Abstract
Research was aimed on microemulsion-based hydrogel for voriconazole. Oleic acid and isopropyl myristate as lipid phases; tween 20: tween 80 as surfactants and PEG600 as cosurfactant were selected to formulate voriconazole microemulsions. The promising microemulsions in terms of zeta potential, pH, viscosity, and drug release were selected and developed into hydrogels using carbopol 934. Resulting microemulsion-based hydrogel (MBH) of voriconazole were evaluated for in vitro diffusion and ex vivo permeation. Antifungal potentials of MBH were assessed against selected fungal strains. Optimal MBH formulations, O6 and O8 had displayed their antifungal potentials with enlarged zone of inhibition against selected fungal strains.
Introduction
Topical administration of therapeutic moieties which possess lipophilicity becomes an attractive mode of drug delivery. The topical administration offers high patient compliance with localized drug concentration and able to target the site of infection (Kaur and Kakkar Citation2010). Among topical formulations, hydrogel systems have posted a phenomenal success in recent past, owing to their effectiveness and convenience in delivery of therapeutics locally and systemically. The success of hydrogels and other topical formulations depended mainly on the physico-chemical principles of therapeutic agents as well as vehicle. Nowadays usage of hydrogel formulations is increased in the clinical management of acute bacterial and fungal infections. Novel hydrogels are highly porous, biocompatible and cross-linked structures of hydrophilic polymers (Goindi et al. Citation2016). In aqueous environment, they absorb substantially high amounts of water and thereby acquire biocompatibility. Hydrogels also acquire biocompatibility due to the resemblance of hydrogels with the extracellular matrix both physicochemically and mechanically. The porosity of hydrogels controls the density of the cross-links in the matrix of gel which aids in enhancing drug loading capacity and subsequent drug release in a rate-dependent manner. Nowadays, microemulsion-based hydrogels (MBH) generated much attention as potential topical drug-delivery system (Feng et al. Citation2009, Lawrence and Rees Citation2012). The concept of microemulsion was introduced in early 1940s by Hoar and Schulman who produced clear single-phase water-in-oil dispersions and coined them as microemulsions (Hoar and Schulman Citation1943). Microemulsions are clear, stable mixtures of oil, water and surfactant mostly in combination with a cosurfactant. In recent past, researchers are keen on these systems because of their potential to act as drug delivery vehicles for a wide range of drugs. Microemulsions, with micro domains of different polarity within the single phase promote solubility of hydrophilic as well as lipophilic drugs. Microemulsion facilitates to higher diffusion and absorption rates. Moreover, microemulsions act as permeation enhancers by reducing the diffusion barrier of stratum corneum (Kogan and Garti Citation2006). Thus, microemulsion plays a prominent role in dermal and transdermal drug delivery. Though it has many advantages, the major drawback with microemulsion is its low viscosity which limits its transdermal applications (Santos et al. Citation2008). In order to avoid this, microemulsion-based hydrogels, have been developed with combined characteristics of both hydrogel/microemulsion so as to enable effective transdermal drug delivery (Raj et al. Citation2016).
Voriconazole, a broad-spectrum triazole antifungal agent, was approved by the US FDA in 2002 (Jeu et al. Citation2003). It is a fluconazole derivative mainly used to treat serious fungal infections in immunocomprimised patients, which include aspergillosis, candidema, candida esophagitis etc., caused by Scedosporium apiospermum and Fusarium spp (Saravolatz et al. Citation2003). Oral usage of voriconazole leads to severe adverse effects such as photophobia, hallucinations, thrombocytopenia, acute renal failure etc (Denning et al. Citation2002). Intravenous administration of voriconazole is reported to have problems with heart rhythms such as arrhythmia, prolongation of Q-T interval, ventricular fibrillation, supraventricular tachycardia, palpitation, atrial fibrillation, and cerebral hemorrhage. Drug interactions were reported with rifampicin, rifabutin, carbamazepine, and long-acting barbiturates (Ullmann Citation2003). Voriconazole has low aqueous solubility which in turn leads to erratic bioavailability. As a mean to address the poor water solubility and bioavailability, voriconazole complexed with β-cyclodextrin have been developed for oral and intravenous delivery (Harding Citation2003). Furthermore, reports reveal that β-cyclodextrin containing intravenous formulations limit the application due to its nephrotoxicity and hemolysis. Hence, it is highly desirable to formulate voriconazole for transdermal delivery. Previous studies report that various gelling agents such as chitosan, xanthum gum, carbopol 940, carbomer, gelatin, carrageenan were used singly or in combination to produce MBH (Hajiabbas et al. Citation2015, Koop et al. Citation2012, Zhu et al. Citation2009;). Cross-linked alginate-gelatin and polymers of acrylic acid, such as Carbopols are some of the most widely used excipients as a thickening agent in topical preparations such as creams and gels, and they form hydrogel with water (Yuan et al. Citation2016). Carbopol, an anionic polyacrylic derivative is used as a gelling agent in this study (Thorgeirsdottir et al. Citation2005). With this background, the research was focused to develop voriconazole-loaded microemulsion-based hydrogel for antifungal activity.
