Abstract
The controlled synthesis of silver nanoparticles (AgNPs) using cell-free filtrate of Fusarium oxysporum fungus was investigated. The effect of fungal incubation period on nanoparticle formation and nitrate reductase enzyme activity was studied using UV–visible spectroscopy and Harley assay, respectively. The highest AgNP formation was observed in the cell-free filtrate of biomass harvested at the early stationary phase where the NR enzyme activity is the maximum. Mixing of the cell-free filtrates of fungal cultures obtained at 23, 28, and 33 °C with silver nitrate solution confirms the higher productivity of AgNP biosynthesis using the cell-free filtrate of fungus incubated at 28 °C. The effect of some factors such as carbon and nitrate sources and light in fungal incubation period on nitrate reductase induction and AgNP formation was also evaluated. In conclusion, increasing nitrate and carbon sources and presence of light induced NR enzyme and produced AgNPs with smaller size, higher monodispersity, and productivity. Results revealed that the presence of ammonium prevents the NR enzyme secretion and causes to the lower productivity of AgNPs.
Introduction
Silver nanoparticles (AgNPs) have attracted considerable attention in recent years due to its wide ranges of applications in biomolecular detection, catalysis, antimicrobial, antiviral, and anticancer actions (Ajitha et al. Citation2015, Krupa et al. Citation2016, Mittal et al. Citation2015, Nayak et al. Citation2015, Pourali and Yahyaei Citation2016). AgNPs have been used for many decades as antibacterial agents in cosmetics, food storage, textile coatings, water treatment, etc. (Du et al. Citation2016, Kejlová et al., Citation2015). A number of chemical and physical methods have been developed for the synthesis of AgNPs. However, these methods suffer from disadvantages such as low yield, high-energy requirements, and difficult separation processes. Also, employing expensive and toxic reagents as reducing and stabilizing agents poses potential risks to human health and the environment (Baker et al. Citation2015, Khatami et al. Citation2016, Pourali and Yahyaei Citation2016). Hence, there is a growing need to develop high yield, low cost, and environmentally being methods that do not use toxic chemicals in the AgNP synthesis protocol (Shanthi et al. Citation2016, Verma et al. Citation2016). In recent years, utilization of biological resources such as bacteria, fungi, and plants for nanoparticle synthesis has become a promising alternative method (Verma et al. Citation2016).
Filamentous fungi are more efficient candidate for biosynthesis of nanoparticles due to their high secretion of proteins, enzymes and metabolites, high growth rates, easy handling in large-scale production, and low-cost requirements for production procedures (Balakumaran et al. Citation2016, Honary et al. Citation2013). Different fungal species have been utilized in the extracellular synthesis of silver nanoparticles including Guignardia mangiferae (Balakumaran et al. Citation2015), penicillium sp. (Honary et al. Citation2013, Maliszewska et al. Citation2014), Aspergillus sp. (Bala and Arya Citation2013, Jain et al. Citation2010, Vigneshwaran et al. Citation2007), and Fusarium oxysporum (Khosravi and Shojaosadati Citation2009, Mohammadian et al. Citation2007, Soni and Prakash Citation2011).
The chemical and physical properties of nanoparticles are strongly related to its size and shape. Thus, the size and the shape of AgNPs were manipulated in recent years by controlling pH, temperature, substrate concentration (metal ions), and reaction time (Balakumaran et al. Citation2015). But, there are few reports in the role of fungal growth condition on the AgNP characteristics. The main objective of this study was to evaluate the potency of nitrate reductase enzyme in controlled synthesis of AgNPs using cell-free filtrate of F. oxysporum. Also, the impact of fungal incubation conditions on AgNP synthesis was investigated using UV–Vis, DLS, and SEM analyses.
Materials and methods
Materials
Fusarium oxysporum (PTCC 5291) was obtained from National Institute of Genetic Engineering and Biotechnology (NIGEB), Tehran, Iran. The pure culture was maintained on a potato dextrose agar (PDA), and the resulting medium was stored at 4 °C. All chemicals, including silver nitrate, potassium nitrate, ammonium nitrate, ammonium sulfate, N-(1-naphthyl) ethylene diamine dihydrochloride (NEED), and sulfanilamide (SA) were of analytical grade and purchased from Sigma-Aldrich (St. Louis, MO).
