Abstract
The present study describes the synthesis of zinc oxide nanoparticles (ZnO-NPs) using an extremophilic actinobacterial cell-free extract, supplied with aqueous zinc acetate solution. Crystalline nature, morphological features, and polydispersed nanoparticles size (15–30 nm) were identified by X-ray diffraction (XRD), atomic force and electron microscopic analysis with dynamic light scattering (DLS) study. The interaction between biomolecules and ZnO-NPs was analyzed using Fourier transform infra-red spectroscopy (FT-IR). Furthermore antibacterial, antioxidant activities, and cell viability test of ZnO-NPs were systematically evaluated. The present study opens a new avenue for the actinobacterial synthesis of oxide nanoparticles.
Introduction
Zinc oxide (ZnO) is a biocompatible nanoparticle and has been used as drug carriers, cosmetics, coatings on medical devices and various biomedical applicants. The zinc oxide nanoparticles (ZnO-NPs) are used commercially in larger extent compared to silver nanoparticles, due to low cost and white appearance (Amornpitoksuk et al. Citation2011). Among the metal oxides, ZnO-NPs have attracted a special consideration as antimicrobial agent. For instance, ZnO-NPs inhibit the growth of Gram positive bacteria than Gram negative bacteria, because the outer peptidoglycan layer present in Gram positive bacteria is not an effective permeability barrier. But, lipopolysaccharides present in the outer membrane of Gram negative bacteria provide resistance to antibacterial substances (Kannathasan et al. Citation2011). In addition, ZnO-NPs reveal good antibacterial activity and reactive oxygen species (ROS) generated (Janaki et al. Citation2015) from the ZnO-NPs actively penetrates the bacterial cell membrane using pores of the cell. The leakage of proteins, minerals, and some matters from the cell has occurred due to the ROS mediated damage of the bacterial cell membrane, so the bacterial growth was inhibited (Brayner et al. Citation2006, Yamamoto, Citation2001). Interestingly, several results propose a selective toxicity of ZnO-NPs preferentially targeting prokaryotic systems, although killing of cancer cells has also been demonstrated (Hanley et al. Citation2008, Reddy et al. Citation2007, Taccola et al. Citation2011). Nanomaterials like liposome, dendrimer, micelle, carbon nanotube, and nanoparticles have widely used in a broad spectrum of cancer studies, such as in diagnostics, monitoring, and therapeutic strategies (Maeng et al. Citation2010, Salata Citation2004). In previous studies, exposure of human bronchoalveolar carcinoma derived cells (A549) to nano- and micro-size zinc oxide particles revealed much steeper dose-dependent cytotoxicity relationships than other metal oxides (Lin et al. Citation2009). Systematic studies suggest that the toxicity is due to the elevated oxidative stress and oxidative DNA damage. BEAS-2B cell lines exposed with ZnO-NPs (13 nm), lead to generation of ROS, excitation of inflammation, and cell death (Xia et al. Citation2008).
Nanoscience and nanotechnology have excellent potential in diverse areas. In day-to-day life, more and more nanomaterials are used in consumer goods. These kinds of nanomaterials have been synthesized by using either physical, chemical, or biological routes for multiple applications. Among which, microbial synthesis of metal and its oxide nanoparticles has received enormous attention due to the advances in cost-effectiveness, environmental friendly, and simple technologies in material science (Tamuly et al. Citation2013). Microbial pathways do not produce any toxic by-products and chemicals during the nanoparticles synthesis, hence it has been proposed as feasible alternatives to physico-chemical methods, due to its unhazardous nature to the environment and also cost-effectiveness (Sinha et al. Citation2009).
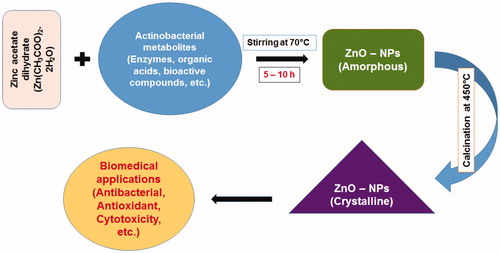
Among the microorganisms, actinobacteria are a reserve source of the secondary metabolites. The value of actinobacteria to society in terms of providing useful drugs especially antibiotics, anticancer drugs, nanomaterials, and enzymes generation in the pharmaceutical industry for revenue is indubitable (Duran et al. Citation2005). The present study describes an eco-friendly synthesis and characterization of ZnO-NPs by microbial technology using Streptomyces sp. (MA30). The role of actinobacterial metabolites as reducing agent in biosynthesis of ZnO nanostructures is shown in . However, there are no proper reports on the synthesis of ZnO-NPs with this extremophilic actinobacteria.
