?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
We proposed an effective and eco-friendly control of dengue, malaria, and filariasis-causing vectors. We tested Ipomoea batatas leaves-mediated silver nanoparticles (AgNPs) against first to fourth instar larvae and adults of Aedes albopictus, Anopheles stephensi, and Culex quinquefasciatus at different concentrations. The synthesized AgNPs showed broad spectrum of larvicidal and adulticidal effects after 48 h of exposure. The characterization of synthesized AgNPs was done using various spectral and microscopy analyses. The maximum efficacy was observed in synthesized AgNPs against the adult of Ae. albopictus with the LC50 and LC90 values were 10.069 and 15.657 μg/mL, respectively, followed by others.
Introduction
Mosquitoes act as a vector for most of the life-threatening diseases like malaria, dengue, chikungunya, filariasis, encephalitis, West Nile virus infection, etc., in almost all tropical and subtropical countries and many parts of the world (Kager Citation2002). Mosquitoes are cause allergic responses in human that include local skin and systemic reactions (Peng et al. Citation1999). Mosquitoes transmit several human diseases, causing millions of deaths every year. India reports 1.4 million malarial cases and about 173 deaths; 1.4 million suspected and 11,985 confirmed chikungunya cases; 5000 Japanese encephalitis cases and approximately 1000 deaths; and 383 dengue cases and 6 deaths during 2006 and 2007 (Kovendan et al. Citation2012; Pavela and Benelli Citation2016). Mosquito-borne diseases are prevalent in more than 100 countries across the world and infecting over 70 crore peoples every year globally and 4 crore of the Indian populations.
Aedes albopictus is a small, dark mosquito with a white dorsal stripe and banded legs. They are strongly attracted to bite humans, cats, dogs, squirrels, deer, and other mammals, as well as birds. They will bite any exposed skin surface. Ae. albopictus is a very aggressive daytime biter (Benelli et al. Citation2015a; Marcondes and Ximenes Citation2015). Its peak feeding times are during the early morning and late afternoon, and its considered to be a vector of dengue fever, a disease endemic to Southeast Asia, Africa, and America (Benelli et al. Citation2015c; Maillard et al. Citation1993; Vasilakis et al. Citation2007). More than 50 million peoples are at risk of dengue virus exposure worldwide. Annually, there are 5 million cases of dengue, 2 million infections, and 12,000 deaths (Kovendan et al. Citation2012). Dengue is the most significant mosquito-spread viral disease and a major international public health concern. It is a self-limiting disease found in tropical and subtropical regions around the world, predominantly in urban and semi-urban areas. DF or DHF is caused by dengue virus which belongs to genus Flavivirus, family Flaviviridae, and includes serotypes 1, 2, 3, and 4 (Den-1, Den-2, Den-3, and Den-4) (Benelli Citation2016b; Benelli and Mehlhorn Citation2016c; WHO Citation2010). The WHO estimates that around 2.5 billion people are at risk of dengue. Infections have dramatically increased in recent decades due to increased urbanization, trade, and travel. No effective drug or vaccine is available so far. The only solution is to prevent the disease carrying mosquito from breeding and biting to humans.
Anopheles species are the most important species as they are a capable vector for malaria parasites. Anopheles stephensi is responsible for the transmission of malaria in urban regions of India (Benelli and Mehlhorn Citation2016c; Rahman et al. Citation1989). In India, malaria is still the most important cause of morbidity and mortality with approximately 2–3 million new cases rising every year (Sharma et al. Citation2009). It begins with a bite from an infected female Anopheles mosquito, which introduces the protists through saliva into the circulatory system. Malaria causes symptoms that typically include fever and headache, which in severe cases can progress to coma or death. About 3.3 billion people – half of the world’s population – are at risk of malaria. In 2010, about 216 million malaria cases (with an uncertainty range of 149–274 million) and estimated 655,000 malaria deaths (with an uncertainty range of 537,000–907,000 occurred in World Wide). Increased prevention and control measures have led to a reduction in malaria mortality rates by more than 25% globally since 2000 and by 33% in the WHO African Region (WHO Citation2012b). Culex quinquefasciatus, a vector of lymphatic filariasis, is widely distributed in tropical Zones with around 120 million people infected worldwide and 44 million people having common chronic manifestation (Benelli et al. Citation2015a; Bernhard et al. Citation2003). Culex mosquitoes are painful and persistent biters and are responsible for filariasis (Conti et al. Citation2010). Lymphatic filariasis is a neglected tropical disease. More than 1.3 billion people in 72 countries worldwide are threatened by lymphatic filariasis, commonly known as elephantiasis. Over 120 million people are currently infected, with about 40 million disfigured and incapacitated by the disease (WHO Citation2012a, Citation2012b). It is a potential vector of bancroftian filariasis and fed on microfilaremia carriers harboured Wuchereria bancrofti larvae, found in waste water disposal systems, and irrigated sites (Mukhtar et al. Citation2003).
Mosquito larvicide, nowadays, includes an organophosphate temephos, methoprene, phytochemicals, soil bacterium, and fungal species. However, the high amount of synthetic chemical larvicides could lead to long-term residual effect to the environment and chronic effects on non-target organisms. Mosquito control is being strengthened in many areas, yet there are significant challenges, including increased mosquito resistance to insecticides and a lack of alternate, cost-effective, and safe insecticides. Nanoparticles play an important role in pharmaceutical, industrial, and biotechnological applications like a drug delivery, diagnostics, image sensing, artificial implants, and tissue engineering (Mishra et al. Citation2013). Nowadays metallic nanoparticles are mostly prepared from noble metals viz., platinum (Pt), gold (Au), silver (Ag), Zinc (Zn), Copper (Cu), and lead (Pb); among those, Ag is the metal of choice in the field of biological system (Kunjiappan et al. Citation2014). Plant constituents such as phenols, flavonoids, terpenoids, tannins, glycosides, alkaloids, proteins, and steroids are capable of reducing metal ions into nanoparticles in a single-step green synthesis process and ensures excellent control over size distribution and crystallinity of the nanoparticles (Velu et al. Citation2015).
Green-synthesized silver nanoparticles (AgNPs) have been recently proposed as newer and safer control tools against mosquito vector of medical and veterinary importance (Benelli Citation2016a; Murugan et al. Citation2015a). The synthesized AgNPs using aerial extract of Ammannia baccifera (Suman et al. Citation2013) showed significant toxic effects against the larvae of An. subpictus and Ae .aegypti. Many plant species have been used in nanotechnology for AgNPs production, which were stable and have significant mosquito larvicidal activity against Cx. quinquefasciatus viz: Mimosa pudica (Marimuthu et al. Citation2010), Tinospora cordifolia (Jayaseelan et al. Citation2011), Eclipta prostrata (Rajakumar and Rahuman Citation2011), Nelumbo nucifera (Santhoshkumar et al. Citation2011), Sida acuta (Veerakumar et al. Citation2013), Heliotropium indicum (Veerakumar et al. Citation2014b), and Cinnamomum zeylanicum (Soni and Prakash Citation2014), respectively.
