Abstract
Hemerythrin is an oxygen-carrying protein found in marine invertebrates and may be a promising alternative to hemoglobin for use in blood substitutes, primarily due to its negligible peroxidative toxicity. Previous studies have shown that glutaraldehyde-induced copolymerization of hemoglobin with bovine serum albumin increases the half-life of the active oxy form of hemoglobin (i.e. decreases the auto-oxidation rate). Here, we describe a protocol for glutaraldehyde copolymerization of Hr with human serum albumin and the dioxygen-binding properties of the co-polymerized products. The copolymerization with HSA results in alteration of hemerythrin’s dioxygen-binding properties in directions that may be favorable for use in blood substitutes.
Introduction
Hemerythrin (Hr) plays the role of dioxygen transporter in marine invertebrates and uses a non-heme diiron active site (Farmer et al. Citation2000, Jin et al. Citation2002, Kryatov et al. Citation2005). The mostly known and best characterized Hr, isolated from Phascolopsis gouldii, is a 108-kDa, octameric protein with four-helix bundle subunits surrounding the eight diiron sites (Farmer et al. Citation2000). Hr diiron sites can exist in (1) the diferrous deoxy form, (2) the diferric oxy form with an oxygen molecule bound to one of the FeIII as hydroperoxide, and (3) the diferric met form which is incapable of dioxygen binding. In addition, a so-called semi-met, FeII FeIII form is known to exist, although its role is obscure (Mot et al. Citation2010, Nocek et al. Citation1984). Differences in UV–vis absorption spectral properties of these forms make possible the evaluation of dioxygen affinity (deoxy → oxy transformation) and auto-oxidation rate (oxy → met transformation, an unavoidable inactivation with time).
Previous studies have reported Hr as a promising alternative to Hb-based blood substitutes, due primarily to the relative inertness of Hr towards oxidative and nitrosative stress agents – hydrogen peroxide, nitric oxide, and nitrite (Kryatov et al. Citation2005, Mot et al. Citation2010). In contrast, Hb-based artificial oxygen carriers (chemical derivatives or encapsulations of Hb (Alayash Citation2004, Chang Citation2004, Citation2009, Tsuchida et al. Citation2009)) participate in undesirable redox side reactions (Alayash Citation2004, Olson et al. Citation2004, Reeder et al. Citation2008) which severely compromises its functioning as an effective blood substitute. Moreover, toxicological tests on human cell cultures and lymphocytes suggest that Hr and its PEGylated and polymerized chemical derivatives have proliferative and cytoprotective effects, whereas GL-polymerized bovine Hb does not (Fischer-Fodor et al. Citation2011).
Chemical derivatizations of Hbs have typically aimed at limiting extravasation and lowering antigenicity, by increasing hydrodynamic volume through polymerization with bifunctional reagents and attachment of PEG-units (Alayash Citation2004, Chang Citation2009, Winslow Citation2004). PEGylation and dialdehyde-cross-linking of Hr were described as being efficacious for developing Hr-based blood substitutes, but these chemical modifications did not totally eliminate the relatively high auto-oxidation rate and oxygen affinity of native Hr (compared with Hb) (Mot et al. Citation2010). For example, GL polymerization of Hr shifted the average molecular weight towards a more optimal region, but led to increased dioxygen affinity, and the native auto-oxidation rate remained unchanged. PEGylation decreased the dioxygen affinity, but led to a higher auto-oxidation rate and a tendency towards subunit dissociation.
Copolymerization of Hb with BSA has been shown to significantly reduce the auto-oxidation rate compared with polyHb (Iacob et al. Citation2011). Copolymerization of Hr with albumin could, thus, conceivably reduce the pro/peroxidant activity of Hr as well. Using HSA for copolymerization could also decrease immunogenicity of Hr.
