?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Curcumin, is a yellow substance extracted from Curcuma longa rhizomes, it is a crystalline compound that has been traditionally applied in culinary practices and medicines in India. The aim of our study is to demonstrate the efficacy of curcumin-loaded magnetic hydrogel nanocomposite in the treatment of heart hypertrophy. 10 rats weighing 150–200 g each were induced with heart failure using 2.5 mg/kg doxorubicin for 2 weeks. The test groups were treated with curcumin-loaded magnetic hydrogel nanocomposite while the control was treated with curcumin alone. malondialdehyde (MDA) levels, superoxide dismutase (SOD), and glutathione peroxidase (GPX) enzymes activities were monitored after two weeks of last the dose. In addition, the expression of three heart failure markers; atrial natriuretic peptide (ANP), B type natriuretic peptide (BNP), and beta major histocompatibility complex (β-MHC) were observed, it was found that the expression of these markers decreases with an increase in the concentration of curcumin (P < 0.05). Curcumin elevated the decreased level of GPX and SOD, and reduced the elevated level of MDA in cardiac tissue. We suggest this combination to be a potent therapy for heart failure and hypertension in the nearest future.
Introduction
The heart secrete two natriuretic peptides; ANP and BNP which are involved in the endocrine regulation of blood pressure (Anand-Srivastava Citation1993, Khosravi et al. Citation2011). Their mechanism of action involves the activation of a common receptor, guanylyl cyclase A (GC-A), which is mainly expressed in the kidneys and vasculature. This is followed by an increase in intracellular cyclic Guanosine monophosphate (GMP), which initiate vasodilatation, diuresis, and natriuresis, thereby decreasing blood pressure (Anand-Srivastava Citation1993, Baharvand et al. Citation2009).
Recent studies suggested that, in addition to the maintenance of volume homeostasis and blood pressure, these peptides are also involved locally in modulating the cardiac growth response to hypertrophic stimuli (Calderone et al. Citation1998, Toolabi et al. Citation2012). Particularly, mice without ANP receptor, and GC-A, have pronounced cardiac hypertrophy and hypertension that is excessive and partly independent of their elevated blood pressure (Knowles et al. Citation2001, Lopez et al. Citation1995, Oliver et al. Citation1997). These experimental observations emphasize the crucial role of this system in cardiovascular homeostasis and suggest that some forms of cardiac hypertrophy and hypertension in humans can be interpreted in part through decreased responsiveness or expression of GC-A (Khosravi et al. Citation2013).
Blunted renal and vascular effects of the exogenous ANP have been demonstrated in patients having arterial hypertension and cardiac failure (Dessì-Fulgheri et al. Citation1997, Hirooka et al. Citation1990). In a recent study, for the first time, the relationship between a functional mutation (deletion) in the region of the promoter of the human GC-A gene and declined GC-A expression, with essential ventricular hypertrophy and hypertension was shown (Nakayama et al. Citation2000). GC-A mice is a very good platform cardiovascular functions study of ANP/GC-A system and its long-term inhibition consequences (Lopez et al. Citation1995, Oliver et al. Citation1997). However, till date, only a few data is available on the effect of GC-A inactivation on cardiac function.
Doxorubicin (Dox) is a powerful, highly efficacious and well established drug in many several cancer therapies, such as leukemia, solid tumors, breast cancer, soft tissue sarcoma, small cell carcinoma of the lung, and esophageal tumor. Inspite of its potentials for cancer therapy, its clinical usefulness is still restricted due to its specific toxicities to cardiac tissues (Zhou et al. Citation2001) resulting in conditions such as hypotension and transient electrocardiographic abnormalities, recorded in up to 41% of patients.
Curcumin (diferuoylmethane), lyphenol, the active component of turmeric in curry, this compound is derived from the plant Curcuma longa. This compound has been shown to be potent in protecting our system against myocardial injury and cardiac function preservation (Srivastava and Mehta Citation2009). Furthermore, curcumin has several therapeutic properties that block the renal and hepatic toxicities induced by DOX, thus, it is a free radical scavenger (Mohamad et al. Citation2009, Swamy et al. Citation2012). Turmeric was first used about 3000 years ago as an anti-inflammatory medicinal agent. There have been several researches about its anti-inflammatory and antioxidative characteristics, and as a potential anti-cancer agent in recent studies. There is also interest in its potential as a cardioprotective agent. Investigators from many countries have studied its effect in vivo models as well in in vitro of myocardial ischemia and cardiac hypertrophy (Srivastava and Mehta Citation2009).
The aim of our study is to demonstrate the cardioprotective effects of curcumin-loaded magnetic hydrogel nanocomposite against doxorubicin-induced cardiac toxicity in rats.
