Abstract
Present investigation deals with, tacrolimus eluting, self-expandable, biodegradable stent fabricated by solvent casting method. The design was based on shape memory polymers, which possess the ability to memorize temporary shape that can substantially differ from their initial permanent shape. A set of biodegradable polymers blend was used such as poly-lactic acid (PLA) and poly-l-glycolic acid (PLGA) to study the shape memory effect of polymer. The prepared stent was assessed for various parameters like Scanning Electron Microscopy (SEM), In-vitro and Ex vivo expansion, Drug content, In-vitro drug release, Haemocompatibility, Differential Scanning Calorimetry (DSC), Fourier Transform Infrared spectroscopy (FTIR), and Textural Characterization.
Introduction
From last few decades bare metal stent is standard protocol and broadly practiced suggested for percutaneous coronary intervention (PCI) includes in cardiac diseases. It is generally observed that, by the use of bare metal stent during PCI can create injury to the blood vessel (Lee et al. Citation2007) and adverse reactions such as delayed endothelialization as well as late thrombosis (Grabow et al. Citation2007), restenosis (Tamai et al. Citation1999; Uurto et al. Citation2003, Citation2005) were reported.
According to recent research, bare metallic stent remained in position may create hindrance, which required an additional treatment (e.g. repeat angioplasty and bypass surgery) (Tamai et al. Citation1999) and further contribute to inflammation (Su et al. Citation2003). A number of studies have addressed to avoid the problems related with bare metal stent by using several modified techniques such as biocompatible material (Raval et al. Citation2007; Shin et al. Citation2009; Uurto et al. Citation2004), drug eluting coating (Bian et al. Citation2012; Mangual et al. Citation2010; Pendyala et al. Citation2012; Shin et al. Citation2009), biodegradable stents (Mangual et al. Citation2010), helical-coiled stents (Bartkowiak-Jowsa et al. Citation2011; Meng et al. Citation2006) and radioactive stents (Hehrlein et al. Citation1995).
A new PLLA (poly‐l‐lactic Acid) coil stent was implanted in porcine coronary arteries, and evaluated further to check possible clinical use (Tamai et al. Citation1999). PLLA-based stent self-expanded preliminary showed that coiled shaped stent regain its original shape in 0.2 s heated by 70 °C, 13 s by 50 °C and 20 min by 37 °C from expanded one.
Expansion study of polymeric stent is generally performed by balloon inflation study with dye heated to 80 °C, which ensures adequate stent expansion heated by almost 50 °C. The balloon inflation study results correlate with the gradually expansion of stent after deployment in-vivo. To defeat the poor compatibility of metallic stent, comprehensive efforts have been committed to develop novel stents by surface modifications (Pan et al. Citation2007). Raval et al., studied mechanisms affecting drug release kinetics from coated cardiovascular stents composed of biodegradable poly-lactide-co-caprolactone and polyvinyl pyrrolidone (Raval et al. Citation2011). A conventional air brush technique was effectively modified so that Co-Cr L605 metallic stents were coated within multiple layers having drug Sirolimus blended together with biodegradable polymer matrix.
A novel type of drug eluting stent (DES) was prepared and checked for its suitability to release an antiproliferative drug in a sustained manner. The developed DES system mimics and minimizes restenosis and remove the possibility of thrombus formation on stent surfaces (Lee et al. Citation2007). In another study, biocompatible stent with a self-expandable mechanism was designed and fabricated. It was fabricated from thin films of chitosan (4% w/v) dissolved in acetic acid solution (2% v/v). This fabricated stent when comes in contact with tissue moisture, it starts expanding and expands completely in few minutes. Similar in vivo results were observed after inserting polymeric stent to rat vasa, it expanded ∼50% over intimal diameter of vessel (Lauto et al. Citation2001).
A new biotherapeutic gene delivery is reported by Paul et al., sustained delivery of biomolecules such as genes to stent surfaces can be achieved efficiently by this method. These types of recombinant baculovirus entrapment to the stent surface allows localized transport to targeted arterial segment which specifically avoid the systemic toxicity issues and gene transfection to adjacent cells (Paul et al. Citation2013). Similarly, other different coating and delivery techniques are explored in literature such as Layer by Layer, Spray coating for effective delivery of therapeutic agents (Pandey et al. Citation2015; Paul et al. Citation2010, Citation2012; Schmitt et al. Citation2012).
The significant complications were found with metallic stent and metal-based polymer-coated stents, such as vessel irritation and delayed endothelialization. Researchers focused towards bioabsorbable stent, which can be implanted easily in patients, and helps in time-dependent degradation (Amin et al. Citation2013).