Ipomoea batatas commonly known as “Sweet potato” is a perennial dicotyledonous plant belonging to the family Convolvulaceae. It is large, starchy sweet-tasting tuberous root as vegetable (FAO Citation1992). Sweet potato is an important food crop in tropical and sub-tropical countries (Tewe et al. Citation2003). I. batatas is an herbaceous perennial vine bearing, simple, entire, and alternate heart-shaped or palmately lobed leaves (Gad and George Citation2009), and it contains protein (0.46% to 2.93%), dietary fiber (0.49% to 4.71%), lipid (0.06% to 0.48%), and ash (0.31% to 1.06%) and essential minerals nutrients such as Ca, P, Mg, Na, K, S, Fe, Cu, Zn, Mn, Al and vitamin A, thiamine, riboflavin, niacin, ascorbic acid, and many other functional compounds (Woolfe Citation1992). The plant tops used in the manufacture of alcohol, vinegar, lactic acid, yeast, acetone, and young leaves used as baby food particularly in Southeast Asia and East Asia (Ma et al. Citation2003). The leaves contain cyanide, tannins, oxalate, and phytic acid as antinutrients and a couple of minerals (Igwenyi et al. Citation2011). Biological activity of I. batatas reported as anti-mutagenic, anti-diabetic (Ijaola et al. Citation2014), anti-bacterial, anti-fungal (Kumar et al. Citation2014), anti-inflammatory, anti-oxidant (Hamsa and Kuttan, Citation2011), cardiovascular (Yamakawa et al. Citation2007), hypertension (Nagamine et al. Citation2014), hyperglycemia and diabetes (Yoshimoto et al. Citation2002), wound healing (Panda and Sonkamble Citation2012), cytotoxic (Lin et al. Citation2008; Yu et al. Citation2013), anti-tumor, Ca2 + antagonist (Pàska et al. Citation1999), antiulcer (Hermes et al. Citation2013), cardiovascular (Jabeen and Aslam Citation2013), hepatoprotective, immunomodulatory, and anticancer activities (Amin et al. Citation2012), respectively. Considering the above information in mind, the present study AgNPs were synthesized using the I. batatas (Convolvulaceae) leaf extract as reducing and stabilizing agent. The AgNPs were characterized by UV–visible spectroscopy, Fourier transform infrared spectroscopy (FTIR), Scanning electron microscopy (SEM), Transmission electron microscopy (TEM), Energy dispersive X-ray spectroscopy (EDX), and X-ray diffraction (XRD). The I. batatas leaf aqueous-mediated AgNPs were tested for its larvicidal and adulticidal potential against the An. stephensi (malaria vector), Ae. albopictus (dengue vector), and Cx. quinquefasciatus (Japanese encephalitis vector).
Materials and methods
Collection of plant material
Fresh and healthy leaves of Ipomoea batatas (Convolvulaceae) () were collected from Vettrilaikaranur kattuvalavu, (Latitude 11°00′N, Longitude 78°00′E) Salem, and the taxonomic identification was done by Dr. D. Natarajan, Assistant Professor, Department of Biotechnology, School of Biosciences, Periyar University, Salem. The voucher specimen was numbered and kept in NDRL research laboratory for further reference. Silver nitrate was purchased from Hi-Media chemicals Pvt. Ltd, Mumbai, India.
Preparation of aqueous plant extract
The fresh and healthy plant leaves were washed thoroughly in tap water rinsed in distilled water, dried with paper towel, and shade-dried at room temperature (35 ± 1 °C). These dried leaves were powdered mechanically using electrical mixer. Aqueous extract was prepared by mixing 10 g of dried powder in 100 mL of double-distilled water. This suspension was mixed well and left for 5 h without disturbance, then filtered through Whattman filter paper (No. 1), and the filtrate was used to check the larvicidal and adulticidal activity against the target mosquito vectors (Minjas and Sarda Citation1986).
Collection of mosquitoes
Different larval instars and pupa of Ae. albopictus, An. stephensi, and Cx. quiquefasciatus were collected from the Centre for Research in Medical Entomology (CRME), Madurai, and were brought to the laboratory without any disturbances. These larvae and pupae were maintained in enamel trays containing deionized water and allowed to feed on brewer’s yeast, and dog biscuits (3:1 ratio) in the laboratory at room temperature for 24 h; before starting of the experiment, the laboratory mosquitoes were maintained according to the modified method of Kamaraj et al. (Citation2009). All the experiments were carried out at 27 ± 2 °C and 75–85% relative humidity under 14:10 light and dark cycles. Pupae were transferred from the trays to a plastic cup (10 × 5 × 3 cm) containing tap water and were maintained in an insectary tray (45 × 45 × 40 cm) where adults emerged. Adults were maintained in plastic cages and were provided with 10% sucrose solution in a jar with a cotton wick. Glass petridish with 50 mL of tap water lined with filter paper was kept inside the cage for oviposition (Lalrotluanga et al. Citation2012; Rahuman et al. Citation2007).
Synthesis of silver nanoparticles from leaves extract
Aqueous leaves extract of I. batatas was prepared by placing 10 g of chopped fresh leaves in a 250-mL Erlenmeyer flask and boiled with 100 mL of sterile double-distilled water upto 20 min at 60 °C in a water bath. The aqueous extract was passed through Whatman filter paper (No.1), and the filtrate (aqueous leaf extract) was stored at 4 °C and used within 3 days. About 10 mL of aqueous leaf extract was treated with 90 mL of prepared 1 mM of Ag NO3 solution incubated in dark at room temperature. The aqueous solution of 1 mM of Ag NO3 was greatly reduced from Ag+ to Ag° by aqueous leaf extract leading to change of pale yellow to dark brown indicate the synthesis (Parashar et al. Citation2009).
Characterizations of green synthesized silver nanoparticles
UV-visible spectroscopy analysis
The bio-reduction of silver ions to silver was monitored by measuring the UV-Visible spectrum of the reaction mixture (silver nitrate + aqueous leaf extract). The samples were diluted with 2 mL of double-distilled water and subsequently analyzed by UV– vis spectroscopy at regular time intervals using a quartz cuvette with water as a reference (Rajesh et al. Citation2009), and surface plasmon resonance (SPR) of AgNPs was characterized using UV–vis spectrophotometer (Shimadzu 1601 model, Japan) at resolution of 1 nm between 200 and 800 nm (Dash et al. Citation2014).
X-ray diffraction analysis
To determine the nature and size of the synthesized AgNPs, X-ray diffraction (XRD) was performed. For this, the reaction mixture was centrifuged (20 000 × g, 15 min, 4 °C), and the pellet was dissolved in deionized sterile water and washed thrice in the same by centrifugation. The powder form of the sample was recovered and coated on the XRD grid; the spectra were recorded at a voltage of 40 kV and a current of 30 mA with Cu Kα1 radiation using Philips PW 1050/37 model. The diffracted intensities were recorded from 10° to 90° at 2θ angles. The full-width at half-maximum (FWHM) from three different peaks were used in Scherrer’s equation to determine the average crystallite size of the nanoparticles (Rao and Biswas Citation2009).
Fourier transform infrared spectroscopy
Dried metallic powder of the AgNPs was subjected to analyze the presence of possible biofunctional groups for the reduction of Ag + ions resulting in formation of AgNPs. The solid residue containing AgNP solution was dispersed in sterile deionized water for three times to remove the unattached biological impurities. The pure residue was oven dried in overnight at 60 °C. The obtained powder was subjected to FTIR measurement using a PerkinElmer Spectrum one instrumental resolution of 4 cm−1 in KBr pellets (Vivek et al. Citation2011).
Scanning electron microscope
The particle size and microstructure were deliberate by high resolution SEM (instrument from Nikon, Japan) (Chattopadhyay et al. Citation2013a). In brief, AgNPs were suspended in deionized water at a concentration of 1 mg/mL and then sonicated using a sonicator bath until the sample form a homogenous suspension. For size measurement, the sonicated stock solution of AgNPs (1 mg/mL) was diluted 20 times. Then, one drop of sonicated aqueous solution was taken on a glass plate and allowed to dry overnight at room temperature under a sterilized condition. Then, the sample was gold coated and images were taken. SEM was used to characterize the size and shape of AgNPs from samples.