Fungal cultivation conditions
Fusarium oxysporum inoculums (107 spore (cfu)/ml) were transferred into 100 ml shake flasks containing 20 ml MGYP media composed of: (w/v) 0.3% malt extract, 1.0% glucose, 0.3% yeast extract, and 0.5%. The pH was set to 6.5 ± 0.2 using 1 N HCl. Forty flasks containing the inoculated medium were incubated at 28 °C with agitation on an orbital shaker operating at 200 rpm for 120 h. Every 6 h, two flasks were removed simultaneously and their biomass was harvested by vacuum filtration. In order to measure the cell dry weight, the resultant wet mycelia were dried at 60 °C to attain a constant weight. The fungal growth curve was obtained by plotting the fungal cell dry weights versus incubation periods. To determine the dependence of AgNP biosynthesis on growth stages of fungus, the biomass-free medium obtained at various incubation periods were mixed with silver nitrate solution and the nanoparticle formation was quantified by using UV–vis spectroscopy. The UV–visible spectra with the highest absorbance and the lowest wavelength were selected and the related incubation periods were considered as the optimal condition for AgNP synthesis.
Effect of incubation temperature on AgNP formation
The flasks containing inoculated media were incubated at 23, 28, and 33 °C to reach stationary phase. The biomass from F. oxysporum cultures was harvested by filtration and then washed extensively with sterilized distilled water to remove any remaining media components. Then, 10 g of wet fungal mycelia were suspended in 100 ml sterilized distilled water and incubated at 28 °C Finally, 50 ml of the cell-free filtrate was added to 50 ml of a 2 mM silver nitrate. The mixtures were incubated at 28 °C under shaking at 200 rpm until complete bioreduction of Ag+ was achieved.
Effect of light in incubation period on AgNP formation
In order to investigate the effect of light on F. oxysporum cultivation and then, on AgNP formation, some flasks containing inoculated MGYP media were exposed to halogen lamp and the others were kept in dark condition. After cultivation period, resultant cell-free filtrate was used for AgNP synthesis, as mentioned above.
Mechanism study of AgNP formation
Nitrate reductase assay
Nitrate reductase in fungal filtrate was assayed according to the procedure described by Harley (Jaidev and Narasimha Citation2010). The mycelia were separated from media by filtration at pre-determined period, and washed extensively with sterile distilled water. For enzyme assay, a mixture of wet mycelia and distilled water (10% w/v) was incubated at 28 °C for 120 h (5 d), and the biomass was separated using Whatman filter paper No. 1. Then, 10 ml aliquot of the 5-d fungal filtrate was mixed with 10 ml of assay medium (30 mM KNO3 and 5% propanol in 0.1 M phosphate buffer of pH 7.5) and incubated at 30 °C in the absence of light for 1 h. Then, 5 ml of SA solution and 5 ml of NEED solution were added, to determine the formation of nitrites in the mixture. Finally, the absorbance of resultant pink solutions at 540 nm was measured using UV–visible spectrophotometer. The enzyme activity was determined based on the increase in the nitrite concentration of the solution over 1 h for the amount of the initial sample (10 ml) and expressed as nmol nitrite h−1 ml−1.
Induction of NR enzyme secretion
In order to increase the secretion of NR enzyme, F. oxysporum was grown aerobically in the modified medium containing (w/v) %: 0.35 yeast extract, 1 peptone, 0.35 potassium nitrate, and 1.5 glucose. Also, flask containing inoculated MGYP medium was used as the control. The flasks containing modified and MGYP media were inoculated and then incubated at 28 °C with agitation on an orbital shaker operating at 200 rpm for 96 h. Then the 5-d cell-free filtrates were assayed for determination of NR activity.
In another set of experiments, the wet mycelia after harvesting from cultures and extensive washing were suspended in (a) sterilized distilled water, (b) 25 mM potassium nitrate, (c) 50 mM potassium nitrate, (d) 1 mM ammonium nitrate and (e) 1 mM ammonium sulfate, with 10% (w/v), separately. Then, the flasks incubated at 28 °C for 120 h and the cell-free filtrate of each flask was assayed for NR activity. Simultaneously, the AgNP formation by 5-d cell-free filtrate of the fungus (each flask) was monitored using UV–visible spectrophotometer.
Characterization of AgNPs
UV–visible analysis
The appearance of color changes of the reaction mixtures were used as initial evidence of AgNP formation. Samples of 1 ml were taken from each flask containing colloidal AgNPs at regular time intervals, and its absorbance was measured using a double beam UV–visible spectrophotometer (Cary 100, Varian, Palo Alto, CA) with a resolution of 1 nm in the range of 200–800 nm.
Dynamic light-scattering analysis
The size distribution and the average size of the synthesized AgNPs were determined by dynamic light scattering (DLS; Malvern Instruments LTD., Malvern, UK). Polydispersity indexes (PDI) determined from DLS experiments were used as a measure of particle aggregation.
