?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In this study, extracellular metabolites of symbiotic bacteria Xenorhabdus stockiae (KT835471) was employed for the synthesis of silver (XsAgNPs) and gold nanoparticles (XsAuNPs). Synthesized NPs were characterized using high throughput instrumentation which confirms the generation of stable, crystalline XsAgNPs and XsAuNPs with the mean size of 14 ± 6 and 14 ± 5, respectively. Further, the NPs exhibits an excellent bactericidal effect against six different pathogens. On the other hand, NPs displayed an outstanding anticancer activity against human lung adenocarcinoma epithelial cells (A549). Therefore, the present study strongly suggests that metal NPs encrusted with functional bio-moieties can be used for different biomedical applications.
Introduction
Nanoparticles (NPs) can be synthesized via physical, chemical and biological routes. Among them, biogenic synthesis of NPs has received much attention due to their improved and interesting physiochemical properties. Bacteria, fungi, yeasts and plants either intra or extracellularly offers higher yields of nanoparticles with low expenses (Kathiresan et al. Citation2009). Intracellularly synthesized NPs require additional downstream processing steps such as ultrasound treatment, exposure to detergents to release the NPs from cells during their purification. Nevertheless, the NPs synthesized through extracellular method were economical with simpler downstream processing as compared to intracellular synthesis (Kalimuthu et al. Citation2008). Diverse bacterial species isolated from various ecological conditions such as Actinobacter sp., Escherichia coli, Klebsiella pneumonia, Lactobacillus sp., Bacillus cereus, Corynebacterium sp., and Pseudomonas sp. were explored to synthesis different metallic nanoparticles (Iravani Citation2014, Sunkar and Nachiyar Citation2012).
However, there was no clear evidence on reduction mechanism of biogenic nanoparticles generation, it was reported that the Pseudomonas stutzeri AG259 bacterium produced nanosilver particles via a mechanism involving the NADH-dependent reductase enzyme that donates an electron and oxidises to NAD+. The electron transfer results in biological reduction of Ag+ ions to AgNPs. In a similar study, Husseiny et al. (Citation2007) were able to reduce Au ions using P. aeruginosa that results in the extracellular synthesis of AuNPs. The bactericidal effect of AgNPs is due to their small size and high surface area to volume ratio, which allows them to interact closely with microbial membrane. Smaller particles with a larger surface area have effective antibacterial activities. For instance, NPs such as AgNPs and AuNPs have been shown to be an effective antibacterial agent against both Gram-positive and Gram-negative bacteria. In addition to antibacterial, anti-cancerous activities of NPs also have also been well established in recent times (Kim et al. Citation2007, Kuo et al. Citation2009). The physiochemical properties of AuNPs are highly size and shape dependent, which has directed too many biomedical applications (Sperling et al. Citation2008).
AuNPs have been extensively used in biomedical applications (Bhattacharya and Murkherjee Citation2008, Cai et al. Citation2008), separation sciences, disease diagnostics, and pharmaceuticals (Bhumkar et al. Citation2007), AgNPs have been found to possess both anti-bacterial and anti-inflammatory properties that can promote faster wound healing. Because of these advantageous properties, AgNPs have been integrated into commercially available wound dressings, pharmaceutical preparations and medical implant coatings (Li et al. Citation2011).
In this study, we firstly demonstrated the extracellular synthesis of XsAgNPs and XsAuNPs using an indigenous entomopathogenic bacteria Xenorhabdus stockiae KT835471 belongs to Enterobacteriacea which is almost found in mutualistic symbiosis with insect-parasitic nematodes, the S. siamyakai (Akhurst and Boemare Citation1988, Poinar and Thomas Citation1966). This study was mainly focused to assess the nanobiotechnological potential of isolated X. stockiae KT835471 to fabricate metal NPs and further the nano-products were evaluated against A549 human lung adenocarcinoma epithelial cell lines and antimicrobial properties against the six bacterial pathogens towards biomedical prospective.
Materials and methods
Materials
Silver nitrate (AgNO3) and Choloroauric acid (HAuCl4) were purchased from Himedia Laboratories. Double-distilled water was used in all experiments. Six bacterial pathogenic genera Escherichia coli (MTCC-443), Enterococcus faecalis (MTCC-3159), Staphylococcus aureus (MTCC-96), Bacillus cereus (MTCC-1272), Proteus vulgaris (MTCC-425) and Bacillus subtilis (MTCC-2387) were obtained from Microbial Type Culture Collection and Gene Bank, Chandigarh, India.