Transmission electron microscope
The size and structure of synthesized AgNPs were characterized by TEM in a JEOL 3010, Japan, operating at 200 kV according to the modified method of Chattopadhyay et al. (Citation2012). In brief, AgNPs were suspended in deionized water at a concentration of 1 mg/mL. Then, the sample was sonicated using a sonicator bath until sample form a homogenous suspension. For size measurement, sonicated stock solution of all AgNPs (0.5 mg/mL) was diluted for 10 times. TEM was used to characterize the size and shape of the AgNPs. A drop of the aqueous AgNPs suspension to make a thin layer on to carbon-coated copper grid (300 mesh size) by slow evaporation and then allowed to dry in vacuum at 25 °C overnight to obtain TEM images.
Energy dispersive X-ray spectroscopy
This technique determines the elemental composition of a sample. In this study, EDX used to confirm the presence of silver in the particles as well as to detect the other elemental compositions of the synthesized silver particles. The particle solution was diluted 100-fold in water, and a drop of 10-μL diluted solution was placed on a carbon stub and air-dried. The EDX spectrum was obtained at an acceleration voltage of 20 kV and collected for 19 s. Mapping was completed using pseudo-colors to represent the two-dimensional spatial distribution of energy emissions of the chemical elements present in the sample. This analysis was done using JEOL JSM 6360 equipped with an EDX (energy dispersive X-ray) analyzer (Majumdar et al. Citation2013).
Larvicidal activity
The larvicidal activity of green synthesized AgNPs was evaluated using WHO protocol (Citation2005) with slight modifications. To test different concentrations of synthesized AgNPs in 200 mL capacity autoclaved Borosil glass beakers, bioefficacy test was conducted against the larvae of target vector at five different concentrations of synthesized AgNPs (25, 50, 75, 100, and 125 μg/mL). To find out the larvicidal activity of synthesized AgNPs, 20 larvae were exposed to each test at different concentrations of extracts. Similarly, each test included a set of control group (Distilled water) with five replicates for each individual concentration of extract. Mortality rate was recorded after 48 h of exposure period. The dead larvae in five replicates were combined and expressed as a percentage of larval mortality for each concentration. The corrected mortality was calculated by Abbott’s formula (Abbott Citation1925):
Dose-response bioassay
Synthesized AgNPs were subjected to a dose-response bioassay for larvicidal activity against Ae. albopictus, An. stephensi, and Cx. quinquefaciatus. Different concentrations (25, 50, 75, 100, and 125 μg/mL) of synthesized AgNPs were prepared for larvicidal activity. The numbers of dead larvae were counted after 48 h of exposure, and the percent mortality was calculated from the average of five replicates. However, at the end of 48 h, the selected test sample turned out to be equal in their toxic potential; this assay was performed as per the modified method of Selvaraj Mohana Roopan et al. (Citation2013).
Adulticidal activity
Adulticidal bioassay of samples was performed by the modified WHO protocol (Citation1981). Based on the wide range and narrow range tests, and AgNPs were tested at 100, 200, 300, 400, 500, and 600 μg/mL concentrations (Veerakumar and Govindarajan Citation2014a). AgNPs were applied on Whatmann no. 1 filter paper (size 12 × 15 cm). Control papers were treated with silver nitrate and distilled water. Twenty mosquitoes were collected and gently transferred into a glass holding tube. The mosquitoes were allowed to acclimatize in the holding tube for1 h, and then exposed to test paper for 1 h. At the end of exposure period, the mosquitoes were transferred back to the holding tube and kept for 24-h recovery period. A pad of cotton soaked with 10% glucose solution was placed on the mesh screen. Each test includes a set control groups (distilled water) with five replicates for each individual concentration. The lethal concentrations [lethal dose (LD50 and LD90)] were calculated by probit analysis (Finney Citation1971).
Statistical analysis
The average larvae and adult mortality data were subjected to probit analysis for calculating LD50, LD90, and other statistics at 95% confidence limits of upper and lower confidence limits, and chi-square values and data were calculated using the Statistical Package of Social Sciences IBM 20.0 software (SPSS Inc., Chicago, IL). Results with P < .05 were considered to be statistically significant.
Results
Synthesis and characterizations of I. Batatas-mediated AgNPs
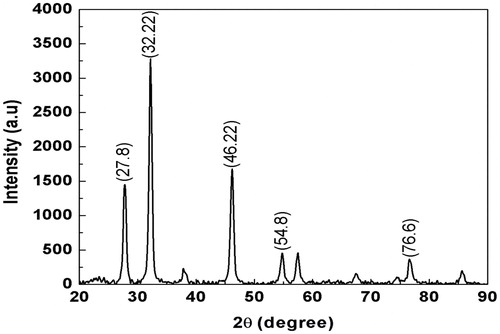
The color change was noted by visual observation in the I. batatas leaf aqueous extract when incubated with AgNO3 solution. I. batatas leaf extract without added AgNO3 did not show any change in color. The aqueous extract mixed with silver nitrate changed to light brown within an hour, and later, it was changed into dark brown during 24-h incubation period, after which no significant changes occurred (). The absorption spectrum of I. batatas leaf extract at different wave lengths ranging from 200 to 800 nm revealed a peak at 410 nm (). XRD spectrum of I. batatas leaf extract reduced AgNPs and revealed the five distinct diffraction peaks observed in the spectra at 2θ values of 27.8°, 32.22°, 46.22°, 54.8°, and 76.6° attributed to (111), (200), (202), (211), (220), and (311) of the cubic silver (). The lattice constant calculated from the diffraction spectrum was a = 4100 Å, and the resultant data were coordinated with the database Joint Committee on Powder Diffraction Standards (JCPDS) file no. 01-087-0717. The resultant XRD spectrum clearly suggests that the AgNPs synthesized from the leaf aqueous extract of I. batatas was crystalline in nature. The average crystal size of AgNPs was calculated by applying Scherrer’s equation and found to be the size of 29 nm.
Figure 2. Formation of AgNPs from I. batatas A: AgNO3 solution, B: AgNO3+ I. batatas leaf extract at 0 h, C: AgNPs from I. batatas at 24 h. (Dark purple coloration indicates the formation of AgNPs).