Scanning electron microscopy (SEM) analysis
The morphological characterization of the synthesized AgNPs using cell-free filtrate of F. oxysporum was performed by SEM (KYKY-EM3200, Mainland, China) at 26.00 kV. The sample was prepared by locating the purified colloidal AgNPs on the SEM holder, followed by gold coating using sputter coater (KYKY-SBC12, Mainland, China). SEM image was recorded at 30,000 × magnification.
Antibacterial activity of AgNPs
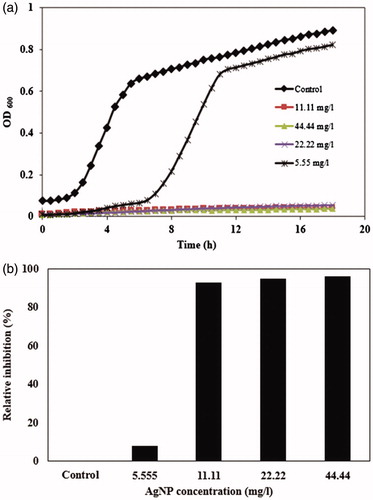
Fresh colonies of Escherichia coli BL21 from a Luria–Bertani (LB) agar plate were inoculated into 10 ml of LB broth medium. The flask was incubated overnight at 37 °C with an agitation rate of 200 rpm. The fresh inoculum was transferred to 10 shake flasks each containing 20 ml of LB media. For the estimation of minimal inhibition concentration (MIC), the culture media of eight flasks was supplemented separately, with 5.55, 11.11, 22.22, and 44.44 mg/L AgNPs synthesized by cell-free filtrate (two replicates for each concentration). Two flasks were considered as the control without an addition of nanoparticles. Finally, all flasks were incubated at 37 °C with continuous shaking at 200 rpm. The optical densities of all media at 600 nm (OD600) were measured every 30 min. The antibacterial activity of silver nanoparticles was assessed by determining the growth curve of E. coli in the presence and the absence of AgNPs. All the experiments were carried out for two times and the average values are reported.
Results
Effect of fungal incubation period on the AgNP formation
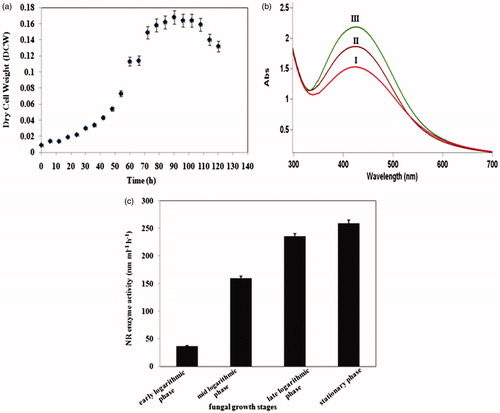
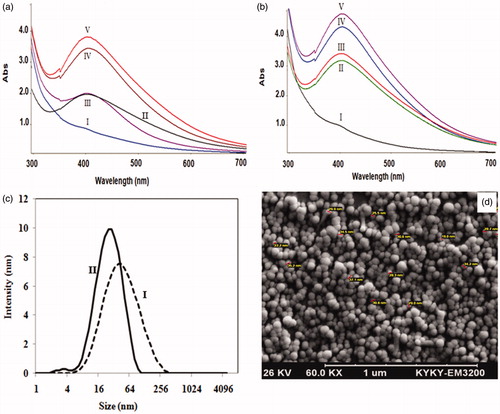
The growth profile shows that after 90 h, there was no considerable change in the dry cell weight of F. oxysporum (). Simultaneously, the effect of fungal incubation period on AgNP formation was investigated using UV–visible spectra of colloidal AgNPs obtained at different growth phases (). As shown in , the NR activities of the fungal filtrates at early, mid, and late logarithmic phases of fungal growth were 37, 160, and 230 nmol h−1 ml−1, respectively. The maximum enzyme activity belongs to the filtrate of biomass which was harvested at the stationary phase (259 nmol h−1 ml−1).
Figure 1. (a) Growth curve of F. oxysporum (PTCC 5291) fungus in MGYP medium at 28 °C; (b) UV–visible spectra of colloidal AgNPs originated from culture medium of F. oxysporum fungus at mid logarithmic (I), late logarithmic (II), and stationary (III) phases; (c) Nitrate reductase enzyme activity in biomass-free filtrates of F. oxysporum acquired at different growth phases.