Isolation of symbiotic bacteria from EPNs
The S. siamyakai was cultured in final instar larvae of Galleria mellonella and incubated at 20 °C in a BOD incubator. The primary stages of symbiotic bacteria X. stockiae was extracted from a pool of freshly emerged infective juveniles (IJs) of S. siamyakai, which was disinfected by dipping them in a 10% sodium hypochlorite solution for 10 min followed by rinsing in sterile distilled water for three times. Secondary stages were isolated from monogenic cultures established with S. siamyakai. The surface-sterilized IJs were destroyed by vortexing and a loop full of suspension was streaked on NBTA (nutrient agar with 0.004% 2, 3, 5-triphenyltetrazolium chloride and 0.025% bromothymol blue) agar plates according to Akhurst (Citation1980). Single colonies were selected and sub-cultured onto new NBTA and this process was repeated until pure colonies of X. stockiae were obtained. Finally, the culture was maintained by glycerol stock at −70 °C.
Molecular identification of X. stockiae KT835471
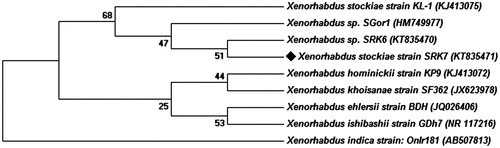
The X. stockiae KT835471 symbiotic bacterial genomic DNA was extracted by Tailliez method. The small subunit (16s) of rRNA was amplified by following primers with both the sense and antisense directions using forward primer indicated as: 16S 20 (5′ AGA GTT TGA TCC TGG CTC 3′) and Reverse primer indicates as: 16S 1390 (5′ GAC GGG CGG TGT GTA CAA 3′). Further, the sequencing was performed by Eurofins Genomics India Pvt. Ltd, Bangalore. BLAST search and multiple sequence alignment was done using CLUSTAL W (Slow/Accurate, IUB) program. Phylogenetic tree was built by the neighbor-joining method and tree topologies were evaluated by performing bootstrap analysis of 1000 data sets using MEGA 6.0 and submitted in the GenBank database.
Optimization and synthesis of XsAgNPs and XsAuNPs
The starting materials were AgNO3 and Chloroauric acid (HAuCl4). Symbiotic bacteria X. stockiae was cultured for 48 h, at 28 ± 2 °C with shaking in a 1 L Erlenmeyer flask containing 500 ml of LB broth. The isolated bacterial strain was screened for its competency to reduce metal ions such as Ag+ and Au3+ into their nano-scale values. Based on the preliminary studies X. stockiae KT835471 was selected as potential strain to synthesis of nanomaterials. Briefly, the strain was inoculated freshly in an Erlenmeyer flask containing LB broth and incubated at 37 °C for 24 h. After incubation period, the culture supernatant was obtained by centrifugation at 6000 rpm for 10 min. Then reaction mixtures for the synthesis of XsAgNPs and XsAuNPs were prepared by blending 10 ml of cell-free supernatant with 90 ml of 1 mM AgNO3 and (1 mM) HAuCl4, respectively, in a 250 ml Erlenmeyer flask and incubated at 30 °C for 24 h. Subsequently, the bioreduction reaction was monitored by visual color change and UV–Vis absorbance of the reaction mixture. Further, to attain narrow size distribution of NPs with maximum yield, different reaction conditions such as pH (5–9), concentration of metal ions (1 mM–5 mM) were optimized based on the intensity of absorbance spectra in UV–Vis spectroscopy.
Instrumentation
The optical characteristics of synthesized NPs were analyzed using UV-visible spectrophotometer, at the wavelength of 300–700 nm using JASCO V-650 UV-Visible Spectrophotometer. FT-IR spectroscopic studies were carried out to study the surface chemistry and role of bacterial proteins in reduction and stabilization of NPs. The wavelength spectrum of the cell free supernatant before and after addition to metal ion solution AgNO3 and HAuCl4, the samples were mixed with KBr powder and pelletized after drying the spectra were recorded using Perkin Elmer make model spectrum 22575927 RX1 (Wavelength range between 4000 cm−1 and 400 cm−1). Size distribution and surface charges of synthesized NPs were measured using dynamic light scattering (DLS) and zeta potential analysis. To perform DLS the colloidal NPs solution was further diluted and allowed for sonication 10–20 min to disperse the particles, size distribution measurements and zeta values were obtained using Malvern Zetasizer, Nano-ZS90 analyzer. The morphology, size and diffraction pattern of XsAgNPs and XsAuNPs were measured at different magnification at 100Kev JEOL JEM 2100 high resolution transmission electron microscope. Energy dispersive electron spectroscopy (EDAX) analysis was performed along with HRTEM analysis to assess the purity and stability of NPs. X-ray diffraction was performed to determine the dimension of biologically synthesized XsAgNPs and XsAuNPs with h, k, l values. The diffraction pattern was obtained with conditions at 40 kV and 30 mA in Cu, K-alpha radiation and particles size (L) using (PAN analytical X pert PRO Model) of the Ag and Au was calculated using following Debye-Scherrrer’s equation.
where, λ is the wavelength of the X-ray, β is full width and half maximum and θ is the Bragg’s angle.