Materials and methods
Chemicals and drugs
Dox, curcumin, and other chemicals were obtained from Sigma Aldrich, Germany. All the chemicals were of the analytical grade and prepare according to the instruction of the manufacturer.
Experimental animals
Ten Albino rats (male) weighing 150–200 g each were procured from animal house of the University. The rats were used for this study after permission from University Animal Ethical Committee. Animals were housed and allowed to adapt for one week to the laboratory conditions prior to the commencement of the experiment, they were allowed free access to standard rat feed and water 12 h before the experiment, they were starved of food but not water.
Preparation of NIPAAM-MAA complexes
The free radical emulsion polymerization method was employed in synthesizing this nanogel. NIPAAM (N-Isopropylacrylamide) and MAA was scattered in water with a molar ratio of 70 and 30, respectively. BIS serve as a cross-linker. Sodium dodecyl sulfate (SDS) was used as the surfactant agent used at a ratio of 3% of monomer weight. To begin the polymerization process, potassium per sulfate (KPS) was used as the initiating agent (at a ratio of 3.5% weight of monomers) (Daraee et al. Citation2014, Daraee et al. Citation2014, Eatemadi et al. Citation2014, Eatemadi et al. Citation2014, Lemke and Dawson Citation2000). Thirty minutes (30 min) following degassing the mixed solution with N2, the increasing temperature of the mixture reaches 70 °C, and the initiator was then poured into it slowly. Polymerization process proceeds for 4 h at this temperature and then the polymer solution (latex) was cooled. Benzolylated dialysis bag membrane with molecular weight of 12—14 kDa was used to purify the synthesized nanocomposite against double-distilled water for 5 days. The polymer synthesized in excess was freeze-dried and stored for further experiments (Seidi et al. Citation2014).
Preparation of curcumin-loaded magnetic hydrogel nanocomposite
Curcumin-loaded magnetic hydrogel nanocomposite was synthesized by dissolving the hydrogel in NaOH solution in the presence of curcumin and magnetic nanoparticles (MNPs) (Berger et al. Citation2004). We dispersed 100 mg of MNPs and 50 mg of curcumin in 40 ml of 25 mM NaOH solution, we sonicate this mixture at 0.5 cycle and 50% amplitude for 15 min. The temperature of the solution increased to 50 °C (Abrams and Gerhardt Citation2000). Consequently, 400 mg of freeze-dried nanogel was scattered in the solution and then sonicated again at the abovementioned conditions for 5 min. The resulting solution was allowed to stand for 48 h in a shaking incubator at 5 °C, to allow for nanogel groove formation, and the encapsulation of curcumin and MNPs into it (Abrams and Gerhardt Citation2000). The next stage involves the centrifugation of the solution; the supernatant was separated and precipitated. The nanocomposite was washed with NaOH, dialyzed against deionized water for a period of 3 days to expel free curcumin and NaOH. The dialyzed solution was centrifuged, freeze-dried, and kept for subsequent research.
Experimental protocol
After a week of acclimatization to the surrounding, the animals were randomly distributed into the control and test group. Five rats in group 1 serving as the normal control group received 5 mg/kg body weight of curcumin each intraperitoneally (i.p.) with 2.5 mg/kg body weight of Dox for cardiac toxicity induction for a period of 2 weeks at every other day interval. Five rats in group 2 received 2.5 mg/kg body weight of Dox each, (i.p.) for cardiac toxicity induction followed by treatment with 5 mg/Kg curcumin-loaded magnetic hydrogel nanocomposite in six equal injections for a period of 2 weeks, to make a total cumulative dose of 30 mg/kg body weight.
Rat heart histology
Rats were sacrificed, the hearts were separated and fixed with 4% formaldehyde, embedded in paraffin, then, 5 μm sections were stained with periodic acid Schiff (to discriminate cell borders) or 0.1% picrosirius red (for collagen). The mean diameter of cardiomyocyte was calculated through photomicrographs of the left and right ventricular free wall using a computer-assisted image analysis system (VIDAS 25, Zeiss, Germany), measuring 100 cells/specimen in the cell nucleus region.
Measurement of drug-loading and encapsulation efficiency
By the following formula, the percentage of curcumin encapsulated on the nanoparticles (EE) (EquationEquation (1))(1)
(1) and drug loading (DL) (EquationEquation (2))
(2)
(2) was measured:
(1)
(1)
(2)
(2)
Morphological study
The morphological characteristics of the synthesized nanocomposite and nanogel were examined using scanning Electron Microscopy (SEM; Model EVO 18, Special Edition, Carl Zeiss Inc.). For analysis of SEM, nanosuspension was dissolved in deionized water, a nanosuspension drop was placed on graphene sheet coated on a stainless steel holder. The samples were then coated using gold particles before visualization in order to boost detection of nanoparticles under 10 kV electron beam.