Materials and methods
Overexpression and purification of recombinant P. gouldii Hr was performed as described in previous studies (Farmer et al. Citation2000, Mot et al. Citation2010, Tomita et al. Citation2013), with slight modifications. BL21 (DE3) Escherichia coli cells, suitable for transformation and protein expression were subjected to heat shock with pDK4–1 plasmid containing the gene of P. gouldii Hr. Transformed cells, resistant to ampicillin, were selected on LB/amp agar plates. A single colony was grown overnight in 50 ml LB/amp medium. This pre-culture was then transferred in 1 L of LB/amp medium and incubated at 37 °C with shaking at 250 rpm. When suitable cell density was reached (OD600 ∼1, approximately 2.5 h), expression was induced with 0.4 mM isopropyl-β-d-thiogalactoside. Cells were further cultured for 5–8 h, then separated by centrifugation, resuspended in a minimal volume of Tris/NaCl and lysed by sonication. A vigorous centrifugation succeeded and the pellet, containing apoHr in the form of inclusion bodies, was dissolved overnight in ∼12 ml GndCl/β-ME. A subsequent centrifugation and the dilution of guanidinic supernatant with a 10-fold volume of Tris/NaCl which was added dropwise resulted in renatured, insoluble apoHr of white color. The suspension was centrifuged, the pellet was redissolved in ∼12 ml of Gnd/β-ME, and the apoHr was renatured again, by very slow addition (over ∼6–10 h) of 10× volume of deoxygenated Tris/NaCl, in the presence of ∼0.1 g of ferrous ammonium sulfate, under anaerobic conditions and continuous stirring, at 4 °C. In this way, soluble deoxyHr was obtained, along with unincorporated FeII and insoluble apoHr. The apoHr was removed by centrifugation, the unicorporated FeII was oxidized by ∼1 h stirring in air, and the insoluble FeIII was removed by dialysis. Finally, excess GndCl/β-ME was removed by dialysis against Tris/NaCl. After these steps, the presence of Hr (mainly in its oxy form) could be verified by its characteristic UV–vis absorption. The Hr was converted to its more stable met form by the addition of a few crystals of potassium ferricyanide and overnight stirring at 4 °C. Ferrocyanide and excess ferricyanide were removed by dialysis against PBS, and the protein was concentrated in an Amicon ultrafiltration unit (Millipore Corp., Billerica, MA). If the UV–vis absorption spectrum of the resulting metHr indicated the presence of any residual ferricyanide (A330 nm/A380 nm absorbance ratio >1.2), then the protein solution was passed over a PD-10 desalting column (GE Healthcare, Chalfont, UK). The A280 nm/A380 nm absorbance ratio of the final product was ≈ 5.8, which is lower by one unit compared with the material used in our previous report on derivatized hemerythrin (Mot et al. Citation2010), suggesting a slightly higher purity. The average yield of recombinant metHr was ∼40 mg protein/L of culture medium. Purified metHr was stored in PBS at −20 °C.
(Co)polymerization reactions were carried out in PBS at a 0.15 mM constant concentration of metHr (protein monomer concentration basis, determined spectrophotometrically with ɛ330 = 6400 M−1⋅cm−1 (1)). A 0.75 mM HSA stock solution was made by dissolving 0.05 g HSA (Sigma-Aldrich, Chicago, IL) in 950 μl PBS. If necessary, successive dilutions of this stock solution were used. GL-cross-linking presumed the reaction of the two (–CHO) carbonyl groups of GL with the (–NH2) amino groups of the proteins. The concentration of lysines was estimated as the molar concentration of protein multiplied by the number of lysine residues/(monomeric) unit (11 in case of Hr, 59 in case of HSA, see ). The relative lysine concentrations of Hr:HSA in copolymerization mixtures were chosen to be within the range of 5–0.25.