Antibacterial activity of XsAgNPs and XsAuNPs
Six human pathogenic bacterial strains such as E. coli (MTCC-443), E. faecalis (MTCC-3159), S. aureus (MTCC-96), B. cereus (MTCC-1272), P. vulgaris (MTCC-425) and B. subtilis (MTCC-2387) were obtained from Microbial Type Culture Collection and Gene Bank, Chandigarh, India. The antibacterial activity of synthesized XsAgNPs and XsAuNPs were determined by well diffusion method. Therefore, six bacterial pathogens E. coli, E. faecalis, S. aureus, B. cereus, P. vulgaris and B. subtilis were sub-cultured. An inoculum of fresh overnight bacterial cultures were spread onto separate sterilized nutrient agar plates, XsAgNPs and XsAuNPs were added into the well of agar plates and incubated at 37 °C for 24 h. The zone of inhibition formed around the well was measured using meter ruler and the results were expressed as mean values (±SD) of three replicates.
In vitro anticancer activity
Cell viability
MTT assay was used to measure the cell viability as a function of redox potential. Actively breathing cells convert the water-soluble MTT to an insoluble purple formazan (Mosmann Citation1983). The formazan is then solubilized and its concentration determined by optical density. The cells were seeded in 96 well flat bottom tissue culture plates at a density of approximately 1.2 × 104 cells per well and allowed to attach overnight at 37° C. The medium was then discarded and cells were incubated with different concentrations (0, 10, 25, 50, 100 and 250 μg/ml) of the NPs containing fresh medium for 24 h. After incubation, the medium was discarded and 100 μl fresh medium was added with 10 μl of MTT (5 mg/ml). After 4 h, the medium was discarded and 100 μl of DMSO was added to dissolve the formazan crystals. Then, the absorbance was read at 570 nm in a microtiter plate reader. Cell viability was calculated by the following formula:
AO/EtBr staining
An acridine orange/ethidium bromide (AO/EtBr) staining method was adopted to detect XsAgNPs and XsAuNPs-induced apoptosis in A549 cells. Briefly, after the treatment of with desired IC50 concentration of NPs the cells were fixed in 3:1 ratio of methanol and glacial acetic acid for 1 h at room temperature. The cells were labelled with 1:1 ratio of AO and EtBr in PBS and incubated for 5 min then the excess unbinding dye was removed by washing with PBS. Stained cells were visualized under UV illumination using the 40x objective (Nikon 80i Eclipse, Japan) and the digitized images were captured.
DNA fragmentation
DNA fragmentation assay was also adopted to assess NPs triggered DNA damage in A549 cells. For that cells were harvested after 24 h of the treatment with NPs at their desired IC50 concertation. To isolate DNA fragments, harvested cells were counted and washed with 1X PBS at 4 °C. Further the cells were pelleted by centrifugation at 200 × g at 4 °C and suspended in DNA lysis buffer (1 M Tris (pH 8.0), 0.5 EDTA and 75% sodium lauryl sarcosine) and incubated overnight with proteinase K (0.5 mg/ml) at 50 °C. After overnight incubation, RNase (50 μg/ml) was added and again incubated for 1 h at 50 °C. Finally DNA was extracted using phenol: chloroform (1:1) and then, electrophoresed in 2% agarose gel for 2 h at 50 V. The gel was stained with ethidium bromide (0.5 μg/ml) and photographed in Gel Doc™ XR + (Bio-Rad, CA, USA).
Results and discussion
Molecular characterization and synthesis of XsAgNPs and XsAuNPs
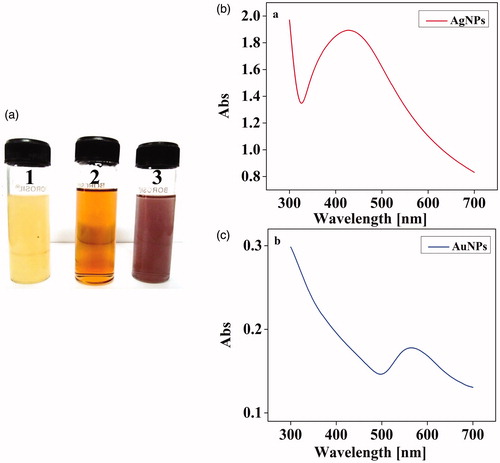
After isolation and initial morphometric authentication, the phylogenetic tree was constructed and the species was grouped into particular genus clade (). Molecular authentication confirmed that the isolated strain was X. stockiae KT835471 as compared with other species in phylogenetic tree. Further X. stockiae KT835471 was extended for nanomaterial preparation at desired structure. The extracellular products of X. stockiae KT835471 were employed for sustainable green synthesis of XsAgNPs and XsAuNPs. After the addition of extracellular components to the metal ions (1 mM AgNO3 and 1 mM HAuCl4) the reaction mixtures were shown color change due to the excitation of surface plasmon resonance (SPR) (AbdelRahim et al. Citation2016); XsAgNPs exhibited dark brownish color after 24 h, whereas XsAuNPs showed pink color within 24 h of incubation ().
