation2016, Lu et al. Citation2014). Thus, the biosynthesized nanoparticles possess higher antioxidant activity as compared to precursor salts and demonstrate potentials as free radical scavengers in biomedical applications. It is well documented that the phenolic compounds may contribute directly to antioxidative action (Kharat and Mendhulkar Citation2016, Lu et al. Citation2014). Antioxidant activities are attributed to the phenolic contents in plants probably due to their redox properties, which allow them to act as reducing agents, hydrogen donors, and singlet oxygen quenchers.
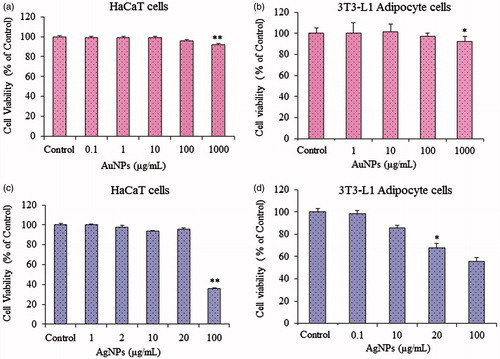
Effects of gold and silver nanoparticles on cell viability
The MTT assay was used to determine the effects of gold and silver nanoparticles on the cell viability of HaCaT and 3T3-L1 pre-adipocytes cell lines. As shown in , gold nanoparticles at concentrations ranging from 1 to 100 μg/ml had no effect on the viability of either cell type. For silver nanoparticles, in HaCaT skin cell lines, cells were viable and did not exhibit any significant cytotoxic effects at 20 μg/mL, which supports the biocompatibility of the silver nanoparticles till 20 μg/mL in HaCaT skin cell lines. However, silver nanoparticles showed significant toxicity at 20 μg/ml on 3T3-L1 pre-adipocytes cells. Thus, taken together, till 10 μg/ml, the silver nanoparticles are non-toxic on the cell viability of HaCaT and 3T3-L1 pre-adipocytes cell lines.
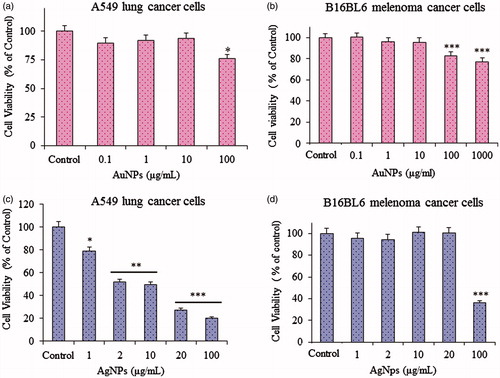
Anti-cancer activity of gold and silver nanoparticles
To observe the in vitro anticancer activity of nanoparticles, we examined the cytotoxicity of gold and silver nanoparticles in A549 lung cancer and B16BL6 melenoma by MTT assay. For gold nanoparticles, as shown in , the gold nanoparticles shows toxicity in cancer cell lines including A549 lung cancer and B16BL5 melenoma cell lines at 100 μg/mL. For silver nanoparticles , in A549 lung cancer cells, the silver nanoparticles show significant cytotoxicity at 2 μg/mL. In B16BL6 melenoma cancer cells, the silver nanoparticles showed cytotoxicity at 100 μg/mL. Therefore, the results supports the biocompatibility of gold and silver nanoparticles for drug delivery, due to non-cytotoxic nature in HaCaT and 3T3-L1 pre-adipocytes cell lines, however, silver nanoparticles, can be apply as anticancer agent in A549 lung cancer and B16BL5 melenoma cell lines. Additionally, It has been reported that the biological green nanoparticles are quite potent and high in terms of efficacy as compared with physio-chemically synthesized nanoparticles (Patra et al. Citation2015). Importantly, gold nanoparticles described in this study may be a potential candidate to act as a carrier the drug delivery to cancer cells and silver nanoparticles can act as an anticancer agent in order to treat cancer (Abbai et al. Citation2016).