A shape memory polymer (SMPs) comes under the class of “actively moving” polymers. They are smart polymers that can memorize temporary shapes and recovers to permanent one (Shen et al. Citation2011; Small et al. Citation2010). Catheter-based interventional demand has increased now a days, SMP techniques will help to reduce the steps as they are least invasive, and X-ray fluoroscopically guided (Jaros et al. Citation2010; Small et al. Citation2010). Venkatraman et al. used well-accepted biodegradable polymers to fabricate a new multilayered stent that have the partial elastic memory (PEM) to gain self-expandability at body temperature. The patented bi-layered prototype stent design allows rapid self-expansion at body temperature (Venkatraman et al. Citation2006).
Current investigation aims to develop fully biodegradable, self-expandable biomedical stent using shape memory polymers (SMPs) by solution casting method. We have used well-accepted biodegradable polymers with thermal activation principle to achieve shape memory effect (SME). The resulting biomedical stent was evaluated for platelet adhesion, ex-vivo expansion, haemolysis study and in-vitro drug release behavior, etc.
Material and methods
Poly-lactic acid (PLA) and poly (d,l-lactic-co-glycolide) (PLGA) with a 50/50 copolymer ratio (generously gifted by Purac Biomaterials, Netherland) were used in the study. Tacrolimus was provided by Glenmark Pharmaceuticals Ltd., R&D Center, Sinnar, Nashik, The solvent Chloroform was purchased from Merck Specialities Pvt. Ltd., Mumbai and Phosphate Buffered Saline (BioPerformance certified, pH 7.4) from Sigma Aldrich Chemicals, Mumbai. All other chemicals and reagents used were of analytical grade.
Fabrication of stent
PLA and PLGA in (as shown in ) were dissolved in Chloroform. Further polymeric solution poured by solution-casting method and thin film of ∼0.40 mm thickness was prepared after evaporation of the solvent under atmospheric conditions. Then the film was cut into strips, wound onto glass mandrel and subjected to a temperature of 40 °C for 2 h to fix the helical shape. Right before the implantation the helical coiled stent deformed at small diameter mandrel at freezing temperature for 1 h.
Table 1. Optimization of bare polymeric stent.
Drug loading
For the loading of drug, above procedure was repeated, firstly drug was dissolved into the chloroform, the polymers were dissolved into the same solution for casting of film, with proper mixing. Finally, the drug containing polymeric solution was poured into the mold.
Optimization of bare polymeric stent
Concentration of PLA and PLGA in bare polymeric stent was optimized by formulating various batches, as shown in the . The concentration of PLA and PLGA were either decreased or increased gradually in every batch. Quantity of chloroform kept same for all the formulated batches. From the performance characteristics of formulated batches, the final formula/concentration of PLA and PLGA for the fabrication of drug loaded polymeric stent was identified. Critical process parameters, which may affect the quality of the final product, were also identified. The optimization was based on self- expandability and thickness of the stent. After assessment of both the parameters, batch V5 was considered as optimized batch with 3:2 ratio of PLA to PLGA, respectively. Self-expandability and thickness was found to be 5.06 ± 0.4 min and 0.25 ± 0.02 mm, respectively, and batch V5 was subjected for further characterization.
Characterization of fabricated and drug-loaded polymeric stent
Thickness
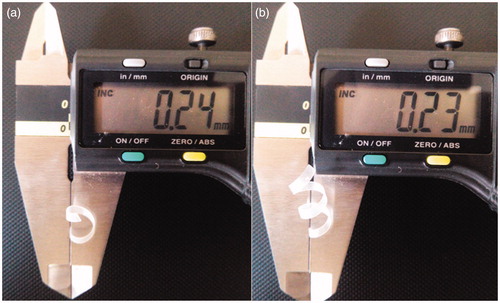
The thickness of drug-loaded polymeric film (stent) was determined by using digital vernier calliper (Mitutoyo Digimatic Calliper, Japan). The measurement of thickness of the stent was performed at three different places and the mean value was calculated.
Fourier transform infrared spectroscopy (FTIR)
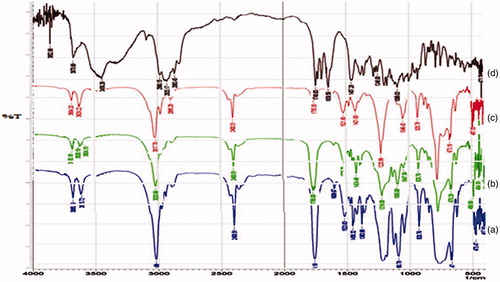
FTIR spectrum of drug was measured in the solid state as potassium bromide dispersion. FTIR spectrum of tacrolimus, PLA, PLGA and physical mixture were recorded by using FTIR spectrophotometer (IR-Affinity-1, Shimadzu, Japan) with diffuse reluctant substance (DRS) technique. The samples were dissolved in chloroform at 1:10 (sample: chloroform) ratio, respectively. One to two drops were added in between the sodium chloride (NaCl) discs. Prepared thin film was scanned in the range of 500–4000 cm − 1.