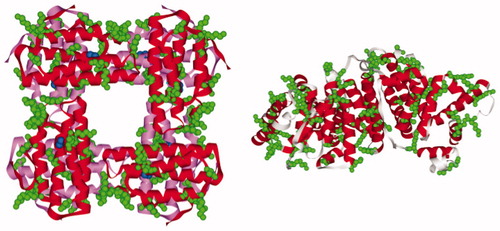
Figure 1. The three-dimensional structure of the P. gouldii Hr (PDB ID: 1I4Y) and HSA (PDB ID: 1GNJ); lysine residues are colored in green; the two layers of four subunits of Hr octamer are colored in red and pink, respectively; di-iron centers are represented as blue spheres.
GL stock solutions at 100 mM were prepared from a 25% (2.65 M) solution (Sigma-Aldrich, Chicago, IL). The concentrations of GL used for cross-linking were between 2 and 10 mM. The steps of derivatization were very similar to those described in a previous study (Mot et al. Citation2010). GL-cross-linking lasted for 2 h at 4 °C with continuous stirring. The reaction was stopped by addition of a 2-fold molar excess (with respect to GL concentration) of sodium borohydride from a freshly prepared, 1 M stock solution (or dilutions made from it). Twenty minutes after the addition of the borohydride, 1/15 volume of 1 M Tris-HCl, pH 7.4 was added, and the solution was allowed to react for a further 20 min. Removal of excess reagents and/or buffer exchange was accomplished using either a PD-10 desalting column (GE Healthcare, Chalfont, UK), or dilution-reconcentration steps (Amicon Ultra-4 or -15 Centrifugal Filter Devices, Millipore Corp., Billerica, MA).
Electrophoresis was carried out in 15% polyacrylamide gels at 200 V limiting voltage and 25 mA limiting current. Spectroscopic measurements were performed on a Cary 50 (Varian, Inc., Palo Alto, CA) UV–vis spectrophotometer. For gel filtration size exclusion chromatography analyses, an FPLC system controlled, SuperdexTM 200 5/150 GL column (GE Healthcare, Chalfont, UK) was used with Tris/NaCl as the elution buffer. Anion exchange chromatography was performed on an 1 ml HiTrap Q FF column (GE Healthcare, Chalfont, UK) using a 20 mM Tris-HCl, pH 7.5 buffer as the mobile phase and a gradient of 20 column volumes was applied to elute the components (20 mM Tris, 1 M NaCl, pH 7.5 elution buffer).
The dioxygen affinity and auto-oxidation rate measurements of the underivatized and (co)polymerized Hrs were carried out in PBS solution at room temperature and must have been preceded by the reduction in Tris/NaCl of diferric met form to deoxyHr under anaerobic conditions, in the presence of sodium dithionite. In order to avoid the dilution of protein solution during the anaerobic dialysis step, the protocol used at this stage was slightly different from that previously described (Mot et al. Citation2010). A 10-fold molar excess (with respect to Hr concentration) of sodium dithionite from a concentrated stock solution in Tris/NaCl was added via a Hamilton microsyringe to a deoxygenated ∼0.3 mM metHr solution at 4 °C. After 48 h, excess dithionite and any protein precipitate were removed by the passage of solutions through Micro Bio-Spin Columns (Bio-Rad Laboratories, Hercules, CA) that had been filled with Sephadex G-25 Medium from PD-10 Desalting Columns (GE Healthcare, Chalfont, UK). For the conversion of the resulting oxyHr into deoxyHr, the protein solution was added to an anaerobic cuvette and purged with a mild flow of argon until the UV–vis absorption spectrum became constant and characteristic of deoxyHr.
Estimation of oxygen affinities and auto-oxidation rates was based on UV–vis absorption spectroscopy measurements, using previously described procedures with only slight modifications (Mot et al. Citation2010, Nocek et al. Citation1984).
A FEI Tecnai F20 field emission, high-resolution transmission Electron Microscope (TEM) operating at an accelerating voltage of 200 kV and equipped with Eagle 4 k CCD camera was employed to obtain the TEM images. Proteins of 5 mg/mL were deposited on carbon film-coated Cu grids. The PBS samples tended to precipitate with the staining material so that these were dialyzed in MOPS buffer solution (10 mM, pH 7.4) which offers a uniform distribution of the clusters on the grid surface (Tomita et al. Citation2013).