Chemical characterization
Characterization of the hydrogel and drug-loaded nanocomposite was carried out by Fourier transform-infrared (FTIR) spectroscopy using the KBr disk. The sample infrared (IR) spectra were scanned between the range of 400 to 4000 cm−1 and recorded on an FTIR (Equniox 55 LS 101 Bruker, Germany). FTIR spectra were obtained at a resolution of 4 cm−1 with a minimum of 256 scans per spectrum. All measurements were taken at room temperature. The spectra of CO2, water, and KBr were deducted from the sample spectrum and the procedure was executed under nitrogen gas to avoid humidity interference.
The nanogel was dispersed in dimethyl sulfoxide (DMSO) and characterized with NMR spectroscopy.
DAPI staining
Induction of apoptosis by samples (curcumin and encapsulated curcumin) was reviewed by morphological fluorescence microscopic studies of condensed and fragmented nuclei of the cells following staining with DAPI. H9c2 cells were grown on a six-well plates (7 × 104 cells = well) containing 12 mm coverslips and consequently treated for various samples in a way that final concentration of drugs alone or in combination with each other was 800 mg/mL. Cells were allowed to attach overnight at 37 °C. Cells were washed using PBS buffer and fixed with 4% paraformaldehyde for 1 h at room temperature. The cells were further washed thrice with PBS buffer, permeabilized with 0.1% (w/v) Triton X-100 for 5 min. It was washed again with PBS buffer solution and stained by incubation with 200 ng/mL DAPI for 20 min (Zhang and Xu Citation2000). DNA fragmentation and condensation of apoptotic cells were analyzed under a fluorescence microscope (Olympus microscope Bh2-RFCA, Japan). Minimum 300 cells were studied in each well and the percentage of apoptotic bodies was calculated. All experiments were done in triplicate.
RNA extraction and cDNA synthesis
For extraction of RNA Trizol isolation reagent (Invitrogen, Carlsbad, CA) was used according to the manufacturer’s protocol for cell lines. After extraction of RNA, sample RNA content was quantified by measuring absorbance at 260 nm. Then, the firmness of extracted RNA was defined by electrophoresis in 0.5 μg/ml ethidium bromide contained agarose gels. Complementary DNA (cDNA) was designed using random primers (N6) and hexamer primers with purchased reverse transcriptase kit from fermentas.
Real-time PCR (qRT-PCR) assay
Quantitative real-time PCR technique was used for the analysis of ANP, BNP, β-MHC, and GAPDH genes expression. The forward (F) and reverse (R) primer sequences of ANP, BNP, β-MHC, and GAPDH (which was used as endogenous control) are summarized in . The quality of real-time PCR reactions was monitored by running standard samples as duplicated. Negative controls were prepared each time, consisting of 2 μl ddH2O as a substitute for cDNA template in test samples. The sample tubes were placed in the Rotor-Gene 6000 Corbet (Hilden, Germany) with the following settings according to the manufacturers’ protocol ().
Table 1. Forward and reverse primer specific for ANP, BNP, β-MHC, and GAPDH genes, which were used in this study.
Table 2. The PCR program for ANP, BNP, β-MHC, and GAPDH.
Enzyme assay
The rat heart was isolated each from the test and control group, it was ice-cooled, homogenized in 1 mL phosphate buffer. The homogenate is then centrifuged at 3000 g for 15 min. Supernatant was collected, MDA levels (Preuss et al. Citation1998), SOD, and GPX enzymes activities were measured as illustrated in previous studies (Marklund and Marklund Citation1974).
Statistical analysis
Data are shown as means ± SE in the measurement (n = 3). Statistical data were analyzed by one-way ANOVA test at the significance level of P = 0.05, 0.01, and 0.001. Furthermore, Tukey’s post test was performed to compare all groups using GraphPad Prism 5 software (La Jolla, CA).
Results
Chemical structure of NIPAAM-MAA
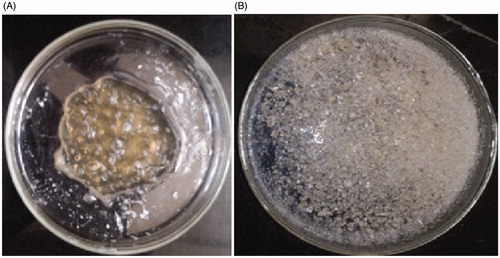
As shown in , the NIPAAM-MAA copolymers were successfully synthesized. and indicated the NIPAAM-MAA copolymers in gel and solid state, respectively.