Materials and methods
Description of test strain
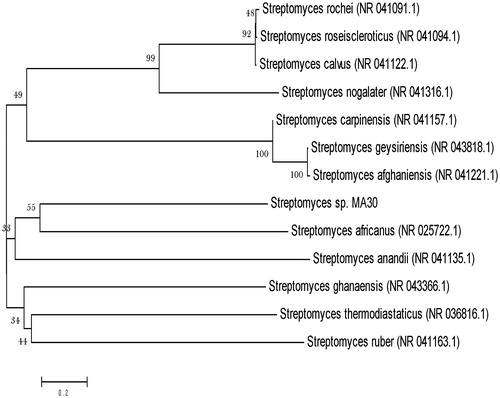
The test strain was isolated from the magnesite mine region (Lat. 11°43′51″N and Long. 78°06′16″E) in Salem, Tamil Nadu, India. The actinobacterial strain was isolated using starch casein agar (SCA) medium supplemented with nalidixic acid (20 μg/mL) and nystatin (100 μg/mL) to reduce bacterial and fungal populations (Radhakrishnan et al. Citation2007). The culture was maintained in International Streptomyces Project (ISP) 2 medium for further studies. The molecular characterization of the potential strain was carried out by 16S rRNA sequencing method. The 16S rRNA gene was annealed with 27F (5′-AGT TTGATCCTGGCTCAG-3′) and 1492R (5′-ACGGCTACCTTG TTACGACTT-3′) primers and further amplified by polymerase chain reactions (Ramya et al. Citation2015). Dideoxy chain termination method was used for sequencing the purified DNA from PCR products in automated instrument on ABI 310 genetic analyzer (Applied Biosystems, Foster City, CA). Subsequently, the relatively retrieved sequences were aligned and phylogenetic tree was constructed using Bootstrap method (MEGA 5.0).
Microbial synthesis of ZnO-NPs
Pure culture of Streptomyces sp. (MA30) was inoculated into Erlenmeyer flask containing 250 mL of ISP 2 broth. The flask was incubated in a rotary shaker at 28 °C at 120 rpm for 72 h. After 72 h of incubation, flask was removed from the shaker, and mycelium was separated by centrifugation at 8000 rpm for 30 min and supernatant was collected. Zinc acetate dihydrate (Zn(CH3COO)2.2H2O) solution (100 mL) was prepared in 2 mM concentration and to react with 50 mL of the supernatant solution for 5–10 h in an aqueous bath system under continuous stirring at 70 °C. The white solid powder was collected through centrifugation at 5000 rpm for 15 min and then dried at 120 °C overnight. The pure crystalline nanoparticles were obtained by heating the ZnO-NPs at 450 °C for 5 h.
Characterization of ZnO-NPs
Structural characterization and phase purity was carried out by X-ray diffraction (XRD) spectroscopy (Shimadzu XRD 6000, Kyoto, Japan), in the operating voltage of 40 kV and a current of 30 mA with Cu Ka radiation (k = 1.540 Å). EDX measurements and SEM analysis of ZnO-NPs were performed by SEM instrument attached with EDX unit (JEOL JFSM 6390, Tokyo, Japan). HR-TEM (Model JEOL JEM 2100, Tokyo, Japan) analysis as well as SAED pattern was performed to identify the size and shape of the ZnO-NPs. The 2D and 3D topography of ZnO-NPs was identified by AFM analysis (Model Nanosurf A). The size distribution of the nanoparticles was identified by using DLS analysis (Model SHIMADZU SALD-7500 nano, Kyoto, Japan). The functional groups and their interactions in between the reactions were carried out by using Fourier transform infra-red (FT-IR) spectroscopic analysis (FT-IR model EXI).
Biomedical applications of ZnO-NPs
Antibacterial activity
Test bacterial pathogens such as, Staphylococcus aureus (NCIM 2079), Bacillus subtilis (NCIM 2063), Escherichia coli (NCIM 2256), Klebsiella pneumoniae (NCIM 2706), Enterobacter aerogenes (NCIM 5139), Salmonella abony (NCIM 2257), and Proteus vulgaris (NCIM 2027) were procured from Actinobacterial Research Laboratory, Periyar University, Salem.