Effect of fungal incubation temperature on AgNP formation
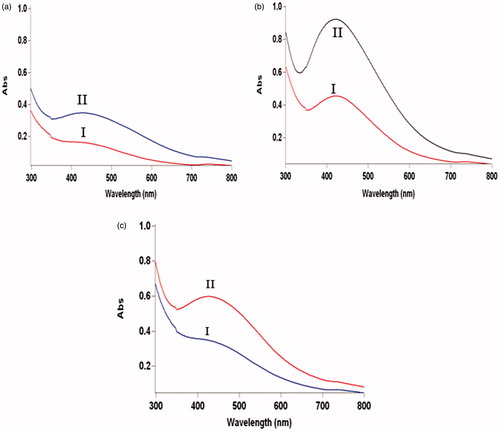
UV–vis spectra of colloidal AgNPs synthesized using cell-free filtrates of fungal cells incubated at three different temperatures of 23, 28, and 33 °C are presented in . The absorption intensity of the spectrum obtained from AgNPs synthesized using the cell-free filtrate of fungus cultured at 28 °C is considerably higher than that obtained using the cell-free filtrates of fungus which was grown at 23 and 33 °C. As shown in these figures, a strong SPR is observed near 440, 420, and 435 nm for AgNPs synthesized using cell-free filtrates of fungus cultured at 23, 28, and 33 °C, respectively.
The effect of light and carbon source in fungal incubation period on AgNP synthesis
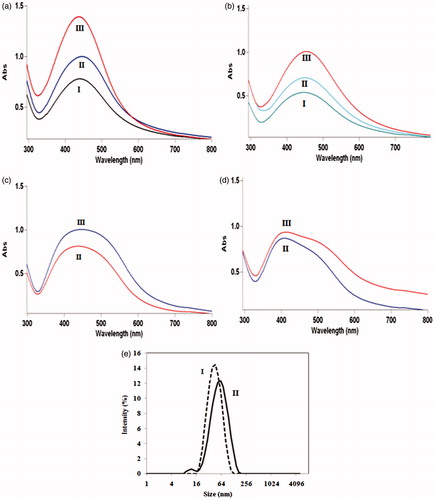
UV–visible spectra of colloidal AgNPs synthesized using cell-free filtrates resulted from fungal cells cultured, in two different media of MGYP and PDB, both in the presence and in the absence of light are presented in . This figure revealed that the production rates of AgNPs synthesized using cell-free filtrates of incubated fungal cells in darkness are lower than the cell-free filtrates of fungal cells incubated in the presence of light. DLS analysis () showed that the average sizes of AgNPs produced by cell-free filtrates of incubated fungal cells in MGYP and PDB media are 34 nm and 44 nm, and their PDI are 0.14 and 0.27, respectively.
Figure 3. UV–visible spectra of colloidal AgNPs produced using cell-free filtrates of F. oxysporum which incubated in (a) MGYP and (b) PDB media in the presence of light; after 24 h(I), 48 h (II), and 72 h (III); (c) MGYP and (d) PDB media in absence of light; after 48 h (II) and 72 h (III); (e) DLS analyses of colloidal AgNPs generated by mixing of AgNO3 solution and cell-free filtrates of F. oxysporum cells which cultured in presence of light in (I) MGYP and (II) PDB media.
The total proteins, as well as, the NR enzyme activity of cell-free filtrates obtained from fungal cells incubated in two different media of MGYP and PDB both in the presence and in the absence of light are measured by Bradford and Harley assays, respectively. Results are presented in . The enzyme activities of the cell-free filtrates resulted from incubated fungal cells in the presence of light in MGYP and PDB media were measured as 151.34 and 32 nmol h−1 ml−1, respectively. NR enzyme activities were measured as 53.44 and 10.67 nmol h−1 ml−1 in cell-free filtrates of fungus incubated in MGYP and PDB media in the absence of light, respectively.
Table 1. Comparison of total proteins and NR enzyme secretions in cell-free filtrates of F. oxysporum cultured in MGYP and PDB media both in the presence and in the absence of light.
Stimulation of nitrate reductase enzyme
The nitrate reductase enzyme activity was measured in cell-free filtrates of the fungal cells which were grown in MGYP medium and modified medium containing potassium nitrate, based on the Harley method. The NR activities were measured as 170 and 220 nmol h−1 ml−1 in the filtrates obtained from fungal cells incubated in MGYP and modified media, respectively. UV–visible spectra of colloidal AgNPs originated from cell-free filtrates of fungal cells which were incubated in two different media are presented in . DLS results () revealed that the average sizes of colloidal AgNPs produced by cell-free filtrates of fungus incubated in MGYP and modified media are 38 and 24 nm, and their PDI are 0.30 and 0.23, respectively.