FTIR analysis showed that curcumin crystallinity did not change upon fabrication. Bands as a result of carbonyl bond stretches were studied to deduce the physical state of the drug. FTIR data in the region (1100–1700 cm−1) were compared for curcumin-loaded magnetic hydrogel nanocomposite and pure curcumin () (Sanphui et al. Citation2011). As anticipated, three main properties C = O bands () were noticed for crystalline curcumin at 1100, 1650, and 1700 cm−1. The amine peak at 1550cm−1 found in the FTIR spectra of curcumin nanogel shifted to 1520 cm−1. This finding showed that minor structural alterations occurred at the molecular level. Magnetic hydrogel nanocomposite fabrication also gave peaks at the same wavelengths ().
Figure 2. FTIR spectrum of (A) NIPAAM-MAA nanogel, and (B) NIPAAM-MAA drug-loaded magnetic hydrogel nanocomposite.
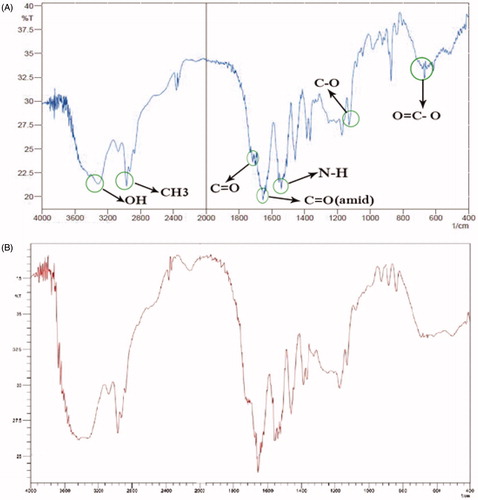
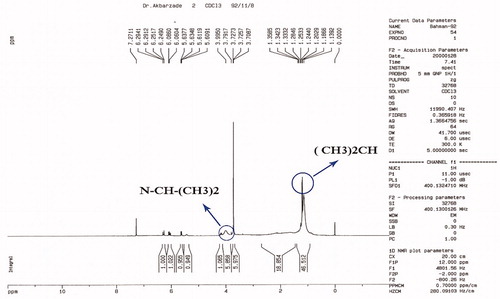
represents the NMR spectrum of the nanogel. The terminal isopropyl methyl protons show a high peak at 1.1 ppm. The peak at 3.8 ppm represents isopropyl methylene protons. The MAA protons carboxylic group is shown in a weak peak at 12 ppm. Nanogel backbone of methylene protons was shown at 1.3–2.2 ppm. The amide N–H group’s peak was also shown at 8.1–8.2 ppm. These NMR and FTIR results revealed a comparable chemical structure as expected. The NMR peak displayed the high purity of the final product.
Morphological study
shows the SEM images of the Fe3O4 (), nanogel (Fe3O4-NIPAAM-MAA copolymers) () and the curcumin-loaded nanocomposite ().
Figure 4. SEM image of (A) Fe3O4 (B) NIPAAM-MAA drug-loaded magnetic hydrogel nanocomposite, and (C) NIPAAM-MAA-Fe3O4 nanogel.
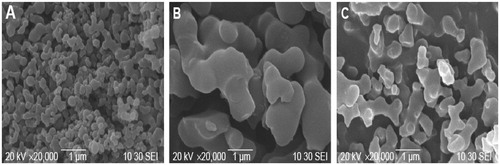
SEM results reveal that Fe3O4 nanoparticles () were aggregated. Fe3O4 nanoparticles without surface modification created particles aggregation and formation of large clusters, leading to increase in particle size. For removing these clusters Fe3O4 nanoparticles were modified by NIPAAM-MAA polymers. The prepared Fe3O4-NIPAAM-MAA nanoparticles were uniform spherical nanoparticles with a mean diameter of approximately 18–23 nm without obvious aggregation (). After loading curcumin in Fe3O4-NIPAAM-MAA nanoparticles, the obvious differences in the particles size and shape were not observed ().
As it has been shown, the nanocomposite size is slightly greater than that of the nanogel. This could be related to the fact that some of the MNPs are absorbed on the surface of the nanogel and the interaction between their surface charges acts as a signal for assembling the nanocomposite, and its increase in size. Micrograph of the nanocomposite depicts that the particles are nearly mono dispersed. These characteristics are crucial for MNPs application in hyperthermia, to generate more heat equivalent to the applied magnetic field.
Entrapment efficiency (EE) and drug loading (DL)
The curcumin content in the curcumin-NIPAAM-MAA copolymer was calculated using the ultraviolet spectrophotometer. The curcumin-loading content and drug entrapment efficiency were calculated based on the EquationEquations (1)(1)
(1) and Equation(2)
(2)
(2) , respectively. The drug loading for curcumin was 21%. The entrapment efficiency for curcumin was 91%.