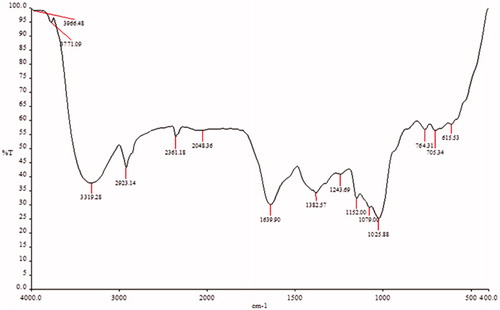
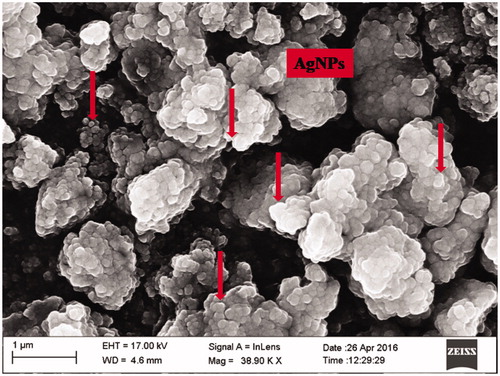
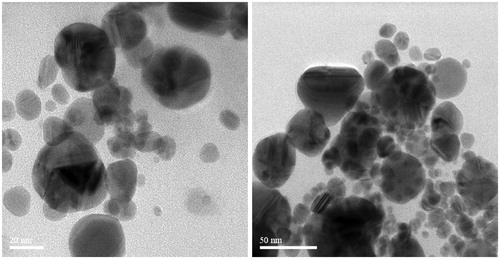
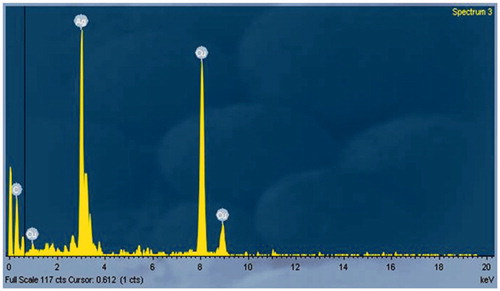
FTIR has become a significant tool in understanding the involvement of functional groups in relation linkage of metal particles and biomolecules. In the current study, FTIR measurements were performed to identify the promising functional group of biomolecules responsible for capping and stabilizing the AgNPs. FTIR spectral peaks suggest the occurrence of bands relevant to alcohols OH stretching (3966.48 cm−1), A weak band appears around 3771.09 cm−1 may be due to the stretching frequency of the O-H bond possibly arising from the protein present in the sample. The weak bands at 3319.28 cm−1 may be assigned due to N-H stretching of the primary amines which possibly arise from the sugars and protein present in the extract. A peak around 2923.14 cm−1 can be assigned to the alkanes C-H stretching. The prominent peak around 2048.36 cm−1 alkynes could be attributed to a peak responsible for reduction of silver nitrate into silver metal. The peaks at 1639.90 cm−1 may correspond to carbonyls correlating to proteins. The peaks at 1243.69 cm−1 can be attributed to the C-N stretching frequency arising from the aliphatic amines. The absorption peak at 1079.00 cm−1 may be due to carboxylic acids strong bonds. The bands at 764.31 cm−1 indicate the presence of N-H stretching primary amine. A peak was observed around 705.34 cm−1 that could be assigned to C-H stretching vibration of alkane (; ). After reduction, the AgNPs were precipitated at the bottom of conical flask. This precipitate was washed out twice with sterile double-distilled water and then analyzed by employing SEM. According to the SEM analysis, the morphology of the AgNPs was observed in spherical shape and aggregate form (). The samples of AgNPs synthesized using I. batatas liquids were prepared by placing a drop of reaction mixture over copper grid and allowing water to evaporate in sterile condition. TEM highlighted the presence of spherical and oval shapes of AgNPs, with 20–50 nm-sized AgNPs were observed (). Energy dispersive x-ray spectroscopy (EDX) results show silver (Ag) signal arise from the Ag nanoparticles, and the atomic percentage of silver is 58.44%. Except for Ag, there are some other peaks observed in EDX. The atomic percentage of Carbon (C), Chlorine (Cl), and Copper (Cu) are 6.79%, 1.63%, and 34.76%, respectively. The carbon (C) signal obtained from the adsorbed components of the leaf extract as well as coating material of the instrument. Except copper, other elements have a very low atomic percentages compared to silver, and suggests the formation of pure AgNPs ().
Table 1. FTIR spectrum of I. batatas leaf extract.
The larvicidal bioassay of I. batatas synthesized AgNPs was observed at various concentrations against the first to fourth-instar larvae of the mosquito vectors Ae. albopictus, An. stephensi, and Cx. quinquefasciatus. Considerable mortality was evident after the treatment of I. batatas synthesized AgNPs for all larval instars. It was observed that Cx. quinquefasciatus is the most susceptible strain followed by An. stephensi and Ae. albopictus, respectively. The mortality rate was increased based on the concentration of extract; for example, in the An. stephensi first-instar stage at 25 μg/mL concentration, the larval mortality was 34.3% whereas at 125 μg/mL concentration, it was increased to 81.0%. The LC50 and LC90 values were represented as follows: LC50 4.03, 4.305, 3.490, 3.16; LC90 6.42, 6.794, 5.84, and 5.192 μg/mL, respectively (). A similar observation has been noted in Ae. albopictus; the mortality rate was totally concentration dependent. In first-instar stage at 25 μg/mL concentration, the larval mortality was 29.0%, whereas at 125 μg/mL concentration, it was increased to 74.0%. Similarly, fourth-instar larvae stage at 25 μg/mL concentration, the larval mortality was 34.6%, while at 125 μg/mL concentration, it was improved to 82.3%. The LC50 and LC90 values were presented as follows: LC50 value of the first instar of Ae. albopictus, i.e. LC50 8.62, 5.95, 6.40, 4.74; LC90 13.52, 9.27, 9.80, 7.46 μg/mL (). In the control group, no mortality could be recorded. The larvae of Cx. quinquefasciatus were found highly susceptible to the synthesized AgNPs. The time-dependant effect shows the maximum mortality (>85%) at 125 μg/mL against fourth-instar larvae of Cx. quinquefasciatus. At higher concentration, mortality starts within the first 18 h of exposure. More than 50% mortality occurs within the first 24 h. The LC50 and LC90 values were represented as follows: Cx. quinquefasciatus LC50 7.55, 8.02, 5.63, 6.31; LC90 11.77, 12.03, 8.60, and 9.04 μg/mL, respectively (). The decreasing concentrations of AgNPs (after 48 h) of the lived larvae of mosquitoes were pupated or emerged as adults before they died. The results of larvicidal activity clearly show that the percentage of mortality being directly proportional to the concentration of the AgNPs. The outcome of study proves that concentration of extract plays a significant role in larvicidal activity.
Table 2. I. batatas AgNPs against different larvae stages of An. stephensi.
Table 3. Larvicidal activity of green synthesized I. batatas AgNPs against different instar larvae of Ae. albopictus.
Table 4. I. batatas AgNPs against different larvae stages of Cx. quinquefasciatus.
The results of adulticidal activity of synthesized AgNPs of I. batatas against the Ae. albopictus, An. stephensi, and Cx. quinquefasciatus adults (shown in ). Among three vectors tested, the highest mortality was observed in followed by Ae. albopictus, An. stephensi, and Cx. quinquefasciatus. At higher concentrations, the adult showed restless movement for some times with irregular wagging and died. The rates of mortality were directly relative to the concentrations. The maximum efficacy was observed in synthesized AgNPs against the adult LC50 and LC90 values were 10.069 and 15.657 μg/mL, Ae. albopictus; 17.578 and 26.040 μg/mL, An. stephensi; 12.568 and 19.510 μg/mL, respectively. No mortality was observed in the control (χ2 value was significant at the P < .05 level).
Table 5. Adulticidal activity of I. batatas aqueous leaf extract synthesized AgNPs against Ae. albopictus, An. stephensi, and Cx. quinquefasciatus.
Discussion
The nanoparticle synthesis results of AgNPs solution with leaf aqueous extract may be easily observed after bioreduction of Ag+ was taken into further AgNPs confirmation using UV–Visible absorption spectrum of I. batatas highlighted changes in color intensity. After 24 h, the UV-spectrum had showed a maximum absorption peak at 410 nm. This result is agreement with earlier work using different plant species, such as Aloe vera (Dinesh et al. Citation2015), Phyllanthus niruri (Suresh et al. Citation2015), Moringa oleifera (Sujitha et al. Citation2015), and Caulerpa scalpelliformis (Murugan et al. Citation2015b). FTIR spectra clearly indicate that the biomolecules particularly proteins present in aqueous leaf extract are responsible for synthesis and stabilization of AgNPs (Dhanasekaran and Thangaraj Citation2013). The functional groups such as alcohol, amines, amides, alkanes, aliphatic, and halides confirmed their presence in AgNPs, and these are the main classes in most of the functional groups. The similar kind of results were obtained from the present study; very strong broad absorption peaks at 3966.48 cm−1 (O–H stretch and sharp); very strong and sharp peaks at 3771.09 cm−1 (phenols) were observed. The FTIR results indicate that the secondary structure of the proteins is not affect as a consequence of reaction with the Ag + ions or binding with the AgNPs. This result suggests that the biological molecules could probably perform a function for the formation and stabilization of AgNPs in an aqueous solution. It is well known that proteins can bind to AgNPs through free amine groups in the proteins (Gole et al. Citation2001) and therefore stabilization of the silver by surface-bound protein (Logeswari et al. Citation2013).