Figure 4. UV–visible spectra of colloidal AgNPs in reaction solutions containing 1:1 volume ratio of AgNO3 solutions and cell-free filtrates originated from F. oxysporum cells incubated at (a) MGYP medium, (b) modified medium after 12 h (I), 24 h (II), 36 h (III), 60 h (IV), and 72 h (V); (c) DLS analysis of colloidal AgNPs generated by the mixtures of AgNO3 and cell-free filtrates of F. oxysporum incubated in (a) MGYP medium; (b) modified medium containing potassium nitrate; (d) SEM image of synthesized AgNPs in reaction mixture containing silver nitrate, and cell-free filtrate of incubating F. oxysporum in modified medium containing nitrate source.
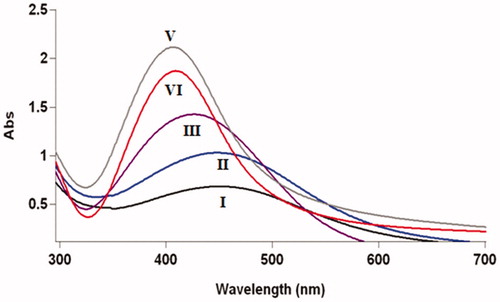
UV–vis spectra of colloidal AgNPs synthesized using filtrates of harvested fungal cells which submerged in sterilized distilled water, 25 mM potassium nitrate, 50 mM potassium nitrate, 1 mM ammonium nitrate, and 1 mM ammonium sulfate are presented in .
Figure 5. UV–vis analysis of colloidal AgNPs synthesized using mixtures of silver nitrate solutions and filtrates of submerged F. oxysporum biomass in ammonium sulphate (I); ammonium nitrate (II); distilled water (III); 25 mM potassium nitrate (IV); 50 mM potassium nitrate (V); after 72 h (reaction completion).
Antibacterial activity of AgNPs
The antibacterial activity of synthesized AgNPs using the cell-free filtrate of F. oxysporum is presented in . The presence of all concentrations of AgNPs in culture media reduces the growth rate of E. coli (). The final optical density of the bacterial cells after 18 h exposing to different concentrations of AgNPs is presented in .
Discussion
The presence of a single SPR band in all UV–visible spectra corresponds to the production of isotropic nanoparticles (Sadhasivam et al. Citation2010). The lower-wavelength region of the UV–visible spectrum implies the formation of smaller nanoparticles (Ibrahim Citation2015). The absorbance intensity of UV–visible spectra provides insight into the bioreduction of Ag+ and consequently, the productivity of each method (Ghaseminezhad et al. Citation2012). UV–visible spectra of solutions () showed that the peak area and the height of the spectra originated from the biomass-free medium after 90 h of incubation (i.e. the early stationary phase), were considerably higher. Thus, nanoparticle formation depends on the growth phase of the fungal cells and it reaches maximum at the stationary phase. These results are in agreement with our previous report on Neurospora intermedia fungus (Hamedi et al. Citation2014). Comparing the NR enzyme activities in cell-free filtrates obtained from different growth phases of fungus () revealed that the increased formation of AgNPs at the early stationary phase is likely due to the higher secretion of extracellular enzymes, especially NR enzyme, in this phase.
Comparing the absorbance intensities in confirms the higher productivity of AgNP biosynthesis using the cell-free filtrate of fungus incubated at 28 °C. The shift of the SPR band to lower wavelengths (420 nm) indicates that the AgNP synthesis using cell-free filtrate of fungal cells at 28 °C led to the formation of smaller nanoparticles. The presence of a symmetrical plasmon band in UV–visible spectra can be attributed to the narrower size distribution of the produced nanoparticles. Comparing the UV–visible spectra revealed that using cell-free filtrate of cultured fungal cells at 28 °C for AgNP synthesis resulted in the formation of particles with higher monodispersity than using cell-free filtrates of cultured fungal cells at 23 and 33 °C . These observations could be attributed to the higher secretion of proteins, enzymes as well as other metabolites, at 28 °C, which act as reducing agents. The Bradford assay confirmed the presence of higher amount of total proteins in fungal filtrate obtained at 28 °C (0.5524 mg/ml) compared with others obtained at 23 (0.3919 mg/ml) and 33 °C (0.4617 mg/ml). Increasing the reducing agents results in increased formation of silver atoms as nucleation centers. These nucleation centers catalyzed the reduction of remaining Ag+ ions for cluster formation. This stage can control the cluster size. Thus the nuclei increment as a consequence of higher consumption of reducing agents reduces the cluster growth and led to the increased formation of AgNPs (Hamedi et al. Citation2014).