DAPI staining
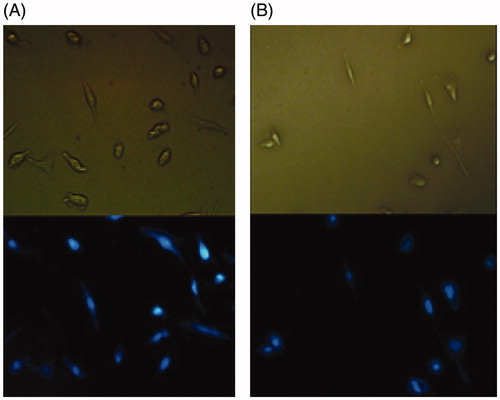
Chromatin morphology and its density are well-known criteria for the determination of healthy, apoptotic, and necrotic cells. In this study, inductions of apoptosis were investigated by DAPI staining using fluorescent microscopy. Representative microscopic images of the cells following a 72 h exposure to media (negative control), the curcumin and curcumin-NIPAAM-MAA sample and NIPAAM-MAA are shown in . Our results showed that all samples (untreated cells, the cells treated with polymer, free curcumin, and curcumin-NIPAAM-MAA) did not show significant chromatin degradation.
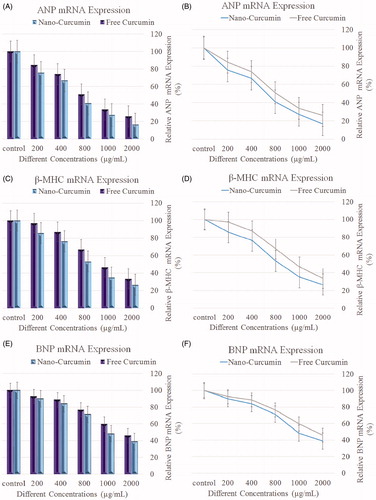
Decrease in the expression of ANP, BNP, and β-MHC mRNA by nano-curcumin
The upregulation of BNP, ANP, and β-MHC are often used as molecular markers for cardiac hypertrophy (Khosravi et al. Citation2011). ANP, β-MHC, and BNP mRNA levels were measured via real-time PCR. The level of ANP, β-MHC, and BNP mRNAs were normalized against the uniformly expressed housekeeping gene, GAPDH, within each sample, and each procedure was repeated three times for each samples. The H9c2 cells were co-incubated with four different concentrations of nardosinone (200, 400, 800, 1000, and 2000 μg/mL). Calculation of 2-ΔΔCT values demonstrated an increasing amount of 2-ΔΔCT, and mRNA expression decreases. demonstrated that with increasing concentration of NIPAAm curcumin, a decreasing trend in the mRNA levels of β-MHC, ANP, and BNP was shown.
Biochemical markers of oxidative stress
To study the effect of DOX-induced oxidative stress in the rat heart, we analyzed the MDA levels, GPX, and SOD activities. depicted that the myocardial MDA level in rats that was administered with DOX and then treated with curcumin showed significant increase (96.70 ± 1.66 nM/g tissue), while SOD and GPX activities were significantly decreased (39.43 ± 3.23 and 8.76 ± 0.87 U/g) compared with the curcumin-loaded magnetic hydrogel nanocomposite (31.25 ± 1.95 nM/g tissue) and (80.89 ± 4.52 and 19.99 ± 1.66 U/g), respectively (P < 0.05). The treatment group showed an amelioration in the parameters analyzed (P < 0.05). These results indicated that administration of curcumin-loaded magnetic hydrogel nanocomposite blocked DOX-mediated lipid peroxidation in rat heart tissue as a result of their antioxidant potential.
Table 3. Effect of curcumin-loaded magnetic hydrogel nanocomposite on oxidative stress parameters; MDA concentration, SOD, and GPX, activities.
Discussion
Magnetic nanoparticles are a unique type of nanomaterials which are routinely applied in the field of biomedical technology. They are currently designed for the detection of disease and treatment, specifically as drug carriers in drug-targeted delivery systems (Adabi et al. Citation2016, Mou et al. Citation2015). Due to their superparamagnetic properties and biocompatibility, MNPs as a next-generation drug-delivery vehicle have great attraction. Although the possible benefits of MNPs are considerable, any possible toxicity related to these MNPs should be identified differently. The biomedical characteristics and drug-loading capacity of MNPs obtained as a result of different surface coatings are the most sensitive parameters in toxicity. A lot of organic and inorganic materials are employed as coating materials for reducing toxicity and surface functionalization of MNPs. Temperature or pH sensitive materials are widely employed to monitor drug loading and targeted release. Furthermore, MNPs can be directed and controlled to a chosen pathological region via external magnetic files (EMF). The advent of targeted drug delivery has reduced the dosage and increased the efficiency of drugs, resulting in reduced side effects to normal tissues (Mou et al. Citation2015). In our study, we loaded curcumin into magnetic hydrogel nanocomposite, for the treatment of heart problem. Result indicated that when curcumin-loaded nanoparticles concentration is increased, the heart failure markers decreases, in addition, curcumin-loaded nanoparticles has been shown to give a better results as compared to curcumin alone.