Materials and methods
Materials
Voriconazole was obtained as a gift sample from M/s. Unichem Labs, Goa, India. Glycerin, methanol, oleic acid, tween 20, and tween 80 were procured from Himedia Pvt. Ltd., Mumbai. Carbopol 934, isopropyl myristate, PEG 600, propylene glycol, and triethanolamine were purchased from SD Fine Chem. Ltd., Mumbai, India. Double refined oils used in the study were purchased from the local supermarkets.
Solubility studies
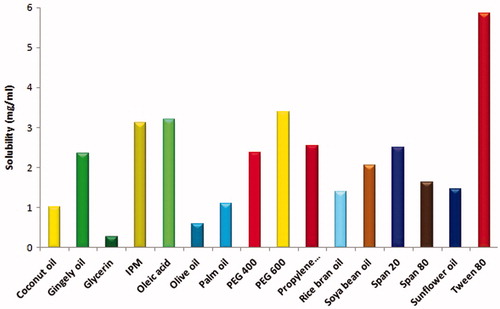
Liquid vehicles such as coconut oil, gingelly oil, isopropyl myristate (IPM), oleic acid, olive oil, palm oil, rice bran oil, sun flower oil, and soya bean oil; butanol, glycerin, PEG 400, PEG 600, propylene glycol, span 20, span 80, tween 20, and tween 80 were used to determine the solubility of voriconazole. They were assessed for their suitability as solvent, surfactant, and co-surfactant systems. Voriconazole was added in excess into 5 ml of each vehicle followed by vortex mixing for 30 s. Mixtures were equilibrated on a thermostatically controlled shaker for 72 h at 27 °C. After equilibration, the mixtures were centrifuged at 3000 rpm for 20 min and filtered through a 0.45 μm nylon filter to remove undissolved voriconazole. Dilutions were suitably made with methanol and its drug content was assayed at 256.5 nm (Xiao et al. Citation2011).
Drug-loaded microemulsion
Appropriate pseudo-ternary phase diagrams were constructed by water titration method to establish the possible compositions of oil, surfactant, and cosurfactant systems for the formulation and development of microemulsions (Gannu et al. Citation2010). Different ratios of oil to surfactant/cosurfactant were selected within the range of 1:1, 1:2, 2:1, and 2:3. Specified amount of voriconazole was added to the mixture with selected composition of microemulsions containing oil, surfactant, and cosurfactant systems. The contents were vortexed until drug was dissolved completely. The resultant clear microemulsions were stored in vials suitably.
Optimization of microemulsion
The prepared microemulsion systems (O1–O16 and I1–I16) were analyzed for their drug content and drug release. The systems with promising drug release profiles were further developed into their respective hydrogels. The optimized liquid microemulsion systems were characterized for globule size, zeta potential, and viscosity (Chen et al. Citation2006, Liu et al. Citation2011).
Drug content
A measured volume (1 ml) of each voriconazole containing microemulsion formulation was diluted with methanol. After suitable dilutions, absorbance was measured at 256.5 nm and the percent of drug content was calculated.
In vitro dissolution studies
Dissolution testing of voriconazole microemulsions were carried out by USP-II dissolution tester (rotating paddle) with 900 ml of 0.1 N HCl at 37 ± 0.5 °C and 75 rpm to determine drug release profiles. Voriconazole-loaded microemulsions filled in capsules equivalent to 50 mg were placed in the basket of dissolution medium. The 5 ml aliquot was withdrawn at regular intervals of 5, 15, 30, 45, and 60 min. The experiment was set for sink condition. The samples were then filtered and their concentrations were assessed using UV-Spectrophotometer (Shimadzu UV 1700). The measurements were done for six independent samples. To investigate the possible release mechanism of voriconazole from the capsule formulation, the drug release data were fitted to various kinetic models such as the zero-order, first-order, and Hixson Crowell.