Comparison of the wavelengths and intensities of spectra in shows that using the cell-free filtrate of incubated fungal cells in MGYP medium in the presence of light led to the formation of AgNPs with smallest size and highest productivity. DLS analysis () indicated that cell-free filtrate resulted from incubated fungal cells in MGYP medium produced AgNPs with smaller size and narrower size distribution in comparison with the cell-free filtrate obtained from cultured fungus in PDB medium. An increase in C:N ratio led to the induction of nitrate reductase enzyme and improvement of fungal growth (Raudabaugh et al. Citation2013). NR enzyme secretion was induced by increasing the glucose concentration (Vaidyanathan et al. Citation2010). Therefore, the presence of glucose in MGYP medium induced the secretion of NR enzyme, and consequently, leads to the formation of AgNPs with smaller size and narrower size distribution. Based on the results presented in , the effect of light on total proteins secreted by fungus is not very significant. However, on one hand, the presence of light in fungal incubation period leads to increased secretion of NR enzyme. On the other hand, the presence of light induces the metabolic pathways of the NR enzyme production. As presented in , the maximum NR enzyme activity was observed in cell-free filtrate of fungus incubated in MGYP medium in presence of light. This may be the main reason of AgNP formation with the smallest size and the highest monodispersity in this case. These results reveal that the AgNP characteristics can be controlled and improved by the induction of NR enzyme. There is no any report about the influence of light, in fungal incubation period, on NR enzyme activity and nanoparticle formation. Although studies showed that the NR enzyme of plants can be increased in the presence of light (Ali et al. Citation2011).
Comparing the NR enzyme activities in cell-free filtrates of fungus grown in MGYP and modified media verified that the presence of nitrate source cause to increased secretion of NR enzyme in cell-free filtrates which is in agreement with previous report (Raudabaugh et al. Citation2013). The intensities of these spectra () steadily increased as a function of time, indicating the formation of increased number of AgNPs in solutions. The absorption intensity of the spectra obtained from AgNPs generated using the cell-free filtrate of fungus incubated in modified medium is considerably higher than that obtained using cell-free filtrate of MGYP medium, which confirms the higher productivity of AgNP biosynthesis using the cell-free filtrate originated from modified medium. This may be attributed to the higher secreted enzymes, acting as reducing agents, in the fungal filtrate originated from modified medium. The presence of a symmetrical plasmon band in UV–visible spectra can be attributed to the narrower size distribution of the produced particles (Hamedi et al. Citation2012). Comparing the plasmon bands in reveals that the size distribution of AgNPs formed using cell-free filtrate of fungus incubated in modified culture medium was narrower than that those synthesized using the filtrates of fungal cells incubated in MGYP medium. As indicated in , the smaller AgNPs with narrower size distribution were generated using cell-free filtrate originated from incubated fungal cells in modified medium. This could be due to the higher secretion of proteins, especially nitrate reductase enzymes, in the presence of potassium nitrate.
Based on the DLS results, the smallest nanoparticles were obtained using cell-free filtrate of fungus that cultured in modified medium. SEM image of produced AgNPs in this case () verified that the most of the nanoparticles are spherical.
A comparison of absorbance intensities under various conditions () reveals that using the filtrates of submerged fungal cells in 50 mM potassium nitrate and 2 mM ammonium sulfate solutions produce AgNPs with the highest and the lowest monodispersity, respectively. As shown in , the highest and lowest plasmon resonance peaks were obtained for the AgNPs produced using the filtrates of biomass submerged in potassium nitrate (50 mM) and ammonium sulfate (2 mM) solutions, respectively. Therefore, using filtrates of fungal cells suspended in 50 mM potassium nitrate and 2 mM ammonium nitrate solutions led to the formation of the smallest and largest AgNPs, respectively. The stability of AgNPs synthesized was assessed by measuring the absorbance intensities and λmax of the spectra of the reaction solutions over a period of 1 month at room temperature. The absorbance intensities of synthesized nanoparticles using filtrates of fungal cells suspended in two solutions of ammonium sulfate and ammonium nitrate decreased with no significant changes in maximum wavelengths (data not shown). No significant changes in the absorbance intensity and λmax of the colloidal AgNPs prepared using three others were observed during this period. These observations verified that the colloidal AgNPs prepared using filtrates of fungal cells submerged in distilled water and potassium nitrate solutions were extremely more stable than those filtrates obtained by submerged fungal cells in ammonium sulfate and ammonium nitrate solutions.