In XRD analysis of plant-mediated synthesized AgNPs showed intense peaks at values of 27.8°, 32.22°, 46.22°, 54.8°, and 76.6°. They indexed the full width half maximum (111), (200), (202), (211), (220), and (311) regarded as an attributive indicator of biosynthesized silver nanocrystallites. The XRD spectrum results of present study agreed with the Bragg’s reflection of silver nanocrystals (Ouda Citation2014), as well as with other XRD report of AgNPs were done by Suresh et al. (Citation2015). Vivek et al. (Citation2011) observed that the SEM analysis of AgNPs synthesized by the help of Gelidiella acerosa extract having an average mean size of the AgNPs and seems to be spherical in morphology. Similarly, the present results show the scanning electron microscopic observation has confirmed the occurrence of spherical shape nanoparticle in the sample. EDX spectroscopy revealed a strong signal in the Ag region, which confirm the presence of elemental silver. Similarly, Amkamwar et al. (Citation2005) reported the strong signal in AgNPs synthesized by Embilica officinalis.
The present results express that the I. batatas AgNPs were effective against the all larvae stages of selected mosquitoes. Highest mortality rate was observed in Cx. quinquefasciatus, followed by An. stephensi and Ae. albopictus. A dose-dependent effect was observed in the entire investigation and this statement was supported by previous reports that were performed by Amer and Mehlhorn (Citation2006), Bagavan et al. (Citation2009), Subramaniam et al. (Citation2015); Murugan et al. (Citation2016) stated that an increasing number of plant-mediated synthesized AgNPs showed larvicidal toxicity against different mosquito vectors; Marimuthu et al. (Citation2010) studied the larvicidal effect of M. pudica aqueous leaf synthesized AgNPs, showing highest mortality for synthesized AgNPs against the larvae of An. subpictus (LC50 8.89, 11.82, and 0.69 ppm) and Cx. quinquefasciatus (LC50 9.51, 13.65, and 1.10 ppm). Srinivasan et al. (Citation2014) reported the larvicidal activity of phyto-synthesized AgNPs of Avicennia marina leaf aqueous extract against Ae. aegypti (LC50 4.374 and LC90 4.928 ppm) and An. stephensi (LC50 7.40 and LC90 9.865 ppm). The larvicidal activity of synthesized AgNPs of Ficus racemosa was evidence against Cx. quinquefasciatus and Cx. gelidus (LC50 67.72 and LC90 63.70 ppm). The better larvicidal activity was observed in AgNPs of Tinospora cordifolia against fourth-instar larvae of An. subpictus and Cx. quinquefasciatus LC50 6.43 ppm. The AgNPs showed (using Eclipta prostrata) highest efficacy of larvicidal activity against the fourth-instar larvae of Cx. quinquefasciatus (LC50 27.49 and LC90 70.38) and An. subpictus (LC50 27.85 and LC90 71.45 ppm) (Velayutham et al. Citation2013). A dose-dependent effect was found as earlier described for other plant-borne compounds (Benelli Citation2015b). The findings of present study was comparable with the report of Muthukumaran et al. (Citation2015) proved the mosquito larvicidal action of AgNPs fabricated using Gmelina asiatica against Cx. quinquefasciatus (LC50 27.83 μg/mL), Ae. aegypti (LC50 25.77 μg/mL), and An. stephensi (LC50 22.44 μg/mL). Similarly, Govindarajan et al. (Citation2016) who find out the toxicity of Hugonia mystax-synthesized AgNPs exhibited larval toxicity against Cx. quinquefasciatus (LC50 17.46 μg/mL), Ae. aegypti (LC50 15.86 μg/mL), and An. stephensi (LC50 14.45 μg/mL).
The mosquitocidal properties of AgNPs may be due to structural deformation of genetic material, digestive tract enzymes, and generation of reactive oxygen species (Patil et al. Citation2012) and non-target aquatic species (Benelli Citation2016a). The extensive larvicidal toxicity against mosquito vectors could be due to the synergistic insecticidal action of AgNPs and capping biomolecules (Rajan et al. Citation2015). It has been hypothesized that the toxicity of AgNPs against targeted vectors possibly will be attributed to the small size of these nanoparticles, which allows passageway through the insect cuticle and into individual cells where they interfere with molting and disturb other biochemical processes (Arjunan et al. Citation2012).
The adults emerged from all the treatments were deformed due to inhibition and disturbance of regular physiological and metabolic processes (Murugan et al. Citation1996). Our results have shown that synthesized AgNPs of I. batatas have significant adulticidal activity against the Cx. quinquefasciatus, Ae. Albopictus, and An. stephensi. The results are comparable with an earlier report by Veerakumar et al. (Citation2014c) who reported that the maximum adultcidal activity observed in Feronia elephantum leaf extract synthesized AgNPs against filariasis, malaria, and dengue vectors with the following lethal dose values of An. stephensi had LD50 and LD90 values of 18.041 and 32.575 μg/mL; Ae. aegypti had LD50 and LD90 values of 20.399 and 37.534 μg/mL; and Cx. quinquefasciatus had LD50 and LD90 values of 21.798 and 39.596 μg/mL, respectively. Similarly, Veerakumar et al. (Citation2014b) reported Heliotropium indicum synthesized AgNPs against the adult of An. stephensi (lethal dose LD50 26.712 μg/mL; LD90 49.061 μg/mL), Ae. aegypti (LD50 29.626 μg/mL; LD90 54.269 μg/mL), and Cx. quinquefasciatus (LD50 32.077 μg/mL; LD90 58.426 μg/mL), respectively. On the other hand, Suresh et al. (Citation2015) investigated that adultcidal activity of AgNPs synthesized using an aqueous extract from Phyllanthus niruri, against Ae. aegypti resulted better LC50 and LC90 values (LC50 422.29 and LC90 23.58 ppm/mL).
Conclusions
In conclusion, the synthesized AgNPs were confirmed by analyzing the excitation of SPR using UV–Vis spectrophotometer at 410 nm. The results of the FTIR were used to identify the possible biomolecules like an aliphatic amine and primary amine groups which are responsible for the presence of the enzymes are responsible for the reduction synthesis and stabilization of the silver metal ions. Bio-fabricated AgNPs were mostly revealed in spherical shape; crystalline in nature, with face-centered cubic geometry, and their mean size was 20–50 nm. AgNPs have admirable larvicidal activity against third-instar larvae of three tested mosquito vectors, with LC50 values ranging from 3.49 to 6.40 μg/mL. The maximum efficacy was observed in synthesized AgNPs against the adult of Ae. albopictus LC50 and LC90 values were 10.069 and 15.657 μg/mL; An. stephensi 17.578 and 26.040 μg/mL; Cx. quinquefasciatus 12.568 and 19.510 μg/mL, respectively. Green synthesized AgNPs as promising representative for vector control and we oberved the morphological changes in the cuticular membrane of the fourth-instar larvae due to the toxicity of the AgNPs.