Results and discussion
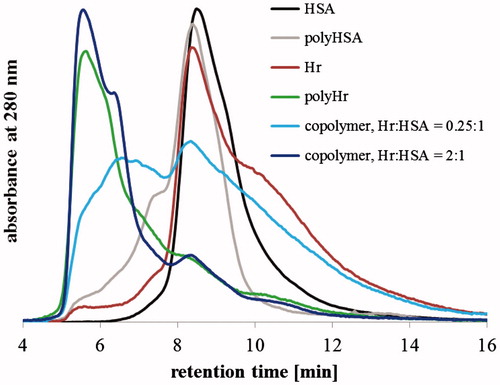
In preliminary experiments, copolymerization at a wide range of [Hr]Lys:[HSA]Lys ratios was monitored via SDS-PAGE (see and ). For a particular ratio of lysine concentrations, the band of copolymer and the band belonging to the mix of polymers are not the same, indicating that copolymerization did occur. To ensure that GL would not be a limiting reactant in this preliminary screening of protein ratios, a relatively high concentration of GL (8 mM) was used.
Figure 2. 15% SDS-PAGE gel of the reaction mixtures of copolyHr-HSA (lanes a) and of polyHr + polyHSA (lanes b) at 0.15 mM Hr, 8 mM GL, and varying HSA concentrations (listed in ).
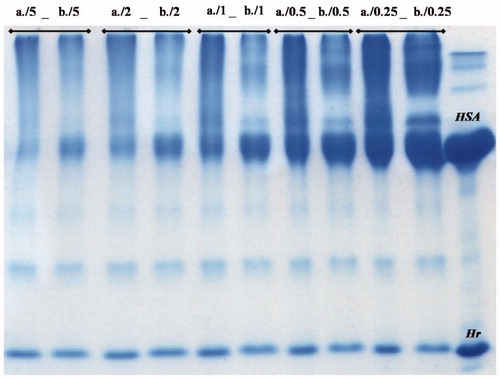
Table 1 . Protein and lysine residue concentrations in the GL-polymerization reaction mixtures subjected to the 15% SDS-PAGE as shown in Figure 1. Hr concentration was kept constant at 150 μM (monomer basis) in all reaction mixtures.
Two of these copolymers, those with [Hr]Lys:[HSA]Lys = 2:1 and 0.25:1, were further investigated via gel filtration chromatography. Chromatograms co-polymerized samples with 6 mM GL () revealed the formation of high(er) molecular weight aggregates and a difference between the two copolymer mixtures: for the [Hr]Lys:[HSA]Lys = 2:1 sample, the molecular weight distribution of products situates closer to polyHr than that for [Hr]Lys:[HSA]Lys = 0.25:1 sample, where species of high and lower molecular weights are in approximately the same proportions. Based on this observation, [Hr]Lys:[HSA]Lys = 2:1 was chosen for further experiments. This ratio corresponds to a [Hr]: HSA] ≈ 10:1 molar ratio (per monomers) and 2.2:1 mass ratio.
Next, the effect of 2–10 mM range of GL concentration was analyzed using SDS-PAGE, UV–vis absorption spectroscopy, and gel filtration chromatography methods. Under denaturing conditions, the relative quantity of monomeric, dimeric, and trimeric Hr can serve as a surrogate for the degree of co-polymerization. Based on this criterion, the SDS-PAGE in shows that co-polymerization occurs even at 2 mM GL. Furthermore, in the case of co-polymers, the relative intensity of higher molecular weights (above 67 kDa of HSA) is increased compared with the intensity of the mixture of polymers, which is indicative of covalently linked Hr-HSA species formed during the copolymerization. Additional evidence for covalent Hr-HSA polymers is that the quantity of unreacted HSA is always higher in the individual HSA polymer reaction than in the copolymer mixture. Gel filtration chromatograms (see ) also indicated an increasing degree of (co)polymerization as the GL concentration was raised. Based on chromatograms, a nearly total consumption of low molecular weight units can be approximated to occur at ≥6 mM GL, where only low levels of unpolymerized Hr were observed; most of the derivatized Hr eluted in the void volume of the column (molecular weight >1300 kDa). In contrast, ≤4 mM GL-co-polymerized mixtures retained a significant portion of unpolymerized protein.