Characterization of microemulsion
pH and viscosity
The pH of promised formulations was estimated by pH meter using a glass membrane electrode (ELICO LI 200, Hyderabad, India). Viscosity was determined using Brookfield viscometer, Middleboro, MA with spindle # 63 at 10 rpm (Gulsen and Chauhan Citation2005).
Zeta potential and droplet size analysis
Zeta potential and droplet size distribution patterns of optimized microemulsions were determined using Zeta sizer (HAS 3000, Horiba Scientific, Singapore). Samples were placed in clear disposable zeta cells and results were recorded. Polydispersity index (PDI) values of microemulsions were computed with help of ĐM= Mw/Mn formula, in which Mw is the weight-average molar mass and Mn is the number-average molar mass (Tavano et al. Citation2011).
Excipients compatibility
Infrared (IR) spectroscopy studies of voriconazole and promised formulations (O2, O4, O6, O8, I1, I2, I3, and I4) were recorded by ATR-FTIR spectrophotometer (Agilent CARY 630 ATR-FTIR) and background spectrum collected under similar conditions (Hathout et al. Citation2010). A sample of material was placed on the diamond ATR crystal and analyzed using Agilent resolutions pro software. Each spectrum of sample was collected from 32 single average scans at a resolution of 4 cm−1 in the absorption region of 600–4000 cm−1.
Design of microemulsion-based hydrogel (MBH)
Microemulsions with promising drug content values and drug release profiles were chosen to formulate MBH. In this method, carbopol 934 (0.5%) was allowed to swell in distilled water for 6 h under continuous agitation until a viscous solution (gel consistency) was obtained. Selected microemulsion formulation was added to the viscous gel under thermostatic agitation. Small amount of triethanolamine was added to neutralize the formulation. Permeation enhancers such as propylene glycol (1%) and glycerin (<1%) were also added to MBH (Chen et al. Citation2007).
Evaluation of MBH
Viscosity
Dynamic viscosity of MBH was assessed by using Brookfield synchro electric viscometer (model LVDV, Middleboro, MA) attached with #64 spindle. In this test, MBH systems were filled in graduated propylene jar and spindle was lowered perpendicularly taking care that spindle do not touch the bottom of the jar. The spindle was rotated in the hydrogel at increasing shear rates of 0.5, 1, 2.5, and 5 rpm. At each speed, the corresponding dial reading was noted.
In vitro and ex vivo studies
In vitro diffusion studies were performed with vertical Franz diffusion cell using phosphate-buffered saline (PBS 7.4) as the medium (Elshafeey et al. Citation2009). Voriconazole-loaded MBH (200 mg) was gently placed onto donor chamber and thereby drug was allowed to diffuse through cellophane membrane into receptor chamber. At regular intervals of 0.5, 1, 2, 3, 4, 5, and 6 h, 0.5 ml of samples were withdrawn from the receptor chamber. The whole experiment was carried out at 37 ± 0.5 °C with 300 rpm speed and by marinating sink condition. The experiment was also carried out in similar manner by replacing the cellophane membrane with excised animal skin membrane (Nair and Nair Citation2015). The amount of drug permeated through the membrane was plotted as the function of time. Permeability parameter, membrane flux (J) was obtained from the slope and intercept of the straight line obtained by plotting the cumulative amount of voriconazole permeated versus time in steady state conditions. Permeation coefficient (KP) was calculated from the equation, Kp = J/C, where, C is the initial concentration of drug in the formulation applied to the membrane. The drug release kinetics of various voriconazole-loaded MBH were assessed (Ustundag Okur et al. Citation2011).
Skin irritation test
The MBH systems were evaluated for skin irritation. The skin irritation test was carried out on eight Wistar albino rats. The selected MBH system was carefully applied on depilated portion of the animal skin. The treated rats were observed for the changes on their skin surface for a period of 36 h (Prasanna Raju et al. Citation2015).