Physio-chemical characterization of NPs
UV- visible spectroscopy
Interestingly, the UV-visible spectroscopic analysis have showed an intense SPR peak at 430 nm for XsAgNPs and 560 nm for XsAuNPs (). The synthesized particles were highly stable at 37 °C ± 2. In order to sustain the reproducibility, optimal conditions to synthesis narrow size distributed NPs within shorter period was fixed as pH (9 for AgNPs and 6 for AuNPs), concentration of metal ion (1 mM for both AgNPs and AuNPs) and incubation time 24 hrs (Figure S1 a1&b1 and a2&b2). The NPs synthesized at the optimal conditions have generated a strong resonance centered at about 430 nm and 560 nm and the intensity was getting increased with incubation time. The results observed for the culture extract of X. stockiae is a highly significant of developing a prompt method for XsAgNPs. Earlier reports have shown that the formation of AgNPs by a strong and broad peak between 430 nm and 450 nm after 72 h of incubation period by utilizing P. aeruginosa (Husseiny et al. 2013). In the present study a similar peak for XsAgNPs was observed at 430 nm after 24 h of incubation period. Similarly, studies on biosynthesis of AuNPs using S. maltophilia has proposed an absorbance spectra at 530 nm (Nangia et al. Citation2009) whereas, we found a sharp shift in peak maxima with a maximum absorbance at 560 nm. Colloidal solutions of XsAuNPs shows intense color due to SPR arising from united oscillation of permitted conduction electrons encouraged by an interrelating electromagnetic field. Proteins and amino acid residues in proteins such as cysteine, tyrosine and tryptophan are reported to play an important role in biosynthesis and stabilization of XsAuNPs. Free amino or cysteine groups in proteins can bind to the Au ions and thus, surface-bound proteins can stabilize them (Mishra et al. Citation2012, Sastry et al. Citation2003).
FT-IR analysis
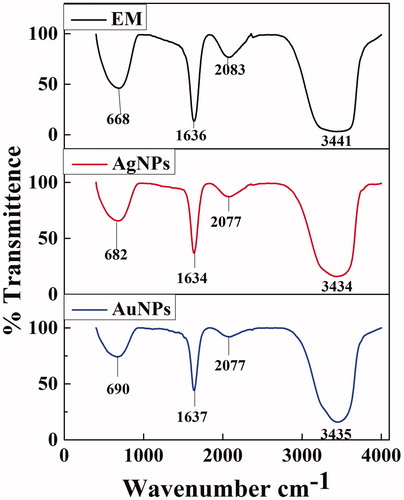
FT-IR analysis was performed to ascertain the involvement of possible biomolecule responsible for reduction and stabilization of NPs. The IR spectrum of XsAgNPs manifests prominent transmittance located at 3441, 2083, 1636, and 668 cm−1. In the IR spectrum of XsAuNPs the intense bands are observed at 3434, 2077, 1634 and 682 cm−1. The strong bands at 1640 cm−1 and 1639 cm−1 corresponds to the –C = C– stretches and transmittance at 3441 cm −1 and 3434 cm −1 indicates the –N-H– stretches (). Comparing the resulting spectrum between bacterial and synthesized XsAgNPs a major shift was observed in 3379 to 3435 cm −1 the amine functional groups that mainly contribute the synthesis of XsAgNPs and XsAuNPs. It was observed from the FT-IR spectrum of XsAgNPs that the bands at 1639 cm−1 corresponds to primary amine C–N stretches vibrations of the proteins, respectively (Gole et al. Citation2001). The amide linkages between amino acid residues in proteins give rise to well-known signs in the infrared region of electromagnetic spectrum. The bands seen at 3418 cm−1 were assigned to the stretching vibrations of primary and secondary amines, respectively. As compared with the FTIR transmittance of extracellular metabolites, synthesized NPs shows variation in the stretches mainly due the interaction of metal ions with bacterial proteins (Vignesh et al. Citation2015)
XRD
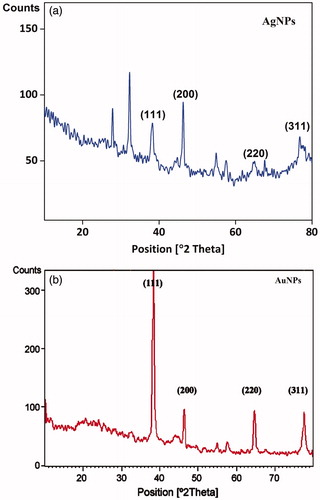
XRD pattern of XsAgNPs and XsAuNPs were interpreted with JCPDS intensities, after reduction the diffraction peaks at 2θ = 38.03θ, 46.18θ and 63.43θ were indexed as (111) (200) and (220) planes highly matches with the standard (JCPDS) file No. 04–0783 of a faced centered cubic (fcc) lattice of silver (Ag°) (). The XRD patterns displayed here are consistent with earlier reports (Shankar et al. Citation2003). Likewise, for AuNPs the XRD peak corresponding to three peaks (JCPDS file No. 04–0784) at (38.17°) (44.36°) and (64.65°) which are found to be an identical with those reported for the standard gold metal (Au°) (). The mean size of AgNPs and AuNPs was calculated using Debye–Scherrer’s equation by determining the width of (111) peak and found to be 8 and 6 nm, respectively, which is fairly in agreement with the TEM measurement. XRD results suggested that, narrow-ranged sizes of nanoparticles agreed with the SPR results (Philip and Unni Citation2011).