Figure 4. 15% SDS-PAGE of GL-copolymerized Hr-HSA (lanes a) and mixtures of separately GL-polymerized Hr and HSA (lanes b); reaction conditions: 0.15 mM Hr, [Hr]Lys: [HSA]Lys = 2:1 (∼14 μM HSA), GL concentrations varying from 2 to 10 mM as indicated by the number in the labels above the lanes.
![Figure 4. 15% SDS-PAGE of GL-copolymerized Hr-HSA (lanes a) and mixtures of separately GL-polymerized Hr and HSA (lanes b); reaction conditions: 0.15 mM Hr, [Hr]Lys: [HSA]Lys = 2:1 (∼14 μM HSA), GL concentrations varying from 2 to 10 mM as indicated by the number in the labels above the lanes.](/cms/asset/adc9dd67-c8d7-4956-94c7-da360896717a/ianb_a_1269118_f0004_c.jpg)
Figure 5. Gel filtration chromatograms of Hr-HSA copolymers; reaction conditions: 0.15 mM Hr, [Hr]Lys:[HSA]Lys = 2:1 (∼14 μM HSA), GL in concentrations varying from 2 to 10 mM.
![Figure 5. Gel filtration chromatograms of Hr-HSA copolymers; reaction conditions: 0.15 mM Hr, [Hr]Lys:[HSA]Lys = 2:1 (∼14 μM HSA), GL in concentrations varying from 2 to 10 mM.](/cms/asset/033f3828-479b-470f-83a2-4f5bf6790bdb/ianb_a_1269118_f0005_c.jpg)
In order to qualify spectral alterations produced by co-polymerization, all spectra recorded were anchored at two points – 800 and 310 nm (see ). Examining the bands that correspond to the diferric site of metHr (300–450 nm region), a tendency of flattening can be noticed. This tendency becomes enhanced as the GL concentration increased, but was always stronger for polyHrs than for copolymers. At the highest GL concentrations (8 and 10 mM), the polymerized Hr totally lost its spectral characteristics, indicating significant iron loss and/or drastic di-iron site structural alterations (Farmer et al. Citation2000, Farmer et al. Citation2001, Mot et al. Citation2010), whereas copolymers with the same GL concentration retain the bands at 330 and 380 nm () .
Figure 6. UV–vis absorption spectra of Hr-HSA (co)polymerization mixtures; reaction conditions: 0.15 mM Hr, [Hr]Lys:[HSA]Lys = 2:1 (∼14 μM HSA), GL in concentrations varying from 2 to 10 mM (note: neither HSA nor polyHSA absorb in the visible region of the spectrum).
![Figure 6. UV–vis absorption spectra of Hr-HSA (co)polymerization mixtures; reaction conditions: 0.15 mM Hr, [Hr]Lys:[HSA]Lys = 2:1 (∼14 μM HSA), GL in concentrations varying from 2 to 10 mM (note: neither HSA nor polyHSA absorb in the visible region of the spectrum).](/cms/asset/6dab540a-01fe-435f-ad8b-c7031379fc6b/ianb_a_1269118_f0006_c.jpg)
Figure 7. Anion-exchange (left panel) and gel filtration size exclusion (right panel) chromatograms of GL-polymerized Hr and GL-co-polymerized Hr-HSA; reaction conditions: 0.15 mM Hr, [Hr]Lys: [HSA]Lys = 2:1 (∼14 μM HSA), GL concentrations varying from 2 to 6 mM. A, B, and C labels in the left panel correspond to fractions A, B, and C in the right panel.