In vitro antifungal activity
The optimal MBH formulations O2, O4, O6, O8, and plain drug solution were assayed for antifungal activity against strains of Actinomyces, Candida, Rhizopus, and Sacchromyces species. The fungal culture suspensions were made according to the ATCC protocol of microbiologics and they were incubated for 24 h to establish the growth of fungi before transferring onto the solid agar medium as the strain was available in lyophilized form (Celebi et al. Citation2015). The sterile petri-dishes were filled with agar growth medium and allowed to solidify. The inoculums of fungal strains were spread uniformly over the solid agar surface by spreader glass rod. Two wells were made in each agar medium with the help of a sterile cork borer. The wells were filled with samples of standard voriconazole and MBH; and samples were incubated. The antifungal activity of MBH was evaluated under strict aseptic conditions by measuring the clear rings appeared around the wells as zone of inhibition after the fourth day of incubation (Dobler et al. Citation2016).
Results
Solubility study
Solubility studies were performed to identify the suitability of vehicles in the microemulsion formulation, shown in . From the solubility profiles, oleic acid and isopropyl myristate exhibited superior solubility and therefore chosen as oil phases; tween 20 and tween 80 were selected as surfactant systems; while PEG 600 and propylene glycol were selected as co-surfactant systems.
Selection of formulation from ternary diagram
According to the compositions given in , the pseudo-ternary phase diagrams were envisaged to select the clear microemulsion systems (Figure not shown). The coordinates within the colored regions close to the surfactant-rich axis was selected by trial and error process before the addition of the drug.
Table 1. Composition of voriconazole microemulsions.
Drug content
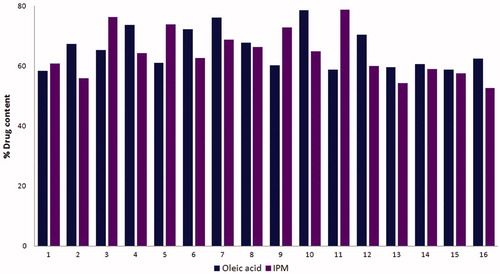
In the oleic acid and IPM systems, drug content of voriconazole-loaded microemulsion was found to be in the range 90–95% () indicating good drug-loading capacity of microemulsion with minimal wastage of drug.
Drug release
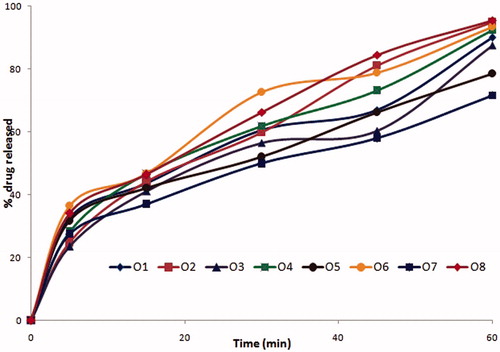
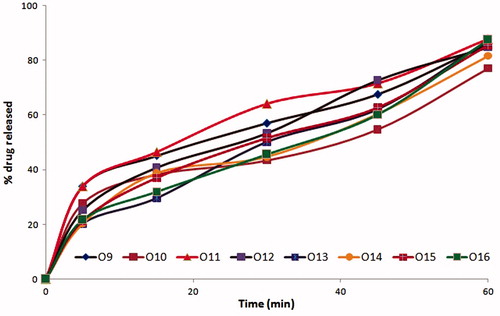
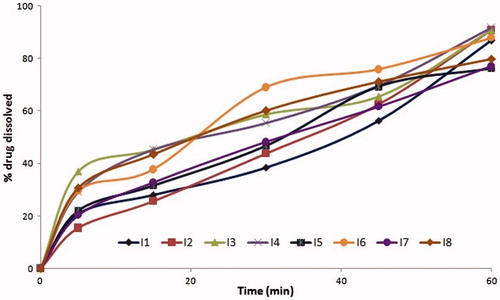
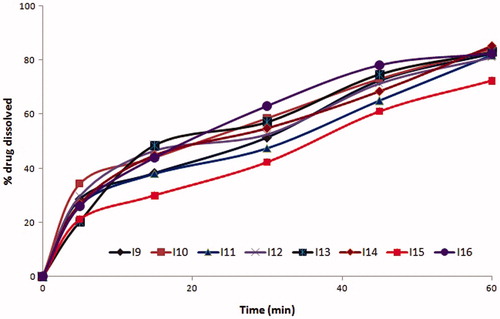
The formulations coded O3, O7, O11, and O15 among oleic acid phase and I3, I7 formulations among IPM as oil phase were found to possess similar drug release profiles, due to similar surfactant concentration. Further O3, O7, I3, and I7 with PEG 600 as cosurfactant had shown slightly enhanced drug release compared to O11 and O15 which contain propylene glycol as cosurfactant at same Smix ratios. The similar trend was exhibited with all microemulsion systems with oleic acid and IPM phases. The formulations with 2:1 of S:CoS ratio were evidenced with better drug release than that of 1:2 and 1:1 compositions of Smix due to rich surfactant concentration, which resulted in reduced interfacial tension. The effect of higher co-surfactant concentrations was evident with mean globule size of 25 nm. The drug release profiles of formulations are shown in . Among the formulations, four formulations O2, O4, O6, and O8 with higher cosurfactant concentrations had shown the highest drug release than others (). Depending on their drug release profiles and phase behavior O2, O4, O6, and O8 formulations were selected for further studies.