HRTEM
Size and morphology of nanoparticles were characterized in TEM micrograph which shows fine configuration of spherical XsAgNPs in size ranges between 10 and 30 nm with an average size of 14 ± 6 nm (). Interestingly TEM micrograph of gold colloids displays both spherical, ovoid and triangle NPs in the size range between 10 and 30 nm with an average size of 14 ± 5 nm (). EDAX analysis shows a strong signal for Ag and Au, respectively ( and ), the cu signal is due the interference of copper grid used for the study. It was also found that both the XsAgNPs and XsAuNPs having a thin layer of biomolecule on its surface, particles were polydispersed and stable for long period of time. TEM data obtained at different magnifications represents the polymorphism with respect to shape and size of XsAgNPs and XsAuNPs.
Figure 5. (a) HRTEM micrographs displays well-distributed spherical AgNPs with an average size of 14 ± 6 nm (b) EDAX analysis gives a strong signal for Ag and (c) Particle size distribution of XsAgNPs.
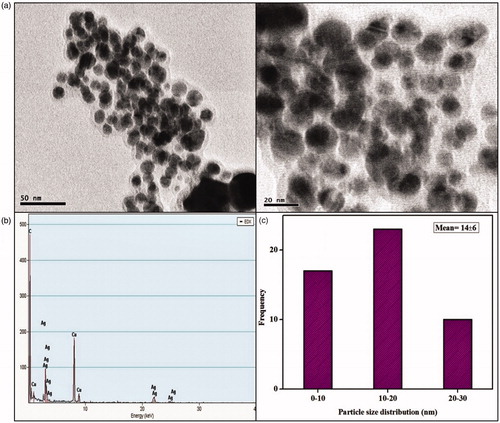
Figure 6. (a) HRTEM micrographs displays well-distributed spherical and triangle AuNPs with an average size of 14 ± 5 nm (b) EDAX analysis gives a strong signal for Au and (c) Particle size distribution of XsAuNPs.
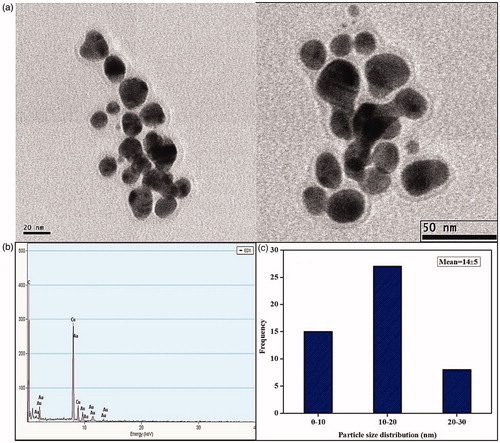
Similar phenomenon has been observed in few earlier studies where the K. pneumonia produces AgNPs in the range from 28.2 to 122 nm and possess an average size of 52.5 nm (Murdock et al. Citation2008). Through exploiting the R. capsulata, spherical AuNPs with 10–20 nm range have been observed at lower metal ion concentration (Shanshoury et al. Citation2011).
DLS (dynamic light scattering)
The hydrodynamic diameter of XsAgNPs 149.6 nm and XsAuNPs 144 nm were evaluated by DLS which confirms the particle size distribution, respectively (Figure S2a & Figure S3a), their corresponding zeta potential value −42.8 mV and −38.6 mV is suggesting high stability of NPs (S2b, Figure S3b). The large negative potential value could be due to the capping agent, which generate repulsive forces between the NPs (Balakumaran et al. Citation2016).