To check the cardioprotective effect of nanocurcumin, H9c2 cell lines were treated with curcumin and the encapsulated curcumin was then stained with DAPI stain. Observation under fluorescent microscope shows that cardiac toxicity treatment with curcumin nanocomposite stained with 4,6-diamidino-2-phenyl indole dihydro chloride (DAPI) had orange-red fluorescent color which gave bright green fluorescent color in relation to curcumin-treated group (control), showing the fact that the treatment with curcumin nanocomposite lead to more cardiotoxicity reduction in the cells like nuclear fragmentation as compared with control cells. Curcumin have been implicated in apoptosis induction in various cell lines (Duvoix et al. Citation2005). Curcumin was earlier presented to induce apoptosis in malignant cancer cell lines including leukemic cell lines (Roy et al. Citation2002). Ghosh et al. (Ghosh et al. Citation2009) showed that curcumin possesses remarkable anti-proliferative activity in lymphoblast leukemic cells by DNA damage induction in cancer cells, resulting in the apoptosis of cancer cells.
Three markers for heart failure was studied; ANP, BNP, and β-MHC. In humans, loss of the ANP receptor, GC-A, function has been reported in approximately two different clinical situations. In patients with heart failure or heart hypertrophy, the plasma concentrations of ANP are elevated but the peptide systemic responses are blunted, indicating decreased responsiveness of GC-A (Hirooka et al. Citation1990). However, in a recent study, it was shown that a functional deletion mutation found in the human GC-A gene and decreased activity of receptor are related to ventricular hypertrophy and hypertension (Nakayama et al. Citation2000).
Several researchers investigated the molecular mechanisms regulating cardiac hypertrophy using primary cardiomyocytes as the standard experimental in vitro system, our study proposes the use of Curcumin-loaded magnetic hydrogel nanocomposite as cardioprotective agent against doxorubicin-induced cardiac toxicity. This conclusion is supported by several pieces of evidence. The cardiac hypertrophy markers, ANP, BNP and β-MHC, at the mRNA and protein levels were constitutively expressed.
One of the device of heart failure and cardiac hypertrophy in patients is the elevation in β-MHC/α- MHC, ANP, and BNP expression levels (Barry et al. Citation2008). Hence, their expressions have been used as a good predictor of the heart ventricular dysfunction and decompensated heart failure. The ability to induce cellular hypertrophy by doxorubicin, in our study, is demonstrated by the induction of the cardiac hypertrophy markers β-MHC, ANP, and BNP in a concentration- and time-dependent manner. Our results are supported by previous studies that cardiac hypertrophy is related to the increased level of β-MHC/α- MHC ratio (Izumo et al. Citation1987, Mahdavi et al. Citation1984, Reiser et al. Citation2001). In addition, increased BNP and ANP expression levels are related to ventricular hypertrophy and heart failure induced by isoproterenol (Zordoky et al. Citation2010). In our study, the highest level of decrease in gene expression was observed for ANP, followed by β-MHC and BNP mediated by curcumin-loaded magnetic hydrogel nanocomposite.
Our study showed an increase in the level of cardiac MDA which could be traced to DOX-induced generation of free radicals oxygen species that causes extensive tissue damage, interacting with membrane proteins, lipids, and nucleic acids (Hamza et al. Citation2008). In addition, the activities of the cardiac GPX and SOD were significantly decreased in curcumin group, this is supported by Dbrowska et al.(Dabrowska et al. Citation2008) Two different ways of synthesizing radical oxygen free species by DOX have been illustrated; the first involves the synthesis of a semiquinone free radical that produces superoxide radicals, and the second way yields iron–DOX complex that is capable of reducing oxygen to hydrogen peroxide and some other active species (De Beer et al. Citation2001). Free radicals are capable of attacking highly unsaturated fatty acids in the cell membrane to generate lipid peroxidation and as a result damage cell membranes (Choi et al. Citation2008, Ghafarzadeh et al. Citation2016; Namdari and Eatemadi Citation2016).
Curcumin-conjugated nanoparticles has been shown to be cardioprotective, this may be traced to its antioxidant properties. Our study proposed that curcumin-conjugated nanoparticles may be considered as a potentially useful candidate to limit and mop-up free radical-mediated organ injury.