![Figure 7. Anion-exchange (left panel) and gel filtration size exclusion (right panel) chromatograms of GL-polymerized Hr and GL-co-polymerized Hr-HSA; reaction conditions: 0.15 mM Hr, [Hr]Lys: [HSA]Lys = 2:1 (∼14 μM HSA), GL concentrations varying from 2 to 6 mM. A, B, and C labels in the left panel correspond to fractions A, B, and C in the right panel.](/cms/asset/fe6fa19e-b75e-46b4-b821-e5dcf10ea56b/ianb_a_1269118_f0007_c.jpg)
Efforts to separate the various oligomers in the co-polymerized mixtures by anion exchange or gel filtration chromatographies in the amounts required for functional measurements were unsuccessful (≈10% yield for 2 mM GL-derivatized copolymer). Therefore, dioxygen affinity and auto-oxidation rate measurements were made on the co-polymerized mixtures without further separation of the various protein components.
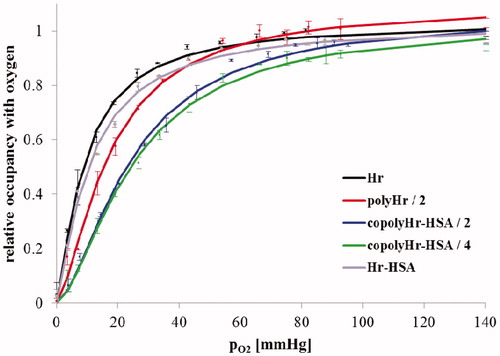
The (de)oxy form of 6 mM GL-polymerized Hr was very unstable; most of it precipitated and was, therefore, removed when transformed into its oxy form (during de desalting/filtration step). UV–vis absorption spectra of the oxy form of the co-polymers were different from the spectra of underivatized Hr (). The characteristic band at 500 nm is so much altered in the case of 4 mM GL polymerized Hr and 6 mM GL-copolymerized Hr-HSA (becoming a shoulder rather than a peak) that it was considered unreliable for quantitative determinations of dioxygen affinity and auto-oxidation rates. Estimated values of the P50 and t1/2 parameters of the unmodified Hr, GL-polymerized Hr, and the rest of the co-polymers are represented in .
Figure 8. UV–vis absorption spectra of the oxy forms of Hr, GL-polymerized Hr, and co-polymerized Hr-HSA.
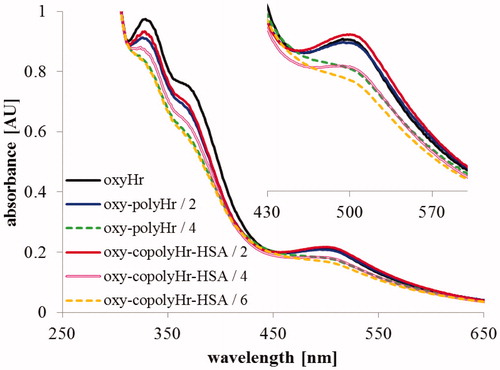
Table 2. Estimated values of dioxygen affinity (P50) and half-life of auto-oxidation (t1/2) of unmodified and GL-derivatized Hrs and Hr-HSA GL-copolymers; reaction conditions: 0.15 mM Hr, [Hr]Lys:[HSA]Lys = 2:1 (∼14 μM HSA), GL in concentrations as indicated (2 or 4 mM).
The P50 values in indicate that co-polymerization with HSA significantly reduces the dioxygen affinity of Hr. also shows that co-polymerization induces a slight co-operativity in dioxygen binding. The copolymerization with HSA increased the auto-oxidation rate of Hr. However, even for the 4 mM GL-copolymerized HSA-Hr, the auto-oxidation half-life was determined to be ∼5 h.
Transmission electron microscopy (TEM) images () show a significant difference between the native and the derivatized Hr, in terms of size and shape. The dimensions of the protein aggregates increase with the derivatization but more so with copolymerization. shows particles with sizes ranging from ∼15 nm for nHr to ∼45 nm for copolyHr-HSA (Figure S1), and to less uniform particles in case of polyHr. The degree to which these differences may translate into advantages or disadvantages in vivo remains to be explored.