Acknowledgements
The authors would like to thanks Periyar University, Department of Biotechnology, Natural Drug Research Laboratory, Salem, Tamil Nadu, India, for providing the excellent facilities to carry out the work. We acknowledge the support extended by SAIF, IIT Madras for TEM and EDX studies. We wish to acknowledge St Joseph’s College, Trichy for helping FTIR analysis. The XRD analysis of the AgNPs sample was carried out in the Department of Physics, Periyar University, Salem, Tamil Nadu is highly acknowledged.
Disclosure statement
The authors declare that they have no conflict of interests.
References
- Abbott WS. 1925. A method of computing the effectiveness of insecticides. J Ecol Entomol. 18:265–267.
- Amer A, Mehlhorn H. 2006. Larvicidal effects of various essential oils against Aedes, Anopheles, and Culex larvae (Diptera, Culicidae). Parasitol Res. 99:466–472.
- Amin ZA, Bilgen M, Alshawsh MA, Ali HM, Hadi AH, Abdulla MA. 2012. Protective role of Phyllanthus niruri extract against thioacetamide induced liver cirrhosis in rat model. Evid Based Complement Alternat Med. Article ID 241583, 9 pages. doi: 10.1155/2012/241583
- Amkamwar B, Damle C, Ahmad A, Sastry M. 2005. Biosynthesis of gold and silver nanoparticles using Emblica officinalis fruit extract, their phase transfer and transmetallation in an organic solution. J Nanosci Nanotechnol. 5:1665–1671.
- Arjunan NK, Murugan K, Rejeeth C, Madhiyazhagan P, Barnard DR. 2012. Green synthesis of silver nanoparticles for the control of mosquito vectors of malaria, filariasis, and dengue. Vector Borne Zoonotic Dis. 12:262–268.
- Bagavan A, Kamaraj C, Elango G, Abduz Zahir A, Abdul Rahuman A. 2009. Adulticidal and larvicidal efficacy of some medicinal plant extracts against tick, fluke and mosquitoes. Vet Parasitol. 166:286–292.
- Benelli G. 2015b. Research in mosquito control: current challenges for a brighter future. Parasitol Res. 114:2801–2805.
- Benelli G. 2016a. Plant-synthesized nanoparticles: an eco-friendly tool against mosquito vectors? In: Mehlhorn H, Ed. Nanoparticles in the Fight against Parasites, Volume 8 of the series? Parasitol Res Monographs. Switzerland: Springer International Publishing, pp. 155–172.
- Benelli G. 2016b. Spread of Zika virus: the key role of mosquito vector control. Asia Pac J Trop Biomed. 6:468–471.
- Benelli G, Bedini S, Cosci F, Toniolo C, Conti B, Nicoletti M. 2015a. Larvicidal and ovideterrent properties of neem oil and fractions against the filariasis vector Aedes albopictus (Diptera: Culicidae): a bioactivity survey across production sites. Parasitol Res. 114:227–236.
- Benelli G, Mehlhorn H. 2016c. Review: declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol Res. 115:1747–1754.
- Benelli G, Murugan K, Panneerselvam C, Madhiyazhagan P, Conti B, Nicoletti M. 2015c. Old ingredients for a new recipe? Neem cake, a low-cost botanical by-product in the fight against mosquito-borne diseases. Parasitol Res. 114:391–397.
- Bernhard L, Bernhard P, Magnussen P. 2003. Management of patients with lymphoedema caused by filariasis in North-eastern Tanzania: alternative approaches. Physiotherapy. 89:743–749.
- Chattopadhyay S, Chakraborty SP, Laha D, Baral R, Pramanik P, Roy S. 2012. Surface-modified cobalt oxide nanoparticles: new opportunities for anti-cancer drug development. Cancer Nanotechnol. 3:13–23.
- Chattopadhyay S, Dash SK, Ghosh T, Das D, Pramanik P, Roy S. 2013a. Surface modification of cobalt oxide nanoparticles using phosphonomethyl iminodiacetic acid followed by folic acid: a biocompatible vehicle for targeted anticancer drug delivery. Cancer Nanotechnol. 4:103–116.
- Conti B, Canale A, Bertoli A, Gozzini F, Pistelli L. 2010. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol Res. 107:1455–1462.
- Dash SK, Ghosh T, Roy S, Chattopadhyay S, Das D. 2014. Zinc sulfide nanoparticles selectively induce cytotoxic and genotoxic effects on leukemic cells: involvement of reactive oxygen species and tumor necrosis factor alpha. J Appl Toxicol. 34:1130–1144.
- Dhanasekaran D, Thangaraj R. 2013. Evaluation of larvicidal activity of biogenic nanoparticles against filariasis causing Culex mosquito vector. Asian Pacific J Trop Dis. 3:174–179.
- Dinesh D, Murugan K, Madhiyazhagan P, Panneerselvam C, Nicoletti M, Jiang W, et al. 2015. Mosquitocidal and antibacterial activity of green-synthesized silver nanoparticles from Aloe vera extracts: towards an effective tool against the malaria vector Anopheles stephensi. Parasitol Res. 114:1519–1529.
- FAO. 1992. Nutrition and development: a global assessment. Proceedings of the International Conference on Nutrition; 1992 Dec 1–2; Rome, Italy.
- Finney DJ. 1971. Probit Analysis. London: Cambridge University Press, pp. 68–78.
- Gad L, George T. (Eds) 2009. The Sweet Potatoes. Springer Netherlands: Springer Science+ Business Media, pp. 391–425. doi:10.1007/978-1-4020-9475-0. Available from: http://zipcodezoo.com/index.php/Special:BookSources/978-1-4020-9475-0.
- Gole A, Dash C, Ramachandran V, Mandale AB, Sainkar SR, Rao M, Sastry M. 2001. Pepsin-gold colloid conjugates: preparation, characterization and enzymatic activity. Langmuir. 17:1674–1679.
- Govindarajan M, Kadaikunnan S, Alharbi NS, Benelli G. 2016. Single-step biological fabrication of colloidal silver nanoparticles using Hugonia mystax: larvicidal potential against Zika virus, dengue, and malaria vector mosquitoes. Artif Cells Nanomed Biotechnol. 9:1–9.
- Hamsa TP, Kuttan G. 2011. Evaluation of the anti-inflammatory and anti-tumor effect of Ipomoea obscura (L) and its mode of action through the inhibition of proinflammatory cytokines, nitric oxide and COX-2. Inflammation. 34:171–183.
- Hermes D, Dudek DN, Maria MD, Horta LP, Lima EN, de Fátima Â, et al. 2013. In vivo wound healing and antiulcer properties of white sweet potato (Ipomoea batatas). J Adv Res. 4:411–415.
- Igwenyi IO, Offor CE, Ajah DA, Nwankwo OC, Ukaomah JI, Aja PM. 2011. Chemical compositions of Ipomea aquatica (green kangkong). Int J Pharm Bio Sci. 2:B593–B598.
- Ijaola TO, Osunkiyesi AA, Taiwo AA, Oseni OA, LanreIyanda YA, Ajayi JO, et al. 2014. Antiiabetic effect of Ipomoea batatas in normal and alloxan-induced diabetic rats. J Appl Chem. 7:16–25.
- Jabeen Q, Aslam N. 2013. Hypotensive, angiotensin converting enzyme (ACE) inhibitory and diuretic activities of the aqueous-methanol extract of Ipomoea reniformis. Iran J Pharm Res. 12:769–776.