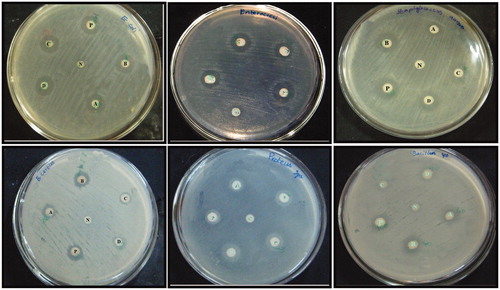
Antibacterial assay
Different dosages of XsAgNPs and XsAuNPs (20 μl and 40 μl) were tested against various human bacterial pathogens such as E. coli, E. faecalis, S. aureus, B. cereus, P. vulgaris and B. subtilis. In well diffusion method, XsAgNPs exhibits comparatively higher inhibitory effect of than XsAuNPs (). Maximum bactericidal effect was found against E. coli, followed by E. faecalis in case of 40 μl of XsAgNPs and XsAuNPs, respectively. The remaining bacterial pathogen such as S. aureus, B. cereus, P. vulgaris and B. subtilis shows moderate inhibitory action against NPs (Table S1). It was clearly demonstrated that AgNPs has the ability to inactivate microorganisms by interacting with their enzymes, proteins, or DNA to obstruct cell proliferation (Amit Kumar et al. Citation2012). The increased antimicrobial activity of AgNPs may be accredited to its distinct characteristics of small size and high surface area to volume ratio. Ag+ are among the most potent and rapidly acting antimicrobial agents (Chernousova and Epple Citation2013, Eckhardt et al. Citation2013). There are different mechanisms of action of AgNPs has been proposed, firstly AgNPs getting attached to the surface of cell membrane and disturb its power of functions, such as permeability and respiration. On the other hand, plasmolysis (cytoplasm separated from bacterial cell wall) in P. aeruginosa and the inhibition of bacterial cell wall synthesis in S. aureus bacteria were reported by Asharani et al. (Citation2009). More excitingly, Guzman et al. (Citation2012) have reported that the AgNPs are able to penetrate the bacteria and cause further damage, possibly by interacting with phosphorus-containing compounds such as DNA. Finally, NPs are capable of binding to cells as well as macromolecules like proteins and DNA. When NPs come in contact with cells, they are taken up by a variety of mechanisms that can lead to activation of cellular signaling processes producing ROS, inflammation and finally cell cycle arrest or cell death (Ahmad et al. Citation2010).
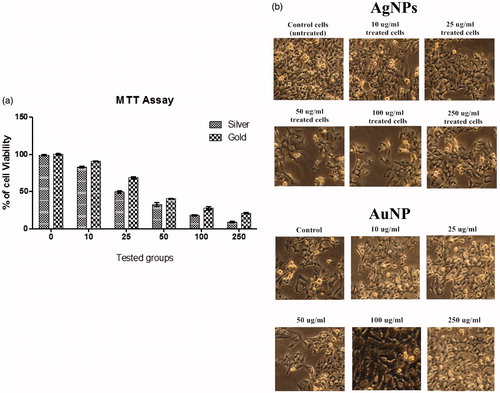
Cell viability (MTT assay)
Cell viability assay describes the cellular response to a toxic material, also it will provide information about cell death, survival and metabolic activities (Rani et al. Citation2009). In this study the cytotoxicity effects of synthesized NPs were evaluated against A549 cell lines and it was noticed that XsAgNPs was more potent than that of XsAuNPs in cellular toxicity against treated A549 cells. Data were collected four replicates and the mean and the standard deviation values were calculated consequently; 50% (IC50) inhibitory concentration of XsAgNPs were fixed to be 29.4 μg/ml and 49.8 μg/ml for XsAuNPs against A549 cells (). As described previously by various investigators, the toxicity of AgNPs could be based on the available exposed surface for reaction with the cell, which increases with decreasing NP size.
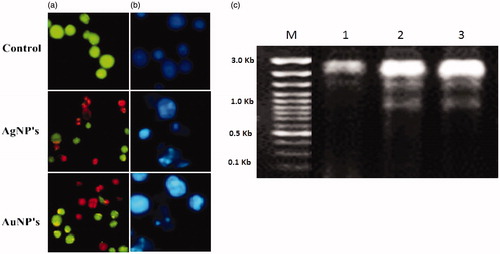
Measurement of apoptosis
The apoptosis studies of NPs on the A549 cells were confirmed in AO/EB-staining method. The apoptosis was noticed in the treated cells with morphological changes and chromatin condensation (). Fluorescence microscopic observations evident that the viable (light green), early apoptotic (bright green fluorescence), late apoptotic cells (orange fluorescence) and nonviable cells (red-colored fluorescence). <AQ6> Also treated NPs on A549 cells were noticed condensed nuclei, membrane blabbing and apoptotic bodies of nuclear morphology compared in the untreated control cells remain intact (). The induction of apoptosis is confirmed by shrunken cells and DNA fragmentation (Lima et al. Citation2012, Sriram et al. Citation2010). Any kind of hindrances to DNA leads the activation of signal transduction pathways and results in apoptosis or cause interventions with regular cellular manners thereby causing cell death. The XsAgNPs and XsAuNPs have induced apoptosis in treated A549 cells through ROS-mediated DNA damage (). Collectively, these observations confirmed that NPs has triggered the cell death through apoptotic pathway (Jeyaraj et al. Citation2015).