Characterization of microemulsions
Viscosity and pH
The pH of the formulations was found to be nearly neutral i.e., in the range of 7–7.4 as a result of non-ionic and neutral excipients of microemulsion formulation. The formulations with neutral pH facilitate the ease of transdermal drug delivery. Due to the presence of dehydrate moieties (tween 20, tween 80, and PEG 600), viscosities of the microemulsion systems were found to be >123 cp.
Droplet size and zeta potential measurement
Globule size of the microdispersion formulations was found in the range of 24–65 nm and polydispersity index (PDI) was less than 1. The size analysis results inferred that globule size of microemulsion formulations was uniform. The formulation, O2 with Smix in the ratio of 1:2 containing PEG600 as cosurfactant possesses larger globule size than the other three selected formulations due to the presence of large quantities of oil. Zeta potential was found to be in the range of 1.2 to −10.7 mV as shown in and indicated the thermodynamic stability of microemulsions.
Table 2. Viscosity, pH, globule size, PDI, and Zeta potential of microemulsions.
FT-IR studies
The FT-IR spectra of pure voriconazole and voriconazole MBH were identical. The –OH stretching around 3189.9 cm−1, C–N peak around 1128.5 cm−1 and C–F peak at 1403.89 cm−1 retained in all the formulations. It indicated that there were no irreversible interactions established between voriconazole and excipients.
Evaluation of MBH
Viscosity
The viscosity coefficient values of selected MBH systems were between 240 to 280 cp as shown in . The higher viscosity was found for the O6-MBH.
Table 3. Viscosity, in vitro, and ex vivo studies of selected MBH formulations.
Permeation studies
In vitro diffusion and ex vivo permeation studies were conducted to determine the release of drug and the capacity of formulations to cross bio-membranes for 6 h. In this study, the amount of drug permeated through bio-membrane was more in MBH formulations than that of pure drug as per the flux and permeability coefficient values demonstrated in . The data revealed that O2, O4, O6, and O8 formulations possess almost similar drug release profiles. However, the drug release from O6 and O8 was slightly higher than O2 and O4 formulations. Release of voriconazole from MBH formulations had followed slow first-order release and obeyed the Higuchi kinetic model.
Skin irritation test
The voriconazole MBH systems did not provoke any gross changes or manifestations of skin irritation or inflammation symptoms in any treated animal.
Antifungal studies
Antifungal studies of MBH formulations were carried out against four different strains of fungi which include Actinomyces, Candida, Rhizopus, and Sacchromyces species (). It was found that O6 and O8 exhibited greater zones of inhibition.
Table 4. Antifungal activity of MBH formulations.
Discussion
In total, 32 number of formulations were prepared by varying the oil phase, surfactant, and co surfactant ratios. Solubility studies of voriconazole in various lipid vehicles were performed to find the suitability of vehicles for the formulation of microemulsion. The vehicles, oleic acid, and IPM had shown high solubility for voriconazole due to their higher HLB values (17 and 11.5, respectively) and were chosen for the formulation (Rhee et al. Citation2001). The microemulsion area and their respective concentrations were determined by envisaging pseudo-ternary phase diagrams (Bagwe et al. Citation2001, Constantinides and Scalart Citation1997). Working formulae was attained on addition of 50 mg of voriconazole to the oil and Smix ratio obtained from the triplots.