- Jayaseelan C, Abdul Rahuman A, Rajakumar G, Vishnu Kirthi A, Santhoshkumar T, Marimuthu S, et al. 2011. Synthesis of pediculocidal and larvicidal silver nanoparticles by leaf extract from heartleaf moonseed plant, Tinospora cordifolia Miers. Parasitol Res. 109:185–194.
- Kager PA. 2002. Malaria control: constraints and opportunities. Trop Med Int Health. 7:1042–1046.
- Kamaraj C, Bagavan A, Rahuman AA, Zahir AA, Elango G, Pandiyan G. 2009. Larvicidal potential of medicinal plant extracts against Anopheles subpictus Grassi and Culex tritaeniorhynchus Giles (Diptera: Culicidae). Parasitol Res. 104:1163–1171.
- Kovendan K, Murugan K, Shanthakumar SP, Vincent S. 2012. Evaluation of larvicidal and pupicidal activity of Morinda citrifolia L. (Noni) (Family: Rubiaceae) against three mosquito vectors. Parasitol Res. 111:1481–1490.
- Kumar A, Paul S, Kumari P, Somasundaram ST, Kathiresan K. 2014. Antibacterial and phytochemical assessment on various extracts of Ipomoea pescaprae (L.) R. Br through FTIR and GC-MS spectroscopic analysis. Asian J Pharm Clin Res. 7:134–138.
- Kunjiappan S, Chowdhury R, Bhattacharjee C. 2014. A green chemistry approach for the synthesis and characterization of bioactive gold nanoparticles using Azolla microphylla methanol extract. Front Mater Sci. 8:123–135.
- Lalrotluanga LN, Senthil-Kumar N, Gurusubramanian G. 2012. Insecticidal and repellent activity of Hiptage benghalensis L. Kruz (Malpighiaceae) against mosquito vectors. Parasitol Res. 111:1007–1017.
- Lin RJ, Chen CY, Lo WL. 2008. Cytotoxic activity of Ipomoea cairica. Nat Prod Res. 9:747–753.
- Logeswari P, Silambarasan S, Abraham J. 2013. Eco-friendly synthesis of silver nanoparticles from commercially available plant products and their antibacterial properties. Scientia Irancia F. 20:1049–1054.
- Ma J, Zhang J, Xiong Z, Yong Y, Zhao XS. 2003. Preparation, characterization and antibacterial properties of silver-modified graphene oxide. J Mater Chem. 21:3350–3352.
- Maillard M, Marston A, Hostettmann K. 1993. Search for molluscicidal and larvicidal agents from plants. In: Balandrin M, Ed. Human Medicinal Agents from Plants. Washington, DC: American Chemical Society, vol. 534, pp. 256–273.
- Majumdar R, Bag BG, Maity N. 2013. Acacia nilotica (Babool) leaf extract mediated size-controlled rapid synthesis of gold nanoparticles and study of its catalytic activity. Int Nano Lett. 3:53.
- Marcondes CB, Ximenes MF. 2015. Zika virus in Brazil and the danger of infestation by Aedes (Stegomyia) mosquitoes. Rev Soc Bras Med Trop. 49:4–10.
- Marimuthu S, Rahuman AA, Rajakumar G, Santhosh kumar T, Kirthi AV, Jayaseelan C, et al. 2010. Evaluation of green synthesized silver nanoparticles against parasites. Parasitol Res. 108:1541–1549.
- Minjas JN, Sarda RK. 1986. Laboratory observations on the toxicity of Swartzia madagascariensis (Leguminosae) extract to mosquito larvae. Trans R Soc Trop Med Hyg. 80:460–461.
- Mishra A, Kaushik NK, Sardar M, Sahal D. 2013. Evaluation of antiplasmodial activity of green synthesized silver nanoparticles. Colloids Surf B: Biointerf. 111:713–718.
- Mukhtar M, Herrel N, Amerasinghe FP, Ensink J, Vander Hoek W, Konradsen F. 2003. Role of wastewater irrigation in mosquito breeding in south Punjab, Pakistan. Southeast Asian J Trop Med Public Health. 34:72–80.
- Murugan K, Aruna P, Panneerselvam C, Madhiyazhagan P, Paulpandi M, Subramaniam J, et al. 2016. Fighting arboviral diseases: low toxicity on mammalian cells, dengue growth inhibition (in vitro) and mosquitocidal activity of Centroceras clavulatum-synthesized silver nanoparticles. Parasitol Res. 115:651–662.
- Murugan K, Benelli G, Ayyappan S, Dinesh D, Panneerselvam C, Nicoletti M, et al. 2015a. Toxicity of seaweed-synthesized silver nanoparticles against the filariasis vector Culex quinquefasciatus and its impact on predation efficiency of the cyclopoid crustacean Mesocyclops longisetus. Parasit Res. 114:2243–2253.
- Murugan K, Eugine Venus JS, Panneerselvam C, Bedini S, Conti B, Nicoletti M, et al. 2015b. Biosynthesis, mosquitocidal and antibacterial properties of Toddalia asiatica-synthesized silver nanoparticles: do they impact predation of guppy Poecilia reticulata against the filariasis mosquito Culex quinquefasciatus. Environ Sci Pollut Res. 22:17053–17064.
- Murugan K, Jeyabalan D, Senthilkumar N, Babu R, Sivaramakrishnan S. 1996. Antipupational effect of neem seed kernel extract against mosquito larvae of Anopheles stephensi (Liston). J Ent Res. 20:137–139.
- Muthukumaran U, Govindarajan M, Rajeswary M. 2015. Mosquito larvicidal potential of silver nanoparticles synthesized using Chomelia asiatica (Rubiaceae) against Anopheles stephensi, Aedes aegypti, and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 114:989–999.
- Nagamine R, Ueno S, Tsubata M, Yamaguchi K, Takagaki K, Hira T, et al. 2014. Dietary sweet potato (Ipomoea batatas L.) leaf extact attenuates hyperglycaemia by enhancing the secretion of glucagon-like peptide-1 (GLP-1). Food Funct. 5:2309–2316.
- Ouda SM. 2014. Antifungal activity of silver and copper nanoparticles on two plant pathogens, Alternaria alternate and Botrytis cinerea. Res J Microbiol. 9:34–42.
- Panda V, Sonkamble M. 2012. Phytochemical constituents and pharmacological activites of Ipomoea batatas I. (Lam) – a review. Int J Res Phytochem Pharmacol. 2:25–34.
- Parashar UK, Saxenaa PS, Srivastava A. 2009. Bioinspired synthesis of silver nanoparticles. Dig J Nanomater Bios. 4:159–166.
- Pàska C, Innocenti G, Kunvári M, László M, Szilágyi L. 1999. Lignan production by Ipomoea cairica callus cultures. Phytochemistry. 52:879–883.
- Patil SV, Borase HP, Patil CD, Salunke BK. 2012. Biosynthesis of silver nanoparticles using latex from few Euphorbian plants and their antimicrobial potential. Appl Biochem Biotechnol. 167:776–790.
- Pavela R, Benelli G. 2016. Ethnobotanical knowledge on botanical repellents employed in the African region against mosquito vectors – a review. Exp Parasitol. 167C:103–108.
- Peng Z, Yang J, Wang H, Simons FER. 1999. Production and characterization of monoclonal antibodies to two new mosquito Aedes aegypti salivary proteins. Insect Biochem Mol Biol. 29:909–914.
- Rahman SJ, Sharma SK, Rajagopal R. 1989. Manual on Entomological Surveillance of Vector Borne Diseases. New Delhi : NICD.
- Rahuman AA, Gopalakrishnan G, Venkatesan P, Geetha K. 2007. Larvicidal activity of some Euphorbiaceae plant extracts against Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 102:867–873.