Conclusions and prospects
Our results show that co-polymerization of Hr with HSA modified Hr’s dioxygen-binding properties in a direction generally considered to be favorable for use in blood substitutes. Copolymerization of Hr lowered its dioxygen affinity while only modestly increasing its auto-oxidation rate. The co-polymerization also induced a small degree of apparent co-operativity in dioxygen binding to Hr. Dioxygen-binding co-operativity is an inherent property of Hb but not of native Hr (Farmer et al. Citation2000, Kurtz Citation1999). Our results, thus, suggest that Hr/HSA copolymers could be a promising alternative to Hb as an artificial oxygen carrier, particularly since HSA is likely to reduce antigenicity of Hr. An engineered substitution of a dioxygen-binding pocket residue was previously shown to lower Hr’s dioxygen affinity and auto-oxidation rate (Farmer et al. Citation2000, Farmer et al. Citation2001). Copolymerization of this Hr pocket residue variant with HSA could further improve the functionality of Hr for its use in blood substitutes.
| Abbreviations | ||
| BSA | = | bovine serum albumin |
| GL | = | glutaraldehyde |
| GndCl/β-ME | = | a mixture of 6 M guanidine hydrochloride/β-mercaptoethanol (6 ml/100 μl) |
| Hb | = | hemoglobin |
| Hr | = | hemerythrin |
| HSA | = | human serum albumin |
| LB/amp | = | Luria-Bertani medium containing 100 mg/L ampicillin |
| PBS | = | phosphate buffered saline (137 mM NaCl, 2.7 mM KCl, 12 mM NaH2PO4, pH 7.4) |
| SDS–PAGE | = | sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| Tris/NaCl | = | 20 mM Tris-HCl, 150 mM NaCl, pH 7.4 buffer |
Supporting_Information__HrHSA.docx
Download MS Word (4.8 MB)Acknowledgements
Funding from the The Romanian Ministry of Education and Research (Grants PN09-440213 and PN-II-ID-PCE-2012-4-0488) is gratefully acknowledged. F. S. thanks the “Babes-Bolyai” University for the financial support via the Sectoral Operational Programme for Human Resources Development 2007–2013, co-financed by the European Social Fund, under the project POSDRU/159/1.5/S/132400 with the title “Young successful researchers – professional development in an international and interdisciplinary environment”. M. A. thanks the Babeş-Bolyai University for a student research scholarship. Augustin Mot and Denisa Hathazi (BBU, Department of Chemistry) are thanked for helpful discussions.
Disclosure statement
The authors declare no conflict of interest.
Additional information
Funding
References
- Alayash AI. 2004. Oxygen therapeutics: can we tame haemoglobin? Nat Rev Drug Discov. 3:152–159.
- Bassan A, Blomberg MRA, Siegbahn PEM. 2004. A theoretical study of the cis-dihydroxylation mechanism in naphthalene 1,2-dioxygenase. J Biol Inorg Chem. 9:439–452.
- Chang TMS. 2004. Hemoglobin-based red blood cell substitutes. Artif Organs. 28:789–794.
- Chang TMS. 2009. Nanobiotechnology for hemoglobin-based blood substitutes. Crit Care Clin. 25:373–382.
- Cooper CE, Silaghi-Dumitrescu R, Rukengwa M, Alayash AI, Buehler PW. 2008. Peroxidase activity of hemoglobin towards ascorbate and urate: a synergistic protective strategy against toxicity of Hemoglobin-Based Oxygen Carriers (HBOC). Biochim Biophys Acta 1784:1415–1420.
- Eike JH, Palmer AF. 2004. Effect of Cl- and H + on the oxygen binding properties of glutaraldehyde-polymerized bovine hemoglobin-based blood substitutes. Biotechnol Prog. 20:1543–1549.