The data indicated that microemulsions possess a reasonable drug-loading capacity. Further low standard deviation values indicate that the microemulsion systems possess uniform drug content, confirms the appropriateness of the method adopted for formulation. However, varied drug release profiles were observed from different microemulsion formulations from in vitro dissolution studies. This could be due to type and efficiency of co-surfactant used. In our study, propylene glycol (PG) as a co-surfactant though it is a small chain alcohol its HLB value was found to be 3.4, which favors the formation of w/o microemulsions but not o/w microemulsions. In comparison to formulations containing PG as co-surfactant, microemulsion systems with PEG600 as co-surfactant displayed better drug release profiles. Furthermore, PG is not completely miscible with IPM, hence the formulations with PG in IPM phase were found to be unstable. Although PEG600 has long chain and being less hydrophilic, maintains the hydrocarbon chains of tween 80 effectively in oil phase through van der Waal forces, resulting in the formation of stronger film that was resistant to coalescence (Chen et al. Citation2004). The co-surfactant at higher concentrations effectively tend to liquefy the interfacial film by increasing its flexibility to mould globular size lower than that of surfactant-rich formulations and it was therefore Smix with 1:2, 2:3 shown better drug release profiles. Nonetheless, the drug release profiles of oleic acid phase formulations were slightly higher than that of IPM phase microemulsion systems. This drug release phenomenon was attributed due to their HLB values that are 17 and 11.5, respectively. Oleic acid with greater HLB value (17) favors formation of o/w microemulsion with much ease than IPM (11.5), possibly the reason for higher drug release. Polyhydric alcoholic co-surfactant PEG600 in association with tween could affect the magnitude of intermolecular attraction in microemulsion systems and resulted with increased dynamic viscosities of microemulsions. The pH values were kept neutral with the addition of triethanolamine, which are important criteria for topical formulations.
The droplet size and PDI of the dispersed phase of microemulsion are pivotal in rendering the thermodynamic stability of microemulsions (Cilek et al. Citation2006, Tenjarla Citation1999). Data on microemulsion droplet size analysis provided by Zeta sizer indicated that the uniform distribution of globules in micro dispersions. As liquid microemulsions contain tween 20 and tween 80 (nonionic surfactants) might have reduced globular interactions by stearic repulsions rather than electro kinetic approach, and resulted in less potential development. However, the type of oil/lipid phase used in the microemulsion systems, produce slight negative charge. This kind of electrical approach was of low substantial reason in maintaining stability of the dispersed system.
The optimized O2, O4, O6, and O8 microemulsions were further developed into MBHs. Rheology of MBH is pivotal since it affects spreadability and adherence of MBH to the skin membrane. The hydrogels showed higher degree of viscosities owing to the presence of carbopol 934, a gelling agent. The viscosity was unaffected by the presence of voriconazole.
From the diffusion profiles, it was found that the MBH formulations exhibited nearly similar drug release profiles owing to porous matrix network of hydrogel on which microemulsified voriconazole was adsorbed with maximized surface area. Due to moderate adsorption of drug molecules onto the porous matrix gel of MBH, voriconazole release from MBH formulations was followed slow first-order rate and obeyed Higuchi kinetic model. Enhanced diffusion and permeation profiles were portrayed in in vitro diffusion and ex vivo permeation studies. Enhancement was due to the formation of micro domains of voriconazole in MBH systems with ultra-low-interfacial tension owing to the presence of non-ionic surfactants such as tween 20 and tween 80.
The voriconazole MBH systems did not affect any animal skin membrane due to emollient property exerted by gelling constituent, carbopol. The results of antifungal studies of optimized formulations against four different strains such as Actinomyces, Candida, Rhizopus, and Sacchromyces species were satisfactory with the zones of inhibition between 0.2 – 2.2 mm. Zone of inhibitions was found to be greater for O6 and O8 MBH formulations. These results proved that MBH systems were with highest fungicidal efficacies due to substantial penetration of microemulsified (lipid-based) voriconazole into fungal cell membranes. In addition, voriconazole MBH systems (O6 and O8) possessed remarkable experimental permeability characteristics of flux and permeability coefficient. Therefore, voriconazole-loaded MBH systems O6 and O8 were found to be superior in binding and inhibiting CYP450-dependent 14-α sterol demethylase and ergosterol synthesis in fungal cell wall. Thus, voriconazole MBH systems had been portrayed profound antifungal potentials in comparison with standard drug.