Zinc oxide nanoparticles were tested against bacterial strains by using well diffusion method (Shirley et al. Citation2010). Pure cultures were subcultured in Muller Hinton broth for 24 h at 37 °C. Each strain was swabbed uniformly onto the individual Muller Hinton agar (MHA) plates using sterile cotton swabs and wells of 5 mm diameter were made using well puncture. Using sterile micropipette, the ZnO-NPs and zinc acetate solutions (50 μg/mL) were poured into each of the wells in all the plates. After incubation at 37 °C for 24 h, the zone of inhibition was measured and the morphologically induced changes in control and ZnO-NPs treated bacterial cells were analyzed by field emission scanning electron microscopy (FE-SEM) with energy dispersive X-ray (EDX) pattern (ZWISS-SIGMA, Jena, Germany).
Antioxidant activity – DPPH radical scavenging activity
DPPH free radical scavenging assay was examined based on the method of Patel Rajesh and Patel Natvar (Citation2011) with some modifications. This assay method is based on the reduction of DPPH, a stable free radical. An odd electron released from the free radical DPPH gives a maximum absorption peak at 517 nm (purple color). The DPPH (1,1-diphenyl-2-picrylhydrazyl (H-A)) was dissolved in 3.3 mL of methanol. It was secured from light sources by covering the test tubes with aluminum foil. Then about 150 μL DPPH solutions were added to 3 mL methanol and absorbance was taken immediately at 517 nm for control reading. About 100, 200, 300, 400, and 500 μL (1:1 w/v) concentrations of ZnO-NP solutions as well as standard compound (ascorbic acid) were taken and then make up with methanol to 3 mL and to each, 150 μL DPPH was added to all the reaction tubes. Absorbance was taken after 15 min at 517 nm using methanol as blank on UV-Visible spectrometer, Cyberlab Model. The DPPH free radical scavenging activity was calculated using the following formula: % scavenging = (T0−T)/T0 × 100, where T0 – absorbance of control, T – absorbance of test sample.
Nitric oxide (NO) free radical scavenging activity
Nitric oxide has been involved in a variety of biological functions, including antimicrobial, vascular homeostasis, neurotransmission, and antitumor activities. The technique is based on the sodium nitro-prusside in aqueous solution, at physiological pH it spontaneously generates NO which interacts with oxygen to produce nitrite ions that can be estimated using the Griess reagent. Scavengers of NO contest with oxygen, leading to reduced production of nitrite ions. Large amounts of nitrite ions may lead to tissue damage. About 100, 200, 300, 400, and 500 μL (1:1 w/v) concentrations of ZnO-NPs previously dissolved in DMSO, as well as ascorbic acid (standard compound) was taken in separated tubes and in each tube 2.0 mL of sodium nitro-prusside in phosphate buffer saline was added. The solution was incubated at room temperature for 150 min. After incubation, 5 mL of the Griess reagent was added in each tube including control. Methanol was used as blank. The absorbance was measured at 546 nm on UV-Visible spectrometer (Patel Rajesh and Patel Natvar Citation2011). % scavenging/reduction = (T0−T)/T0 × 100, where T0 – absorbance of control, T – absorbance of test sample.
Cytotoxicity studies
MG63 (human osteosarcoma) and Vero cell lines were procured from National Centre for Cell Sciences, Pune (NCCS). The cell lines were grown in RPMI-1640 medium incorporated with 10% fetal bovine serum, along with antibiotics, Streptomycin (100 μg/mL). The cultured cells were incubated in biological incubator with 5% CO2, 97% humidity at 37 °C. All the experimental studies were carried out under aseptic conditions.
The cytotoxicity of ZnO-NPs was assessed by MTT assay. The MG63 and Vero cells (1 × 105) were grown in 96-well microtiter plates and they were treated with different concentrations of ZnO-NPs (12.5–200 μg/mL) for 24 h at 37 °C. The cells were further incubated with MTT solution [MTT 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide] (0.5 mg/mL) at 37 °C for 3 h. The medium with MTT was then flicked off and the formed formazan crystals were solubilized in 100 μL of DMSO and then the absorbance was measured at 570 nm using microplate reader. The cell inhibition percentage was determined using the following formula: % Cell Inhibition =100 × [(T−T0)/(C−T0)], where T is the optical density of the test sample, T0 is the optical density at time zero, and C is the optical density of the control (Carmichael et al. Citation1987).