- Rajakumar G, Rahuman A. 2011. Larvicidal activity of synthesized silver nanoparticle using Eclipta prostrata leaf extract against filariasis and malaria vectors. Acta Trop. 118:196–203.
- Rajan R, Chandran K, Harper SL, Yun SI, Kalaichelvan PT. 2015. Plant extract synthesized nanoparticles: an ongoing source of novel biocompatible materials. Ind Crop Prod. 70:356–373.
- Rajesh WR, Jaya RL, Niranjan SK, Vijay DM, Sahelebrao BK. 2009. Phyto synthesis of silver nanoparticles using Gliricidia sepium (Jaeq). Curr Nanosci. 5:117–122.
- Rao CNR, Biswas K. 2009. Characterization of nanomaterials by physical methods. Annu Rev Anal Chem. 2:435–462.
- Santhoshkumar T, Rahuman AA, Rajakumar G, Marimuthu S, Bagavan A, Jayaseelan C, et al. 2011. synthesis of silver nanoparticles using Nelumbo nucifera leaf extract and its larvicidal activity against malaria and filariasis vectors. Parasitol Res. 108:693–702.
- Selvaraj Mohana Roopana R, Madhumithaa G, Abdul Rahuman A, Kamaraj C, Bharathi A, Surendra TV. 2013. Low-cost and eco-friendly phyto-synthesis of silver nanoparticles using Cocos nucifera coir extract and its larvicidal activity. Ind Crops Product. 43:631–635.
- Sharma VK, Ria AY, Lin Y. 2009. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 145:83–96.
- Soni N, Prakash S. 2014. Green nanoparticles for mosquito control. ScientificWorldJournal. Article ID 496362, 6 pages. Available from: http://dx.doi.org/10.1155/2014/496362.
- Srinivasan B, Muthukumaraswamy S, Mohanraj J. 2014. Biosynthesis of silver nanoparticles from mangrove plant (Avicennia marina) extract and their potential mosquito larvicidal property. Parasit Dis. 40:991–996.
- Subramaniam J, Murugan K, Panneerselvam C, Kovendan K, Madhiyazhagan P, Mahesh Kumar P, et al. 2015. Eco-friendly control of malaria and arbovirus vectors using the mosquitofish Gambusia affinis and ultra-low dosages of Mimusops elengi-synthesized silver nanoparticles: towards an integrative approach? Environ Sci Pollut Res. 22:20067–20083.
- Sujitha V, Murugan K, Paulpandi M, Panneerselvam C, Suresh U, Roni M, et al. 2015. Green-synthesized silver nanoparticle as a novel control tool against dengue virus (DEN-2) and its primary vector Aedes aegypti. Parasitol Res. 114:3315–3325.
- Suman TY, Elumalai D, Kaleena PK, Radhika Rajasree SR. 2013. GC-MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind Crop Prod. 47:239–245.
- Suresh U, Murugan K, Benelli G, Nicoletti M, Barnard DR, Panneerselvam C, et al. 2015. Tackling the growing threat of dengue: Phyllanthus niruri-mediated synthesis of silver nanoparticles and their mosquitocidal properties against the dengue vector Aedes aegypti (Diptera: Culicidae). Parasitol Res. 114:1551–1562.
- Tewe OO, Ojeniyi FE, Abu OA. 2003. Sweet Potato Production, Utilization and Marketing in Nigeria. Lima: Social Sciences Department, International Potato Center (CIP), p. 44.
- Vasilakis N, Shell EJ, Fokam EB, Mason PW, Hanley KA, Estes DM, Weaver SC. 2007. Potential of ancestral sylvatic dengue-2 viruses to re-emerge. Virology. 358:402–412.
- Veerakumar K, Govindarajan M, Rajeswary M. 2013. Green synthesis of silver nanoparticles using Sida acuta (Malvaceae) leaf extract against Culex quinquefasciatus, Anopheles stephensi, and Aedes aegypti (Diptera: Culicidae). Parasitol Res. 112:4073–4085.
- Veerakumar K, Govindarajan M. 2014a. Adulticidal properties of synthesized silver nanoparticles using leaf extracts of Feronia elephantum (Rutaceae) against filariasis, malaria, and dengue vector mosquitoes. Parasitol Res. 113:4085–4096.
- Veerakumar K, Govindarajan M, Rajeswary M, Muthukumaran U. 2014b. Mosquito larvicidal properties of silver nanoparticles synthesized using Heliotropium indicum (Boraginaceae) against Aedes aegypti, Anopheles stephensi and Culex quinquefasciatus (Diptera: Culicidae). Parasitol Res. 113:2363–2373.
- Veerakumar K, Govindarajan M, Rajeswary M, Muthukumaran U. 2014c. Low-cost and eco-friendly green synthesis of silver nanoparticles using Feronia elephantum. Parasitol Res. 113:1775–1785.
- Velayutham K, Rahuman A, Rajakumar G, Mohana Roopan S, Elango G, Kamaraj C, Marimuthu S, et al. 2013. Larvicidal activity of green synthesized silver nanoparticles using bark aqueous extract of Ficus racemosa against Culex quinquefasciatus and Culex gelidus. Asian Pac J Trop Med. 6:95–101.
- Velu K, Elumalai D, Hemalatha P, Janaki A, Babu M, Hemavathi M, Patheri KK. 2015. Evaluation of silver nanoparticles toxicity of Arachis hypogaea peel extracts and its larvicidal activity against malaria and dengue vectors. Environ Sci Pollut Res. 22:17769–17779.
- Vivek M, Senthil Kumar P, Steffi S, Sudha S. 2011. Biogenic silver nanoparticles by Gelidiella acerosa extract and their antifungal effects. Avicenna J Med Biotechnol. 3:143–148.
- Woolfe JA. 1992. Sweet Potato. An Untapped Food Resource. Cambridge: Cambridge University Press, pp. 118–187.
- WHO. 1981. Instructions for determining the susceptibility of adult mosquito to organochlorine, organophosphate and carbamate insecticides establishment of base line. Geneva: WHO, WHO/VBC/81.806. pp. 1–7.
- WHO. 2005. Guidelines for laboratory and field testing of mosquito larvicides. Communicable disease control, prevention and eradication, WHO pesticide evaluation scheme. Geneva: WHO, WHO/CDS/WHOPES/GCDPP/1.3. pp. 1–41.
- WHO. 2010. Dengue transmission research in WHO bulletin Zebit CPW (1984): effect of some crude and Azadirachta enriched neem (Azadirachta indica) seed kernel extracts of larvae of Aedes aegypti. Entomol Exp Appl. 35:11–16.
- WHO. 2012a. Lymphatic Filariasis. Available from: http://www.who.int/mediacentre/factsheets/fs102/en/.
- WHO. 2012b. WHO 10 facts on malaria [cited 2014 Mar 10]. Available from: http://www.who.int/features/factfiles/malaria/en/index.html.
- Yamakawa O, Yoshimoto M, Kurata R, Adachi M. 2007. Growth suppression of human cancer cells by polyphenolics from sweet potato (Ipomea batatas) Leaves. J Agr Food Chem. 55:185–190.
- Yoshimoto M, Yahara S, Okuno S, Islam MS, Ishiguro K, Yamakawa O. 2002. Antimutagenicity of mono-, di- and tricaffeoylquinic acid derivatives isolated from sweet potato (Ipomoea batatas L.) leaf. Biosci Biotech Biochem. 66:2336–2341.
- Yu B, Luo J, Wang J, Zhang D, Yu S, K L. 2013. Pentasaccharide resin glycosides from Ipomoea cairica and their cytotoxic activities. Phytochem. 95:421–427.