- Farmer CS, Kurtz DM, Liu ZJ, Wang BC, Rose J, Ai J, et al. 2001. The crystal structures of Phascolopsis gouldii wild type and L98Y methemerythrins: structural and functional alterations of the O2 binding pocket. J Biol Inorg Chem. 6:418–429.
- Farmer CS, Kurtz DM, Phillips RS, Ai J, Sanders-Loehr J. 2000. A leucine residue “gates” solvent but not O2 access to the binding pocket of Phascolopsis gouldii hemerythrin. J Biol Chem. 275:17043–17050.
- Fischer-Fodor E, Mot A, Deac F, Arkosi M, Silaghi-Dumitrescu R. 2011. Towards hemerythrin-based blood substitutes: comparative performance to hemoglobin on human leukocytes and umbilical vein endothelial cells. J Biosci. 36:215–221.
- Herold S, Exner M, Nauser T. 2001. Kinetic and mechanistic studies of the NO-mediated oxidation of oxymyoglobin and oxyhemoglobin. Biochemistry 40:3385–3395.
- Herold S. 1999. Kinetic and spectroscopic characterization of an intermediate peroxynitrite complex in the nitrogen monoxide induced oxidation of oxyhemoglobin. FEBS Lett. 443:81–84.
- Iacob B, Deac F, Cioloboc D, Damian G, Silaghi-Dumitrescu R. 2011. Hemoglobin-albumin crosslinked copolymers: reduced prooxidant reactivity. Artif Cells Blood Substit Immobil Biotechnol. 39:293–297.
- Jin S, Kurtz DM, Liu ZJ, Rose J, Wang BC. 2002. X-ray crystal structures of reduced rubrerythrin and its azide adduct: a structure-based mechanism for a non-heme diiron peroxidase. J Am Chem Soc. 124:9845–9855.
- Kryatov SV, Rybak-Akimova EV, Schindler S. 2005. Kinetics and mechanisms of formation and reactivity of non-heme iron oxygen intermediates. Chem Rev. 105:2175–2226.
- Kurtz DM. 1999. Oxygen-carrying proteins: three solutions to a common problem. Essays Biochem. 34:85–100.
- Mot AC, Roman A, Lupan I, Kurtz DM, Silaghi-Dumitrescu R. 2010. Towards the development of hemerythrin-based blood substitutes. Protein J. 29:387–393.
- Nocek JM, Kurtz DM, Pickering RA, Doyle MP. 1984. Oxidation of deoxyhemerythrin to semi-methemerythrin by nitrite. J Biol Chem. 259:12334–12338.
- Olson JS, Foley EW, Rogge C, Tsai AL, Doyle MP, Lemon DD. 2004. NO scavenging and the hypertensive effect of hemoglobin-based blood substitutes. Free Radic Biol Med. 36:685–697.
- Reeder BJ, Grey M, Silaghi-Dumitrescu RL, Svistunenko DA, Bulow L, Cooper CE, et al. 2008. Tyrosine residues as redox cofactors in human hemoglobin: implications for engineering nontoxic blood substitutes. J Biol Chem. 283:30780–30787.
- Silaghi-Dumitrescu R, Silaghi-Dumitrescu I. 2006. DFT and the electromerism in complexes of iron with diatomic ligands. J Inorg Biochem. 100:161–166.
- Tomita D, Kimura T, Hosaka H, Daijima Y, Haruki R, Ludwig K, et al. 2013. Covalent core-shell architecture of hemoglobin and human serum albumin as an artificial O2 carrier. Biomacromolecules 14:1816–1825.
- Tsuchida E, Sou K, Nakagawa A, Sakai H, Komatsu T, Kobayashi K. 2009. Artificial oxygen carriers, hemoglobin vesicles and albumin-hemes, based on bioconjugate chemistry. Bioconjug Chem. 20:1419–1440.
- Winslow RM. 2004. MP4, a new nonvasoactive polyethylene glycol-hemoglobin conjugate. Artif Organs. 28:800–806.