Statistical analysis
Results were expressed as mean ± SEM and all the data were analyzed using one way analysis of variance (ANOVA) with Dunnett’s post hoc test to determine significance relative to control groups. In all cases, results were considered to be statistically significant at p < 0.05.
Results and discussion
In the present study, the strain MA30 was isolated from magnesite mine region of Salem, Tamil Nadu, India. Magnesite (MgCO3) is a mineral associated with ultra-basic igneous rocks or dolomitic lime-stones. The nutrients, temperature, pH, humidity, and climate of the magnesite area were different from the normal ecosystem. The feature of magnesite soil and their fertility status created an ecosystem which was considered as a rich source for isolation of different extremophilic actinobacteria. For identification, the 16S rRNA sequence of the strain MA30 was compared with existing sequence in the GenBank by BLAST analysis which suggested that the strain belongs to the genus Streptomyces. The phylogenetic tree was constructed based on the BLAST result using MEGA software with closely related genus and species (). Then, ZnO-NPs were successfully synthesized, after exposing actinobacterial extract to zinc acetate dihydrate solution for 5–10 h, the white colored precipitate settles down at the bottom of the reaction flask. The synthesized ZnO-NPs could be conclusively analyzed and confirmed by X-ray based spectroscopy and electron microscopic techniques.
Figure 2. Phylogenetic tree of potent Streptomyces sp. (MA30) based on 16S rRNA sequence analyzed by Bootstrap method.
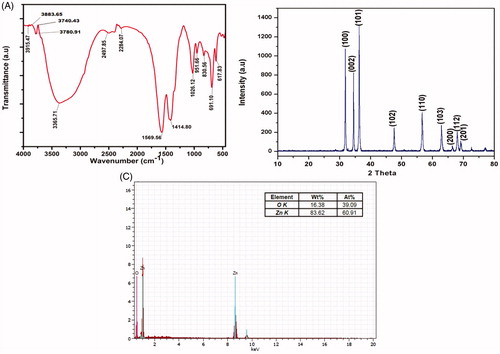
The FT-IR spectrum of synthesized ZnO-NPs showed () strong absorption peaks at 3915.47, 3883.65, 3740.43, 3780.91, 1569.56, 1026.12, 951.66, 830.56, 691.10, and 617.83 cm−1, which were assigned to O‐H stretching of alcohols and phenols, N‐O asymmetric stretching of nitro compounds, -C‐H bending of alkenes, respectively. The weaker bands at 3365.71, 2497.85, 2284.07, and 1414.80 cm−1, correspond to 1°, 2° amines, amides (N‐H stretching), aldehydes (C‐H stretching), nitriles (C≡N stretching), and alkanes (C‐H bending). This results confirms that the bioactive metabolites of actinobacteria could possibly involve in the formation and stabilization of ZnO-NPs. shows the microbially synthesized ZnO-NPs which were confirmed by the characteristic peaks observed in the XRD analysis (JCPDS No. 36-1451). Diffraction patterns were observed at the 2θ angles of 31.86°, 34.41°, 36.38°, 47.56°, 56.59°, 62.87°, 66.38°, 67.95°, and 69.15°, which were equivalent to (100), (002), (101), (102), (110), (103), (200), (112), and (201) respectively. No other characteristic peaks were observed corresponding to the impurities of the zinc acetate. Similarly, Dobrucka and Dugaszewska (Citation2016) reported the XRD pattern of the ZnO-NPs, which were synthesized by using Trifolium pratense flower extracts. The FT-IR spectrum and XRD 2θ angles of the ZnO-NPs, synthesized by Coptidis rhizoma were studied by Nagajyothi et al. (Citation2014). The elemental analysis of ZnO-NPs was confirmed by EDX spectrum. The spectrum () showed strong peak of zinc (83.62%) and oxygen (16.38%) which strongly reveals the presence of ZnO-NPs.
Figure 3. (A) FT-IR spectrum of the ZnO-NPs, (B) characterization of the ZnO-NPs by XRD analysis, and (C) EDX observation of ZnO-NPs.