Conclusion
Xenorhabdus stockiae KT835471 extracellular metabolites have been successfully used as potential reducing and stabilizing agent of XsAgNPs and XsAuNPs formation with narrow size-range particles and superior physiochemical properties. The nano-products were highly-stable, crystalline in nature with an average size of 14 ± 6 and 14 ± 5 nm, respectively. Antimicrobial activity of synthesized NPs demonstrated a tremendous dose-dependent inhibitory activity against all the tested bacterial pathogens. Similarly, NPs have displayed stupendous anticancer activity (IC50–29.4 μg/ml and IC50–49.8 μg/ml) against A549 human lung adenocarcinoma epithelial cells. The overall results suggest that green synthesized NPs can be used for as a potential drug delivery system and also for value added therapy.
Chandrakasan_et_al._supplemental_content.doc
Download MS Word (1.2 MB)Acknowledgements
This work was supported by the Rajiv Gandhi National Fellowship.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
Additional information
Funding
References
- AbdelRahim K, Mahmoud SY, Ali AM, Almaary KS, Mustafa AE, Husseiny SM. 2016. Extracellular biosynthesis of silver nanoparticles using Rhizopus stolonifer. Saudi J Biol Sci. 24:208–216. Available from: http://dx.doi.org/10.1016/j.sjbs.2016.02.025
- Ahmad N, Sharma S, Alam MK, Singh VN, Shamsi SF, Mehta BR, Fatma A. 2010. Rapid synthesis of silver nanoparticles using dried medicinal plant of basil. Colloids Surf B Biointerfaces. 81:81–86.
- Akhurst R. 1980. Morphological and functional dimorphism in Xenorhabdus spp., bacteria symbiotically associated with the insect pathogenic nematodes Neoaplectana and Heterorhabditis. Microbiology. 121:303–309.
- Akhurst RJ, Boemare NE. 1988. A numerical taxonomic study of the genus Xenorhabdus (Enterobacteriaceae) and proposed elevation of the subspecies of X. nematophilus to species. J Gen Microbiol. 134:1835–1845.
- Amit Kumar M, Abhishek K, Uttam Chand B. 2012. Free radical scavenging and antioxidant activity of silver nanoparticles synthesized from flower extract of Rhododendron dauricum. Nano Biomed Eng. 41:18–124.
- Asharani PV, Hande MP, Valiyaveettil S. 2009. Anti-proliferative activity of silver nanoparticles. BMC Cell Biol, 10:65.
- Balakumaran MD, Ramachandran R, Balashanmugam P, Mukeshkumar DJ, Kalaichelva PT. 2016. Mycosynthesis of silver and gold nanoparticles: optimization, characterization and antimicrobial activity against human pathogens. Microbiol Res. 182:8–20.
- Bhattacharya R, Murkherjee P. 2008. Biological properties of “naked” metal nanoparticles. Adv Drug Deliv Rev. 60:1284–1306.
- Bhumkar DR, Joshi HM, Sastry M, Pokharkar VB. 2007. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 24:1415–1426.
- Cai W, Gao T, Hong H, Sun J. 2008. Application of gold nanoparticles in cancer nanotechnology. Nanotechnol Sci Appl. 1:17–32.
- Chernousova S, Epple M. 2013. Silver as antibacterial agent: ion, nanoparticle, and metal. Angew Chem Int Ed Engl. 52:1636–1653.
- Eckhardt S, Brunetto PS, Gagnon J, Priebe M, Giese B, Fromm KM. 2013. Nanobio silver: its interactions with peptides and bacteria, and its uses in medicine. Chem Rev. 113:4708–4754.
- Gole A, Dash C, Ramakrishnan V, Sainkar SR, Mandale AB, Rao M. 2001. Pepsin-gold colloid conjugates: preparation, characterization, and enzymatic activity. Langmuir. 17:1674–1679.
- Guzman M, Dille J, Godet S. 2012. Synthesis and antibacterial activity of silver nanoparticles against gram-positive and gram-negative bacteria. Nanomedicine. 8:37–45.
- Husseiny MI, El-Aziz MA, Badr Y, Mahmoud MA. 2007. Biosynthesis of gold nanoparticles using Pseudomonas aeruginosa. Spectrochim Acta A Mol Biomol Spectrosc. 67:1003–1006.