As shown in , MIC value of AgNPs synthesized using cell-free filtrate of fungus is 11.11 mg/L. Also, the AgNPs with concentration 5.55 mg/L caused to delay the log phase of bacterial growth. In many researches, the antibacterial mechanism of AgNPs is attributed to the penetrating of AgNPs inside the cell membrane and interacting of them with sulfur and phosphorous compounds present within the cell membrane, which caused to inhibition of DNA replication and consequently apoptosis/lysis of bacterial cells (Singh et al. Citation2015, Wang et al. Citation2016).
Conclusion
The controlled synthesis of AgNPs using cell-free filtrate of F. oxysporum was evaluated. By considering parameters of type of carbon and nitrogen sources, cultivation temperature, and presence or absence of light during cell growth on AgNP formation, results showed that the nanoparticle formation increases at the stationary phase which is likely due to the higher secretion of extracellular enzymes, especially NR enzyme. Temperature control of fungal growth at 28 °C can lead to the formation of desirable AgNP size. The presence of glucose in MGYP medium, nitrogen source in modified medium, and presence of light also gives similar results. The smallest AgNPs with the highest productivity was achieved in the case of fungal cultivation in modified medium that the NR enzyme exhibited maximum activity.
Hamedi_et_al._supplemental_material.tif
Download TIFF Image (232.1 KB)Disclosure statement
The authors report that they have no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Ajitha B, Ashok Kumar Reddy Y, Shameer S, Rajesh KM, Suneetha Y, Sreedhara Reddy P. 2015. Lantana camara leaf extract mediated silver nanoparticles: antibacterial, green catalyst. J Photochem Photobiol B: Biol. 149:84–92.
- Ali A, Sivakami S, Raghuram N. 2011. Effect of light, nitrate, and tungstate on the activity of nitrate reductase in rice. Res Dimensions 1:47–55.
- Baker S, Mohan Kumar K, Santosh P, Rakshith D, Satish S. 2015. Extracellular synthesis of silver nanoparticles by novel Pseudomonas veronii AS41G inhabiting Annona squamosa L. and their bactericidal activity. Spectrochim Acta: Part a. 136:1434–1440.
- Bala M, Arya V. 2013. Biological synthesis of silver nanoparticles from aqueous extract of endophytic fungus Aspergillus fumigatus and its antibacterial action. Int J Nanomater Biostruct. 3:37–41.
- Balakumaran MD, Ramachandran R, Balashanmugam P, Mukeshkumar DJ, Kalaichelvan PT. 2016. Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol Res. 182:8–20.
- Balakumaran MD, Ramachandran R, Kalaichelvan PT. 2015. Exploitation of endophytic fungus, Guignardia mangiferae for extracellular synthesis of silver nanoparticles and their in vitro biological activities. Microbiol Res. 178:9–17.
- Du J, Singh H, Yi T-H. 2016. Biosynthesis of silver nanoparticles by Novosphingobium sp. THG-C3 and their antimicrobial potential. Artif Cells Nanomed Biotechnol. 1–7. doi:10.1080/21691401.2016.1178135.
- Ghaseminezhad SM, Hamedi S, Shojaosadati SA. 2012. Green synthesis of silver nanoparticles by a novel method: comparative study of their properties. Carbohydr Polym. 89:467–472.
- Hamedi S, Ghaseminezhad SM, Shojaosadati SA, Soheila S. 2012. Comparative study on silver nanoparticles properties produced by green methods. Iran J Biotechnol. 10:191–197.
- Hamedi S, Shojaosadati SA, Shokrollahzadeh S, Hashemi-Najafabadi S. 2014. Extracellular biosynthesis of silver nanoparticles using a novel and non-pathogenic fungus, Neurospora intermedia: controlled synthesis and antibacterial activity. World J Microbiol Biotechnol. 30:693–704.
- Honary S, Barabadi H, Gharaei-Fathabad E, Naghibi F. 2013. Green synthesis of silver nanoparticles induced by the fungus Penicillium citrinum. Trop J Pharm Res. 12:7–11.
- Ibrahim HMM. 2015. Green synthesis and characterization of silver nanoparticles using banana peel extract and their antimicrobial activity against representative microorganisms. J Radiat Res Appl Sci. 8:265–275.
- Jaidev L, Narasimha G. 2010. Fungal mediated biosynthesis of silver nanoparticles, characterization and antimicrobial activity. Colloids Surf B Biointerfaces. 81:430–433.