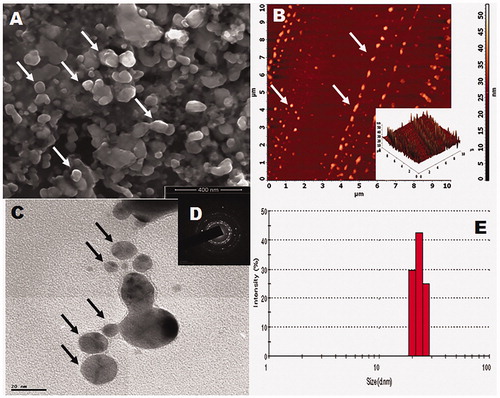
Electron microscopic analysis has been used to determine the size of the materials and to estimate the obtained materials shapes. The FE-SEM and HR-TEM images () of the synthesized ZnO-NPs clearly show the spherical shapes with the average particle size of 16–25 nm. Additionally, AFM images () also shows the 2D topography of the ZnO-NPs. SEAD pattern () reveals the crystal property of the nanoparticles (Dutta et al. Citation2013). The size of the actinobacterially synthesized nanoparticles was analyzed using dynamic light scattering (DLS) analysis. The particle sizes range from 17 to 30 nm with an average size of 25 nm (). The DLS data clearly show the broadening of size and average particle distribution (Selvam et al. Citation2012).
Figure 4. Morphology of synthesized ZnO-NPs (A – SEM image, B – 2D and 3D topography images of AFM, C – HR-TEM image, D – SAED pattern, and E – DLS spectrum).
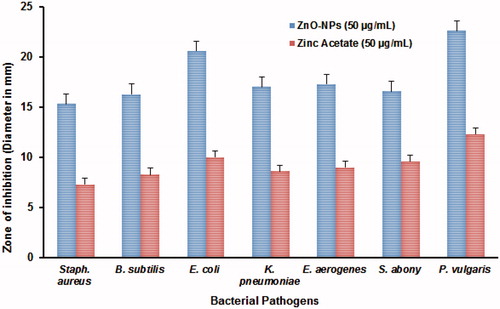
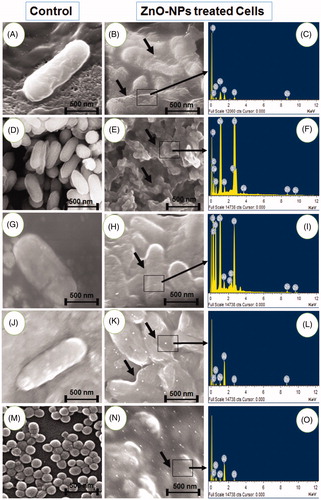
The antibacterial activity of ZnO-NPs was studied against two Gram-positive (Staphylococcus aureus and Bacillus subtilis) and five different Gram-negative (Escherichia coli, Klebsiella pneumoniae, Enterobacter aerogenes, Salmonella typhi, and Proteus vulgaris) bacteria using the well diffusion method with zinc acetate as control (, ). The inhibition zone of antibacterial activity against Gram-positive bacteria ranged from 15 to 16 mm in diameter and Gram-negative bacteria ranged from 16 to 23 mm. The results clearly showed that the antibacterial activity of the ZnO-NPs, synthesized in the present study completely dominates the inhibition of Gram-negative bacterial growth than Gram-positive bacteria. Additionally, the FE-SEM images clearly indicate that the morphologically induced changes like cell wall and cell membrane breakage, cell shrinkage, aggregation of bacterial cells, etc. in ZnO-NPs treated cells, whereas in control cells no changes were observed. EDX analysis also confirmed the morphological changes of ZnO-NP treated cells with strong Zn and O metallic signals. Other minor signals like Cl, C, S, and P were observed due to the bacterial biomolecules. Al signal raised from the aluminum grid was used during FE-SEM analysis (). Normally the activity of the materials was different according to the type of pathogens, synthesis method, shape, size, and the concentrations of ZnO-NPs (Zhang et al. Citation2008). The release of Zn2+ ions is a possible mechanism for antibacterial activity, which damages cell membrane and interacts with intracellular contents (Yamamoto Citation2001). According to Divya et al. (Citation2013), ZnO-NPs cause disruption of bacterial membranes probably by the production of ROS, such as superoxide and hydroxyl radicals. Moreover, ZnO-NPs having positive zeta potential at their surface could be attributed to the damage of the bacterial cell membrane and extrusion of the cytoplasmic contents thereby resulting in the death of the bacterium. Based on these results, it can be concluded that the inhibition of both Gram-positive and Gram-negative bacterial growth by ZnO-NPs could be attributed to the damage of the bacterial cell membrane and the extrusion of the cytoplasmic contents thereby resulting in the death of the bacterial cells (Divyapriya et al. Citation2014).