Conclusions
Voriconazole microemulsions were formulated based on their solubility profiles in varying proportions of oil phase, surfactant, and co-surfactant systems. They were optimized based on drug content and drug release parameters. Among them, formulations of concentrated co-surfactant possessed high drug release profiles owing to their smaller particle size in the range of 25 nm. Further, they were successfully developed into MBH formulations. Oleic acid as lipid phase, tween 80, and PEG600 as surfactant mix containing MBH systems have displayed superior in vitro diffusion and ex vivo permeation profiles. On the whole, MBH formulations are ecumenical and offer superiority in trans-membrane transport of highly lipophilic moieties by which drug-delivery limitations and drug-associated toxic effects can be circumvented. Further attempts on clinical safety and scale-up process should be explored.
Acknowledgements
The authors are grateful to M/s. Hetero Dugs Ltd., Hyderabad for gratis of voriconazole and the management of Sri Padmavathi School of Pharmacy, Tirupati, India for rendering generous support to carry out the research work.
Disclosure statement
The authors report that the article content has no conflict of interest.
References
- Bagwe RP, Kanicky JR, Palla BJ, Patanjali PK, Shah DO. 2001. Improved drug delivery using microemulsions: rationale, recent progress, and new horizons. Crit Rev Ther Drug Carrier Syst. 18:77–140.
- Celebi N, Ermis S, Ozkan S. 2015. Development of topical hydrogels of terbinafine hydrochloride and evaluation of their antifungal activity. Drug Dev Ind Pharm. 41:631–639.
- Chen H, Chang X, Du D, Li J, Xu H, Yang X. 2006. Microemulsion-based hydrogel formulation of ibuprofen for topical delivery. Int J Pharm. 315:52–58.
- Chen H, Chang X, Weng T, Zhao X, Gao Z, Yang Y, Xu H, Yang X. 2004. A study of microemulsion systems for transdermal delivery of triptolide. J Control Release. 98:427–436.
- Chen H, Mou D, Du D, Chang X, Zhu D, Liu J, Xu H, Yang X. 2007. Hydrogel-thickened microemulsion for topical administration of drug molecule at an extremely low concentration. Int J Pharm. 341:78–84.
- Cilek A, Celebi N, Tirnaksiz F. 2006. Lecithin-based microemulsion of a peptide for oral administration: preparation, characterization, and physical stability of the formulation. Drug Deliv. 13:19–24.
- Constantinides PP, Scalart JP. 1997. Formulation and physical characterization of water-in-oil microemulsions containing long-versus medium-chain glycerides. Int J Pharm. 158:57–68.
- Denning DW, Ribaud P, Milpied N, Caillot D, Herbrecht R, Thiel E, et al. 2002. Efficacy and safety of voriconazole in the treatment of acute invasive aspergillosis. Clin Infect Dis. 34:563–571.
- Dobler D, Schmidts T, Zinecker C, Schlupp P, Schafer J, Runkel F. 2016. Hydrophilic ionic liquids as ingredients of gel-based dermal formulations. AAPS PharmSciTech. 17:923–931.
- Elshafeey AH, Kamel AO, Fathallah MM. 2009. Utility of nanosized microemulsion for transdermal delivery of tolterodine tartrate: ex-vivo permeation and in-vivo pharmacokinetic studies. Pharm Res. 26:2446–2453.
- Feng G, Xiong Y, Wang H, Yang Y. 2009. Gelation of microemulsions and release behavior of sodium salicylate from gelled microemulsions. Eur J Pharm Biopharm. 71:297–302.
- Gannu R, Palem CR, Yamsani VV, Yamsani SK, Yamsani MR. 2010. Enhanced bioavailability of lacidipine via microemulsion based transdermal gels: formulation optimization, ex vivo and in vivo characterization. Int J Pharm. 388:231–241.
- Goindi S, Narula M, Kalra A. 2016. Microemulsion-based topical hydrogels of tenoxicam for treatment of arthritis. AAPS PharmSciTech. 17:597–606.
- Gulsen D, Chauhan A. 2005. Dispersion of microemulsion drops in HEMA hydrogel: a potential ophthalmic drug delivery vehicle. Int J Pharm. 292:95–117.
- Hajiabbas M, Mashayekhan S, Nazaripouya A, Naji M, Hunkeler D, Rajabi Zeleti S, Sharifiaghdas F. 2015. Chitosan-gelatin sheets as scaffolds for muscle tissue engineering. Artif Cells Nanomed Biotechnol. 43:124–132.
- Harding VD. 2003. Pharmaceutical formulations containing voriconazole. Google Patents.