- Jain N, Bhargava A, Majumdar S, Tarafdar JC, Panwar J. 2010. Extracellular biosynthesis and characterization of silver nanoparticles using Aspergillus flavus NJP08: a mechanism perspective. Nanoscale 3:635–641.
- Kejlová K, Kašpárková V, Krsek D, Jírová D, Kolářová H, Dvořáková M, Tománková K, Mikulcová V. 2015. Characteristics of silver nanoparticles in vehicles for biological applications. Int J Pharm. 496:878–885.
- Khatami M, Mehnipor R, Poor MHS, Jouzani GS. 2016. Facile biosynthesis of silver nanoparticles using Descurainia sophia and evaluation of their antibacterial and antifungal properties. J Clust Sci. 27:1601–1612.
- Khosravi A, Shojaosadati SA. 2009. Evaluation of silver nanoparticles produced by fungus Fusarium oxysporum. Int J Nanotechnol. 6:973–983.
- Krupa AND, Abigail MEA, Santhosh C, Grace AN, Vimala R. 2016. Optimization of process parameters for the microbial synthesis of silver nanoparticles using 3-level Box–Behnken Design. Ecol Eng. 87:168–174.
- Maliszewska I, Juraszek A, Bielska K. 2014. Green synthesis and characterization of silver nanoparticles using Ascomycota Fungi Penicillium nalgiovense AJ12. J Clust Sci. 25:989–1004.
- Mittal AK, Tripathy D, Choudhary A, Aili PK, Chatterjee A, Singh IP, Banerjee UC. 2015. Bio-synthesis of silver nanoparticles using Potentilla fulgens Wall. ex Hook. and its therapeutic evaluation as anticancer and antimicrobial agent. Mater Sci Eng C Mater Biol Appl. 53:120–127.
- Mohammadian A, Shojaosadati SA, Rezaee MH. 2007. Fusarium oxysporum mediates photogeneration of silver nanoparticles. Sci Iran. 14:323–326.
- Nayak D, Pradhan S, Ashe S, Rauta PR, Nayak B. 2015. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci. 457:329–338.
- Pourali P, Yahyaei B. 2016. Biological production of silver nanoparticles by soil isolated bacteria and preliminary study of their cytotoxicity and cutaneous wound healing efficiency in rat. J Trace Elem Med Biol. 34:22–31.
- Raudabaugh DB, Tzolov MB, Calabrese JP, Overton BE. 2013. Synthesis of silver nanoparticles by a Bryophilous rhizoctonia species. Nanomater Nanotechnol. 3:1–6.
- Sadhasivam S, Shanmugam P, Yun K. 2010. Biosynthesis of silver nanoparticles by Streptomyces hygroscopicus and antimicrobial activity against medically important pathogenic microorganisms. Colloids Surf B Biointerfaces 81:358–362.
- Shanthi S, David Jayaseelan B, Velusamy P, Vijayakumar S, Chih CT, Vaseeharan B. 2016. Biosynthesis of silver nanoparticles using a probiotic Bacillus licheniformis Dahb1 and their antibiofilm activity and toxicity effects in Ceriodaphnia cornuta. Microb Pathog. 93:70–77.
- Singh P, Kim YJ, Wang C, Mathiyalagan R, Yang DC. 2015. Weissella oryzae DC6-facilitated green synthesis of silver nanoparticles and their antimicrobial potential. Artif Cells Nanomed Biotechnol. 44:1–7.
- Soni N, Prakash S. 2011. Factors affecting the geometry of silver nanoparticles synthesis in Chrysosporium tropicum and Fusarium oxysporum. Am J Nanosci. 2:112–121.
- Vaidyanathan R, Gopalram S, Kalishwaralal K, Deepak V, Pandian SR, Gurunathan S. 2010. Enhanced silver nanoparticle synthesis by optimization of nitrate reductase activity. Colloids Surf B Biointerfaces 75:335–341.
- Verma DK, Hasan SH, Banik RM. 2016. Photo-catalyzed and phyto-mediated rapid green synthesis of silver nanoparticles using herbal extract of Salvinia molesta and its antimicrobial efficacy. J Photochem Photobiol B: Biol. 155:51–59.
- Vigneshwaran N, Ashtaputre NM, Varadarajan PV, Nachane RP, Paralikar KM, Balasubramanya RH. 2007. Biological synthesis of silver nanoparticles using the fungus Aspergillus flavus. Mater Lett. 61:1413–1418.
- Wang C, Kim YJ, Singh P, Mathiyalagan R, Jin Y, Yang DC. 2016. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif Cells Nanomed Biotechnol. 44:1127–1132.