Figure 5. (A) Antibacterial activity of synthesized ZnO-NPs against bacterial pathogens, (B) FE-SEM analysis of control and ZnO-NPs treated bacterial cultures with EDX analysis (A, B, C – Proteus vulgaris, D–F – Escherichia coli, G–I – Enterobacter aerogenes, J–L – Salmonella abony, and M–O – Staphylococcus aureus).
Table 1. Antibacterial activity of biosynthesized ZnO-NPs by agar well diffusion method. The results are presented as mean ± (SD) (n = 3).
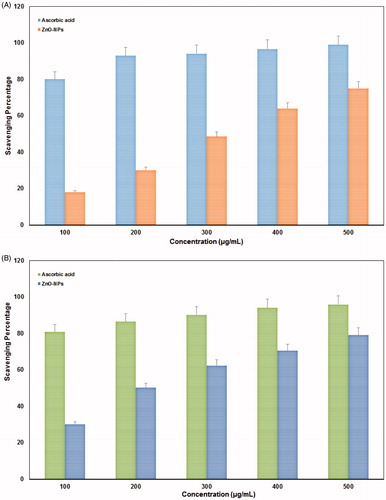
Free radicals (superoxide anions) are generated by the transfer of one electron and play an important role in the formation of other ROS such as hydroxyl radical, hydrogen peroxide, or singlet oxygen in living systems (Stief Citation2003). The DPPH free radical scavenging activity of ZnO-NPs was evaluated with ascorbic acid as standard and graphically represented in . The scavenging effect increased with the increasing concentrations of test samples. From the results, the ZnO-NPs showed maximum scavenging power of 75% at 500 μL (1:1 w/v) concentrations. Similarly, NO scavenging activity was performed with ZnO-NPs and ascorbic acid as standard. The reductive potential of nanoparticle was exhibited on dose dependent manner (). From the graph, when the concentration increases, percentage of scavenging is also increasing linearly for tested nanoparticles and ascorbic acid (standard). The results showed maximum activity of ZnO-NPs was 80% at 500 μL (1:1 w/v) concentration. All the graphs were plotted with standard values of ascorbic acid. Antioxidants can also react with NO to form toxic radicals such as hydroxyl radicals by peroxy nitrite (Halliwell Citation1997). Due to the scavenging power, most of the antioxidants are used in the management of diseases such as neurodegenerative diseases, cancer, AIDS, and so on. Even though, the ZnO-NPs showed better antioxidant nature, a detailed study on its mechanism is needed for the commercialization of these materials in future.
Figure 6. Antioxidant activity of actinobacterially synthesized ZnO-NPs. (A) DPPH radical scavenging activity and (B) nitric oxide free radical scavenging activity.
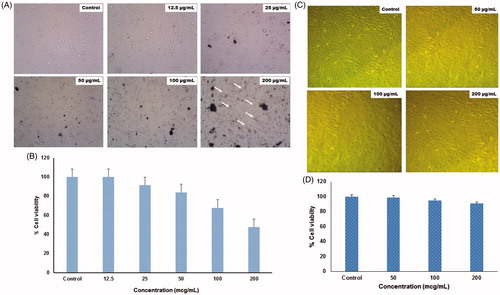
Zinc oxide nanoparticles are used as a novel candidate for excellent anticancer activity with low toxicity and attain much interest today (Nagajyothi et al. Citation2014). MTT assay was used to determine the in vitro toxicity of the synthesized ZnO-NPs on MG63 and Vero cell lines at different concentrations (). The cytotoxicity test showed about 60% of growth inhibition at 200 μg/mL concentration of nanoparticles on the MG63 cell line, whereas only 5–10% of growth inhibition was observed in Vero cell lines at the same concentration. These findings clearly indicate that ZnO-NPs synthesized in this study, may be used as a potent anticancer drug for treating human osteosarcoma effectively without any side effects. Relevant to the evaluation of toxicity of metal and its oxide nanoparticles against many animals, tumor cells have been reported. Akhtar et al. (Citation2012) revealed that three major types of cancer cells (human hepatocellular carcinoma HepG2, human lung adenocarcinoma A549, and human bronchial epithelial BEAS-2B) were killed by the effect of ZnO-NPs, while normal rat astrocytes and hepatocytes were not affected. Additionally, the data confirmed that ZnO-NPs selectively induce apoptosis in cancer cells, which is likely to be mediated by ROS via p53 pathway, through which most of the anticancer drugs activate apoptosis. Pasupuleti et al. (Citation2012) studied that toxicity of ZnO-NPs in Sprague Dawley rats is through oral routes. The incidences of microscopic lesions in liver, pancreas, heart, and stomach clearly indicate that micro-sized ZnO is more toxic than ZnO-NPs. Similarly, ZnO-NPs administrated to Wistar rats at a dose of 10 mg/kg body weight did not affect the exploratory behaviors and the anxious index of the rats (Ben-Slama et al. Citation2015).