- Hathout RM, Woodman TJ, Mansour S, Mortada ND, Geneidi AS, Guy RH. 2010. Microemulsion formulations for the transdermal delivery of testosterone. Eur J Pharm Sci. 40:188–196.
- Hoar T, Schulman J. 1943. Transparent water-in-oil dispersions: the oleopathic hydro-micelle. Nature. 152:102–103.
- Jeu L, Piacenti FJ, Lyakhovetskiy AG, Fung HB. 2003. Voriconazole. Clin Therap. 25:1321–1381.
- Kaur IP, Kakkar S. 2010. Topical delivery of antifungal agents. Expert Opin Drug Deliv. 7:1303–1327.
- Kogan A, Garti N. 2006. Microemulsions as transdermal drug delivery vehicles. Adv Colloid Interface Sci. 123-126:369–385.
- Koop HS, Da-lozzo EJ, de Freitas RA, Franco CRC, Mitchell DA, Silveira JL. 2012. Rheological characterization of a xanthan–galactomannan hydrogel loaded with lipophilic substances. J Pharm Sci. 101:2457–2467.
- Lawrence MJ, Rees GD. 2012. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 64:175–193.
- Liu CH, Chang FY, Hung DK. 2011. Terpene microemulsions for transdermal curcumin delivery: Effects of terpenes and cosurfactants. Colloids Surf B Biointerfaces. 82:63–70.
- Nair RS, Nair S. 2015. Permeation studies of captopril transdermal films through human cadaver skin. Curr Drug Deliv. 12:517–523.
- Prasanna Raju Y, Haitha K, Rao P, Satyanarayana Vandana KR, Thushara Bindu D, Vinesha VHVC. 2015. Formulation and characterization of Aceclofenac -Aloe vera Transemulgel. Curr Drug Deliv. 12:703–708.
- Raj R, Mongia P, Ram A, Jain NK. 2016. Enhanced skin delivery of aceclofenac via hydrogel-based solid lipid nanoparticles. Artif Cells Nanomed Biotechnol. 44:1434–1439.
- Rhee YS, Choi JG, Park ES, Chi SC. 2001. Transdermal delivery of ketoprofen using microemulsions. Int J Pharm. 228:161–170.
- Santos P, Watkinson AC, Hadgraft J, Lane ME. 2008. Application of microemulsions in dermal and transdermal drug delivery. Skin Pharmacol Physiol. 21:246–259.
- Saravolatz LD, Johnson LB, Kauffman CA. 2003. Voriconazole: a new triazole antifungal agent. Clin Infect Dis. 36:630–637.
- Tavano L, Alfano P, Muzzalupo R, de Cindio B. 2011. Niosomes vs microemulsions: New carriers for topical delivery of Capsaicin. Colloids Surf B Biointerfaces. 87:333–339.
- Tenjarla S. 1999. Microemulsions: an overview and pharmaceutical applications. Critic Rev Ther Drug Carrier Syst. 16:461–521.
- Thorgeirsdottir TO, Kjoniksen AL, Knudsen KD, Kristmundsdottir T, Nystrom B. 2005. Viscoelastic and structural properties of pharmaceutical hydrogels containing monocaprin. Eur J Pharm Biopharm. 59:333–342.
- Ullmann AJ. 2003. Review of the safety, tolerability, and drug interactions of the new antifungal agents caspofungin and voriconazole. Curr Med Res Opin. 19:263–271.
- Ustundag Okur N, Apaydın S, Karabay Yavasoglu NU, Yavasoglu A, Karasulu HY. 2011. Evaluation of skin permeation and anti-inflammatory and analgesic effects of new naproxen microemulsion formulations. Int J Pharm. 416:136–144.
- Xiao YY, Liu F, Chen ZP, Ping QN. 2011. Water in oil microemulsions containing NaCl for transdermal delivery of fluorouracil. Yao Xue Xue Bao. 46:720–726.
- Yuan L, Wu Y, Fang J, Wei X, Gu Q, El-Hamshary H, et al. 2016. Modified alginate and gelatin cross-linked hydrogels for soft tissue adhesive. Artif Cells Nanomed Biotechnol. 8:1–8.
- Zhu W, Guo C, Yu A, Gao Y, Cao F, Zhai G. 2009. Microemulsion-based hydrogel formulation of penciclovir for topical delivery. Int J Pharm. 378:152–158.