Acknowledgements
The authors thank Department of Medical Biotechnology, School of advance Science in Medicine, Tehran University of Medical Sciences and Department of Cardiology, Lorestan University of Medical Sciences, Khoramabad, Iran.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- Abrams RM, Gerhardt KJ. 2000. The acoustic environment and physiological responses of the fetus. J Perinatol. 20:S31–S36.
- Adabi M, Naghibzadeh M, Adabi M, et al. 2016. Biocompatibility and nanostructured materials: applications in nanomedicine. Artif Cells Nanomedicine Biotechnol. 1401:1–10.
- Anand-Srivastava MB, Trachte GJ. 1993. Atrial natriuretic factor receptors and signal transduction mechanisms. Pharmacol Rev. 2:455–497.
- Baharvand B, Dehaj ME, Rasoulian B, et al. 2009. Delayed anti-arrhythmic effect of nitroglycerin in anesthetized rats: involvement of CGRP, PKC and mK ATP channels. Int J Cardiol. 135:187–192.
- Barry SP, Davidson SM, Townsend P. a. 2008. Molecular regulation of cardiac hypertrophy. Int J Biochem Cell Biol. 40:2023–2039.
- Berger J, Reist M, Mayer JM, et al. 2004. Structure and interactions in covalently and ionically crosslinked chitosan hydrogels for biomedical applications. Eur J Pharm Biopharm. 19–34.
- Calderone A, Thaik CM, Takahashi N, et al. 1998. Nitric oxide, atrial natriuretic peptide, and cyclic GMP inhibit the growth-promoting effects of norepinephrine in cardiac myocytes and fibroblasts. J Clin Invest. 101:812–818.
- Choi EH, Lee N, Kim HJ, et al. 2008. Schisandra fructus extract ameliorates doxorubicin-induce cytotoxicity in cardiomyocytes: altered gene expression for detoxification enzymes. Genes Nutr. 2:337–345.
- Dabrowska K, Stuss M, Gromadzińska J, et al. 2008. The effects of melatonin on glutathione peroxidase activity in serum and erythrocytes after adriamycin in normal and pinealectomised rats. Endokrynol Pol. 59:200–206.
- Daraee H, Eatemadi A, Abbasi E, et al. 2014. Application of gold nanoparticles in biomedical and drug delivery. Artif Cells Nanomedicine Biotechnol. 1401:1–13. [Internet]. Available from: http://www.ncbi.nlm.nih.gov/pubmed/25229833.
- Daraee H, Etemadi A, Kouhi M, et al. 2014. Application of liposomes in medicine and drug delivery. Artif Cells Nanomedicine Biotechnol. 44:1–11.
- De Beer EL, Bottone AE, Voest EE. 2001. Doxorubicin and mechanical performance of cardiac trabeculae after acute and chronic treatment: a review. Eur J Pharmacol. 415:1–11.
- Dessì-Fulgheri P, Sarzani R, Tamburrini P, et al. 1997. Plasma atrial natriuretic peptide and natriuretic peptide receptor gene expression in adipose tissue of normotensive and hypertensive obese patients. J Hypertens. 15:1695–1699.
- Duvoix A, Blasius R, Delhalle S, et al. 2005. Chemopreventive and therapeutic effects of curcumin. Cancer Lett. 223:181–190.
- Eatemadi A, Daraee H, Karimkhanloo H, et al. 2014. Carbon nanotubes: properties, synthesis, purification, and medical applications. Nanoscale Res Lett. 9:1–13.
- Eatemadi A, Daraee H, Zarghami N, et al. 2014. Nanofiber: synthesis and biomedical applications. Artif Cells Nanomedicine Biotechnol. 44:1–11.
- Ghafarzadeh M, Namdari M, Eatemadi A. 2016. Stem cell therapies for congenital heart disease. Biomed. Pharmacother. 84:1163–1171. [Internet]. Available from: http://www.sciencedirect.com/science/article/pii/S0753332216317942.
- Ghosh AK, Kay NE, Secreto CR, et al. 2009. Curcumin inhibits prosurvival pathways in chronic lymphocytic leukemia B cells and may overcome their stromal protection in combination with EGCG. Clin Cancer Res. 15:1250–1258.
- Hamza A, Amin A, Daoud S. 2008. The protective effect of a purified extract of Withania somnifera against doxorubicin-induced cardiac toxicity in rats. Cell Biol Toxicol. 24:63–73.
- Hirooka Y, Takeshita A, Imaizumi T, et al. 1990. Attenuated forearm vasodilative response to intra-arterial atrial natriuretic peptide in patients with heart failure. Circulation. 82:147–153.
- Izumo S, Lompré AM, Matsuoka R, et al. 1987. Myosin heavy chain messenger RNA and protein isoform transitions during cardiac hypertrophy. Interaction between hemodynamic and thyroid hormone-induced signals. J Clin Invest. 79:970–977.