Conclusions
In this study, for the first time a simple and eco-friendly approach was developed for the biosynthesis of ZnO-NPs by using extremophilic actinobacterial isolate, Streptomyces sp. (MA30). In addition to this, nanostructure and polydispersed ZnO-NPs size (15–30 nm) were confirmed by FT-IR, XRD, EDX, FE-SEM, AFM, DLS, and HR-TEM with SAED pattern. This bio-reduction process can be further explored for the synthesis of various other nanomaterials for versatile applications in future.
Acknowledgements
The authors are indebted to authorities of the Periyar University, Salem for providing all the necessary facilities to carry out this work.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Akhtar MJ, Ahamed M, Kumar S, Khan MM, Ahmad J, Alrokayan SA. 2012. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine. 7:845–857.
- Amornpitoksuk P, Suwanboon S, Sangkanu S, Sukhoom A, Wudtipan J, Srijan K, Kaewtaro S. 2011. Synthesis, photocatalytic and antibacterial activities of ZnO particles modified by diblock copolymer. Powder Technol. 212:432–438.
- Ben-Slama I, Amara S, Mrad I, Rihane N, Omri K, Ghoul JEL, et al. 2015. Sub-acute oral toxicity of zinc oxide nanoparticles in male rats. J Nanomed Nanotechnol. 6:3.
- Brayner R, Ferrari-Iliou R, Brivois N, Djediat S, Benedetti MF, Fievet F. 2006. Toxicological impact studies based on Escherichia coli bacteria in ultrafine ZnO nanoparticles colloidal medium. Nano Lett. 6:866–870.
- Carmichael J, DeGraff WG, Gazdar AF. 1987. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of chemosensitivity testing. Cancer Res. 47:936–942.
- Divya MJ, Sowmia C, Joona K, Dhanya KP. 2013. Synthesis of zinc oxide nanoparticle from Hibiscus rosasinensis leaf extract and investigation of its antimicrobial activity. Res J Pharm Biol Chem. 4:1137–1142.
- Divyapriya S, Sowmia C, Sasikala S. 2014. Synthesis of zinc oxide nanoparticles and antimicrobial activity of Murraya koenigii. World J Pharm Pharm Sci. 3:1635–1645.
- Dobrucka R, Dugaszewska J. 2016. Biosynthesis and antibacterial activity of ZnO nanoparticles using Trifolium pratense flower extract. Saudi J Biol Sci. 23:517–523.
- Duran N, Marcato PD, Alves OL, De Souza GIH, Esposito E. 2005. Mechanistic aspects of biosynthesis of silver nanoparticles by several Fusarium oxysporum strains. J Nanobiotechnol. 3:8.
- Dutta RK, Nenavathu BP, Gangishetty MK. 2013. Correlation between defects in capped ZnO nanoparticles and their antibacterial activity. J Photochem Photobiol B Biol. 126:105–111.
- Halliwell B. 1997. Antioxidants and human disease: a general introduction. Nutr Rev. 55:44–49.
- Hanley C, Janet L, Alex P, Reddy KM, Coombs I, Coombs A, et al. 2008. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology. 19:295103.
- Janaki AC, Sailatha E, Gunasekaran S. 2015. Synthesis, characteristics and antimicrobial activity of ZnO nanoparticles. Spectrochim Acta A Mol Biomol Spectrosc. 144:17–22.