- Iravani S. 2014. Xenorhabdus stockiae KT835471-mediated feasible biosynthesis of metal nanoparticles for their antibacterial and cytotoxic activities. Int Sch Res Notices. 2014:1–18.
- Jeyaraj M, Renganathan A, Sathishkumar G, Ganapathi A, Premkumar K. 2015. Biogenic metal nanoformulations induce Bax/Bcl2 and caspase mediated mitochondrial dysfunction in human breast cancer cells (MCF 7). RSC Advances. 5:2159–2166.
- Kalimuthu K, Babu RS, Venkataraman D, Bilal M, Gurunathan S. 2008. Biosynthesis of silver nanocrystals by Bacillus licheniformis. Colloids Surf B Biointerfaces. 65:150–153.
- Kathiresan K, Manivannan S, Nabeel MA, Dhivya B. 2009. Studies on silver nanoparticles synthesized by a marine fungus, Penicillium fellutanum isolated from coastal mangrove sediment. Colloids Surf B Biointerfaces. 71:133–137.
- Kim JS, Kuk E, Yu KN, Kim JH, Park SJ, Lee HJ, et al. 2007. Antimicrobial effects of silver nanoparticles. Nanomedicine. 3:95–101.
- Kuo WS, Chang CN, Chang YT, Yeh CS. 2009. Antimicrobial gold nanorods with dual-modality photodynamic inactivation and hyperthermia. Chem Commun. 32:4853–4855.
- Li WR, Xie XB, Shi QS, Duan SS, Ouyang YS, Chen YB. 2011. Antibacterial effect of silver nanoparticles on Staphylococcus aureus. Biometals. 24:135–141.
- Lima R, Seabra AB, Duran N. 2012. Silver nanoparticles: a brief review of cytotoxicity and genotoxicity of chemically and biogenically synthesized nanoparticles. J Appl Toxicol. 32:867–879.
- Mishra A, Tripathy SK, Yun S. 2012. Fungus mediated synthesis of gold nanoparticles and their conjugation with genomic DNA isolated from Escherichia coli and Staphylococcus aureus. Process Biochem. 47:701–711.
- Mosmann T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 65:55–63.
- Murdock RC, Braydich-Stolle L, Schrand AM, Schlage JJ, Hussain SM. 2008. Characterization of nanomaterial dispersion in solution prior to in vitro exposure using dynamic light scattering technique. Toxicol Sci. 101:239.
- Nangia Y, Wangoo N, Goyal N, Shekhawat G, Raman Suri C. 2009. A novel bacterial isolate Stenotrophomonas maltophilia as living factory for synthesis of gold nanoparticles. Microb Cell Fact. 8:52.
- Philip D, Unni C. 2011. Extra cellular biosynthesis of gold and silver nanoparticles using Krishna tulsi (Ocimum sanctum) leaf. Phys E. 43:1318–1322.
- Poinar GO, Thomas GM. 1966. Significance of Achromobacter nematophilus Poinar, Thomas (Achromobacteraceae: Eubacteriales) in the development of the nematode, DD-136 (Neoaplectana sp. Steinernematidae). Parasitology. 56:85–390.
- Rani PVA, Mun GLK, Hande MP, Valiyaveettil S. 2009. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 3:279–290.
- Sastry M, Ahmed A, Khan MI, Kumar R. 2003. Biosynthesis of metal nanoparticles using fungi and actinomycetes. Curr Sci. 85:162.
- Shankar SS, Ahmad A, Sastry M. 2003. Geranium leaf assisted biosynthesis of silver nanoparticles. Biotechnol. Prog. 19:1627–1631.
- Shanshoury REL, Elsilk SE, Ebeid ME. 2011. Extracellular biosynthesis of silver nanoparticle using Escherichia coli ATCC, 8739, Bacillus subtilis ATCC, 6633, and Streptococcus thermophilus ESh1 and their antimicrobial activity. ISRN Nanotechnology. 11:1–7.
- Sperling RA, Gil PR, Zhang F, Zanella M, Parak WJ. 2008. Biological applications of gold nanoparticles. Chem. Soc. Rev. 37:1896–1908.
- Sriram MI, Kanth SB, Kalishwaralal K, Gurunathan S. 2010. Antitumor activity of silver nanoparticles in Dalton's lymphoma ascites tumor model. Int J Nanomed. 5:753–762.
- Sunkar S, Nachiyar CV. 2012. Biogenesis of antibacterial silver nanoparticles using the endophytic bacterium Bacillus cereus isolated from Garcinia xanthochymus. Asian Pac J Trop Biomed. 2:953–959.
- Vignesh V, Sathiyanarayanan G, Sathishkumar G, Parthiban K, SathishKumar K, Thirumurugan R. 2015. Formulation of iron oxide nanoparticles using exopolysaccharide: evaluation of their antibacterial and anticancer activities. RSC Adv. 35:27794–27804.