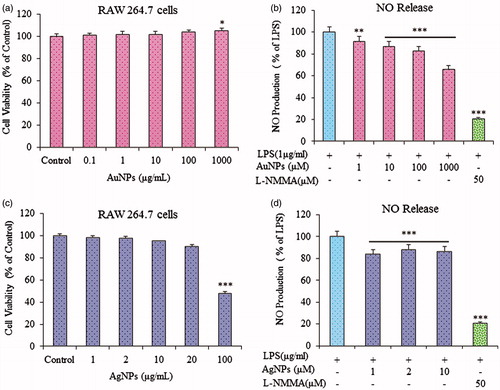
Inhibition of LPS-induced Nitric oxide production
Before evaluating the anti-inflammatory effects of gold and silver nanoparticles, we examined their effects on the viability of RAW264.7 cells. As shown in ; the viability of RAW264.7 cells was not significantly lower than in non-treated cells. Since gold and silver nanoparticles showed no cytotoxicity effect to RAW264.7 cells at 100 and 20 μg/ml, respectively, thus, we used up to 10 μM gold and silver nanoparticles concentration for our study. As previously reported, the macrophage-like RAW264.7 cells describe the action of numerous anti-inflammatory mechanisms at the molecular stage, therefore, to further investigate whether gold and silver nanoparticles can function as an inhibitor of Nitric Oxide production (Ahn et al. Citation2016), in this model, RAW264.7 cells were stimulated with LPS (1 μg/ml) with or without co-treatment with gold and silver nanoparticles. As shown in , the gold and silver nanoparticles dose-dependently repress the NO production induced by LPS. Fascinatingly, it has been observed that gold and silver nanoparticles inhibited LPS-induced NO production in RAW 264.7 cells.
Conclusion
The P. ginseng-mediated gold and silver nanoparticles have been highlighted in this study due to their active functional group. In vitro MTT assay on HaCaT, 3T3-L1 pre-adipocytes cells and Raw 264.7 cell lines showed good cell viability when exposed to high concentration of nanoparticles, however at low concentration, the nanoparticles showed significant anticancer effect on A549 lung cancer, B16BL6 melenoma cell line and anti-inflammatory effect on Raw 264.7 cell lines. The biosynthesized nanoparticles possessed antioxidant activity and demonstrated potentials as free radical scavengers in biomedical applications. High antioxidant activity and low cytotoxicity of biosynthesized nanoparticles were attributed to the protective capping layer consisted of various amino acid residues and surface-bound proteins present in ginseng leaf extract. The results obtained from physicochemical characterization, antioxidant, anticancer, and anti-inflammatory supports its application in the biomedical field. In conclusion, the P. ginseng-mediated green synthesis is cost-effective and advantageous for the development of herbal medicinal plant-mediated, low-cost, and safe nano-drug carriers in targeted drug delivery systems, cancer diagnostic, photothermal therapy, biosensing, and medical imaging. In future, selection of such medicinal plants with high therapeutic importance will create a new platform for the green and effective nanoparticles synthesis and their applications on medical platform.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Funding
This research was supported by Korea Institute of Planning & Evaluation for Technology in Food, Agriculture, Forestry & Fisheries [KIPET NO: 313038–03-3-SB010], and also supported by a grant from the Next-Generation BioGreen 21 Program [SSAC, grant#: PJ0120342016], Rural Development Administration, Republic of Korea.
References
- Abbai R, Mathiyalagan R, Markus J, Kim YJ, Wang C, Singh P, et al. 2016. Green synthesis of multifunctional silver and gold nanoparticles from the oriental herbal adaptogen: Siberian ginseng. Int J Nanomedicine. 11:3131–3143.
- Ahn S, Siddiqi MH, Aceituno VC, Simu SY, Zhang J, Perez ZEJ, et al. 2016. Ginsenoside Rg5: Rk1 attenuates TNF-α/IFN-γ-induced production of thymus-and activation-regulated chemokine (TARC/CCL17) and LPS-induced NO production via downregulation of NF-κB/p38 MAPK/STAT1 signaling in human keratinocytes and macrophages. In Vitro Cell Dev Biol Anim. 52:287–295.
- Debnath R, Purkayastha DD, Hazra S, Ghosh NN, Bhattacharjee CR, Rout J. 2016. Biogenic synthesis of antioxidant, shape selective gold nanomaterials mediated by High altitude lichens. Mater Lett. 169:58–61.
- Guzik TJ, Korbut R, Adamek-Guzik T. 2003. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 54:469–487.
- Guha M, Mackman N. 2001. LPS induction of gene expression in human monocytes. Cell Signal. 13:85–94.
- Huang SH. 2006. Gold nanoparticle-based immunochromatographic test for identification of Staphylococcus aureus from clinical specimens. Clin Chim Acta. 373:139–143.
- Hu SY. 1977. A contribution to our knowledge of ginseng. Am J Chin Med. 5:1–23.
- Jo JH, Singh P, Kim YJ, Wang C, Mathiyalagan R, Jin CG, Yang DC. 2016. Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif Cells Nanomed Biotechnol. 44:1576–1581.