- Kannathasan K, Senthilkumar A, Venkatesalu V. 2011. In vitro antibacterial potential of some Vitex species against human pathogenic bacteria. Asian Pac J Trop Med. 4:645–648.
- Lin W, Xu Y, Huang CC, Ma Y, Shannon KB, Chen DR, Huang YW. 2009. Toxicity of nano- and micro-sized ZnO particles in human lung epithelial cells. J Nanopart Res. 11:25–39.
- Maeng JH, Lee DH, Jung KH, Bae YH, Park IS, Jeong S, et al. 2010. Multifunctional doxorubicin loaded superparamagnetic iron oxide nanoparticles for chemotherapy and magnetic resonance imaging in liver cancer. Biomaterials. 31:4995–5006.
- Nagajyothi PC, Sreekanth TVM, Tettey CO, Jun YI, Mook SH. 2014. Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis rhizoma. Bioorg Med Chem Lett. 24:4298–4303.
- Pasupuleti S, Alapati S, Ganapathy S, Anumolu G, Pully NR, Prakhya BM. 2012. Toxicity of zinc oxide nanoparticles through oral route. Toxicol Ind Health. 28:675–686.
- Patel Rajesh M, Patel Natvar J. 2011. In vitro antioxidant activity of coumarin compounds by DPPH, super oxide and nitric oxide free radical scavenging methods. J Adv Pharm Edu Res (JAPER). 1:52–68.
- Radhakrishnan M, Balaji S, Balagurunathan R. 2007. Thermotolerant actinomycetes from Himalayan Mountain – antagonistic potential, characterization and identification of selected strains. Malay Appl Biol. 36:59–65.
- Ramya S, Shanmugasundaram T, Balagurunathan R. 2015. Biomedical potential of actinobacterially synthesized selenium nanoparticles with special reference to anti-biofilm, anti-oxidant, wound healing, cytotoxic and anti-viral activities. J Trace Elem Med Biol. 32:30–39.
- Reddy KM, Feris K, Bell J, Wingett DG, Hanley C, Punnoose A. 2007. Selective toxicity of zinc oxide nanoparticles to prokaryotic and eukaryotic systems. Appl Phys Lett. 90:2139021–2139023.
- Salata OV. 2004. Applications of nanoparticles in biology and medicine. J Nanobiotechnol. 2:3.
- Selvam S, Rajiv Gandhi R, Suresh J, Gowri S, Ravikumar S, Sundrarajan M. 2012. Antibacterial effect of novel synthesized sulfated β-cyclodextrin crosslinked cotton fabric and its improved antibacterial activities with ZnO, TiO2 and Ag nanoparticles coating. Int J Pharm. 434:366–374.
- Shirley AD, Dayanand A, Sreedhar B, Dastager SG. 2010. Antimicrobial activity of silver nanoparticles synthesized from novel Streptomyces species. Dig J Nanomater Biostruct. 5:447–451.
- Sinha S, Pan I, Chanda P, Sen SK. 2009. Nanoparticles fabrication using ambient biological resources. J Appl Biosci. 19:1113–1130.
- Stief TW. 2003. The physiology and pharmacology of singlet oxygen. Med Hypoth. 60:567–572.
- Taccola L, Vittoria R, Cristina R, Vittorio O, Iorio MC, Vanacore R, et al. 2011. Zinc oxide nanoparticles as selective killers of proliferating cells. Int J Nanomedicine. 6:1129–1140.
- Tamuly C, Hazarika M, Bordoloi M, Das MR. 2013. Photocatalytic activity of Ag nanoparticles synthesized by using Piper pedicellatum C. DC fruits. Mater Lett. 102–103:1–4.
- Xia T, Kovochich M, Liong M, Madler L, Gilbert B, Shi H, et al. 2008. Comparison of the mechanism of toxicity of zinc oxide and cerium oxide nanoparticles based on dissolution and oxidative stress properties. ACS Nano. 10:2121–2134.
- Yamamoto O. 2001. Influence of particle size on the antibacterial activity of zinc oxide. Int J Inorg Mater. 3:643–646.
- Zhang L, Jiang Y, Ding Y, York D. 2008. ZnO nanofluids – a potential antibacterial agent. Prog Nat Sci. 18:939–944.