- Khosravi AR, Pourmoqhadas M, Ostovan M, et al. 2011. The impact of generic form of clopidogrel on cardiovascular events in patients with coronary artery stent: results of the OPCES study. J Res Med Sci. 16.
- Khosravi AR, Raoufi A, Pourmoghadas M, et al. 2013. Late clinical events of drug eluting versus bare metal stenting; OPCES’ ancillary study. Pakistan J Med Sci. 29:258–263.
- Knowles JW, Esposito G, Mao L, et al. 2001. Pressure-independent enhancement of cardiac hypertrophy in natriuretic peptide receptor A-deficient mice. J Clin Invest. 107:975–984.
- Lemke K. a, Dawson SD. 2000. Local and regional anesthesia. Vet Clin North Am Small Anim Pract. 30:839–857.
- Lopez MJ, Wong SK, Kishimoto I, et al. 1995. Salt-resistant hypertension in mice lacking the guanylyl cyclase – a receptor for atrial natriuretic peptide. Nature. 378:65–68.
- Mahdavi V, Lompre AM, Chambers AP, et al. 1984. Cardiac myosin heavy chain isozymic transitions during development and under pathological conditions are regulated at the level of mRNA availability. Eur Heart J. 5:181–191.
- Marklund S, Marklund G. 1974. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 47:469–474.
- Mohamad RH, El-Bastawesy AM, Zekry ZK, et al. 2009. The role of Curcuma longa against doxorubicin (adriamycin)-induced toxicity in rats. J Med Food. 12:394–402.
- Mou X, Ali Z, Li S, et al. 2015. Applications of magnetic nanoparticles in targeted drug delivery system. J Nanosci Nanotechnol. 15:54–62.
- Nakayama T, Soma M, Takahashi Y, et al. 2000. Functional deletion mutation of the 5’-flanking region of type A human natriuretic peptide receptor gene and its association with essential hypertension and left ventricular hypertrophy in the Japanese. Circ Res. 86:841–845.
- Namdari M, Eatemadi A. 2016. Nanofibrous bioengineered heart valve – application in paediatric medicine. Biomed Pharmacother. 84:1179–1188. [Internet]. Available from: http://www.sciencedirect.com/science/article/pii/S0753332216318522.
- Oliver PM, Fox JE, Kim R, et al. 1997. Hypertension, cardiac hypertrophy, and sudden death in mice lacking natriuretic peptide receptor A. Proc Natl Acad Sci USA.94:14730–14735.
- Preuss HG, Jarrell ST, Scheckenbach R, et al. 1998. Comparative effects of chromium, vanadium and gymnema sylvestre on sugar-induced blood pressure elevations in SHR. J Am Coll Nutr. 17:116–123.
- Reiser PJ, Portman M. a, Ning XH, et al. 2001. Human cardiac myosin heavy chain isoforms in fetal and failing adult atria and ventricles. Am J Physiol Heart Circ Physiol. 280:H1814–H1820.
- Roy M, Chakraborty S, Siddiqi M, et al. 2002. Induction of apoptosis in tumor cells by natural phenolic compounds. Asian Pac J Cancer Prev. 3:61–67.
- Sanphui P, Goud NR, Khandavilli UBR, et al. 2011. New polymorphs of curcumin. Chem. Commun (Camb.). 47:5013
- Seidi K, Eatemadi A, Mansoori B, et al. 2014. Nanomagnet-based detoxifying machine: an alternative/complementary approach in HIV therapy. J AIDS Clin Res. 25:304.
- Srivastava G, Mehta JL. 2009. Currying the heart: curcumin and cardioprotection. J Cardiovasc Pharmacol Ther. 14:22–27.
- Swamy AV, Gulliaya S, Thippeswamy A, et al. 2012. Cardioprotective effect of curcumin against doxorubicin-induced myocardial toxicity in albino rats. Indian J Pharmacol. 44:73–77.
- Toolabi T, Vanaki Z, Memarian R, et al. 2012. Quality of nursing documentations in CCU by hospital information system (HIS). J Crit Care Nurs. 5:53–62.
- Zhang JH, Xu M. 2000. DNA fragmentation in apoptosis. Cell Res. 10:205–211.
- Zhou S, Palmeira CM, Wallace KB. 2001. Doxorubicin-induced persistent oxidative stress to cardiac myocytes. Toxicol Lett. 121:151–157.
- Zordoky BNM, Anwar-Mohamed A, Aboutabl ME, et al. 2010. Acute doxorubicin cardiotoxicity alters cardiac cytochrome P450 expression and arachidonic acid metabolism in rats. Toxicol Appl Pharmacol. 242:38–46.