- Kemp MM, Kumar A, Mousa S, Park TJ, Ajayan P, Kubotera N, Mousa SA, Linhardt RJ. 2009. Synthesis of gold and silver nanoparticles stabilized with glycosaminoglycans having distinctive biological activities. Biomacromolecules. 10:589–595.
- Kharat SN, Mendhulkar VD. 2016. Synthesis, characterization and studies on antioxidant activity of silver nanoparticles using Elephantopus scaber leaf extract. Mater Sci Eng C Mater Biol Appl. 62:719–724.
- Kim YJ, Jeon JN, Jang MG, Oh JY, Kwon WS, Jung SK, Yang DC. 2014. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 38:66–72.
- Lu Y, Shipton F, Khoo T, Wiart C. 2014. Antioxidant activity determination of Citronellal and crude extracts of Cymbopogon citratus by 3 different methods. Pharmacol Pharm. 5:395–400.
- Logeswari P, Silambarasan S, Abraham J. 2013. Ecofriendly synthesis of silver nanoparticles from commercially available plant powders and their antibacterial properties. Scientia Iranica. 20:1049–1054.
- Patra S, Mukherjee S, Barui AK, Ganguly A, Sreedhar B, Patra CR. 2015. Green synthesis, characterization of gold and silver nanoparticles and their potential application for cancer therapeutics. Mater Sci Eng C Mater Biol Appl. 53:298–309.
- Patra JK, Baek KH. 2015. Novel green synthesis of gold nanoparticles using Citrullus lanatus rind and investigation of proteasome inhibitory activity, antibacterial, and antioxidant potential. Int J Nanomedicine. 10:7253–7264.
- Radad K, Gille G, Liu L, Rausch WD. 2006. Use of ginseng in medicine with emphasis on neurodegenerative disorders. J Pharmacol Sci. 100:175–186.
- Ramamurthy C, Padma M, Mareeswaran R, Suyavaran A, Kumar MS, Premkumar K, et al. 2013. The extra cellular synthesis of gold and silver nanoparticles and their free radical scavenging and antibacterial properties. Colloids Surf B Biointerfaces. 102:808–815.
- Shah M, Badwaik VD, Dakshinamurthy R. 2014. Biological applications of gold nanoparticles. J Nanosci Nanotechnol. 14:344–362.
- Singh P, Kim YJ, Singh H, Wang C, Hwang KH, Farh Mel A, Yang DC. 2015a. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanomedicine. 10:2567–2577.
- Singh P, Kim YJ, Singh H, Mathiyalagan R, Wang C, Yang DC. 2015b. Biosynthesis of anisotropic silver nanoparticles by bhargavaea indica and their synergistic effect with antibiotics against pathogenic microorganisms. J Nanomater. 2015:234741. doi: 10.1155/2015/234741.
- Singh P, Kim YJ, Yang DC. 2016a. A strategic approach for rapid synthesis of gold and silver nanoparticles by Panax ginseng leaves. Artif Cells Nanomed Biotechnol. 44:1949–1957.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, El-Agamy Farh M, Yang DC. 2016b. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol. 44:811–816.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2016c. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. 44:1150–1157.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2016d. Weissella oryzae DC6-facilitated green synthesis of silver nanoparticles and their antimicrobial potential. Artif Cells Nanomed Biotechnol. 44:1569–1575.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2016e. Microbial synthesis of flower-shaped gold nanoparticles. Artif Cells Nanomed Biotechnol. 44:1469–1474.
- Singh P, Kim YJ, Dabing Z, Yang DC. 2016f. Biological Synthesis of nanoparticles from plants and microorganisms. Trends Biotechnol. 34:7.
- Singh P, Singh H, Kim YJ, Mathiyalagan R, Wang C, Yang DC. 2016g. Extracellular synthesis of silver and gold nanoparticles by Sporosarcina koreensis DC4 and their biological applications. Enzyme Microb Technol. 86:75–83.
- Wang C, Mathiyalagan R, Kim YJ, et al. 2016. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and its anticancer activity. Int J Nanomedicine. 11:3691–3701.
- Wang CZ, Wu JA, McEntee E, Yuan CS. 2006. Saponins composition in American ginseng leaf and berry assayed by high-performance liquid chromatography. J Agric Food Chem. 54:2261–2266.
- Wen J, Zimmer EA. 1996. Phylogeny and biogeography of Panax L. (the ginseng genus, araliaceae): inferences from ITS sequences of nuclear ribosomal DNA. Mol Phylogenet Evol. 6:167–177.
- Yamamoto Y, Gaynor RB. 2004. IkappaB kinases: key regulators of the NF-kappaB pathway. Trends Biochem Sci. 29:72–79.