Differential scanning calorimetry (DSC)
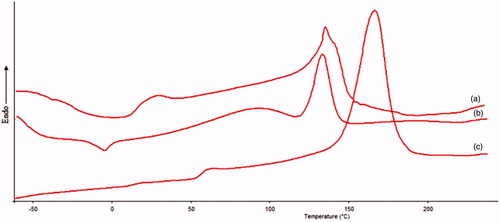
Thermal analysis was performed using DSC (Mettler-Toledo, Zurich, Switzerland). The instrument was calibrated with indium. DSC thermograms were recorded for bare polymeric stent and drug-loaded polymeric stent. The samples weighing 2 mg, were analyzed in sealed and pin-holed standard 40 μL aluminium pan, with a heating rate of 10 °C/min from 30 °C to 300 °C and during the measurement the sample cell were continuously purged with nitrogen at a flow rate of 40 mL/min.
Field emission scanning electron microscopy (FESEM)
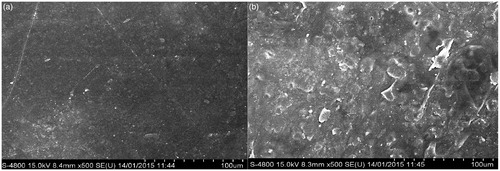
The surface morphology of fabricated polymeric stent was analyzed using scanning electron microscopy (FESEM S 400 TYPE II, Tokyo, Japan) before and after treatment with platelets at acceleration voltage of 15 kV at 1 μm resolution. The samples were gold-plated prior to imaging.
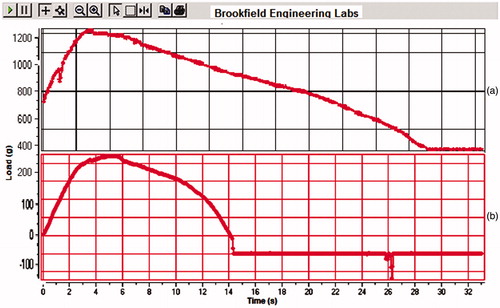
Textural characterization (mechanical property)
Strength of polymeric film was analyzed by using Texture Analyzer (CT3 Texture Analyzer, Brookfield, India), in that a grip was fixed to the base of the texture analyzer, while the another one was attached to the load cell, sample was placed in between the both grips on the texture analyzer. 100 gm load was applied to the film, and graph was plotted as load versus time.
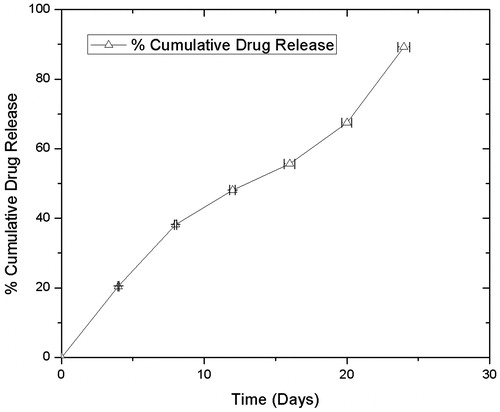
In-vitro drug release
In vitro drug release analysis of tacrolimus-loaded polymeric stent was investigated by incubating the tacrolimus-loaded polymeric stent in 10 mL of standard measuring flasks (SMF) containing phosphate buffer saline (PBS) solution of pH 7.4 maintained at 37 °C with constant agitation at 60 rpm. Samples were withdrawn after 4, 8, 12 and 16 days, respectively, and immediately the media was replaced with equal volume of fresh PBS after each sampling to maintain sink condition. Release of drug was determined using HPLC at 215 nm.
Drug content
Total drug content of tacrolimus-loaded polymeric stent was estimated by HPLC. The HPLC system used for tacrolimus analysis was Agilent 1200 equipped with a UV visible detector. The column used for analysis was C18. Tacrolimus-loaded polymeric stent was analyzed using a mobile phase consisting of acetonitrile at a flow rate of 1.0 mL/min. The detector wavelength was set at 215 nm. To investigate the total drug content, the tacrolimus-loaded polymeric stent was dissolved in 10 mL of mobile phase (acetonitrile) and 20 μl was injected into the HPLC system.
In-vitro expansion
In-vitro expansion study was performed on formulated stent (V5). In this study polymeric stent was kept in PBS maintained at 37 °C and outside diameter of stent was measured at different time intervals like 0, 1, 2, 3, 4, and 5 min.
Ex-vivo expansion
Ex-vivo self-expansion study was carried out on goat vessel. Polymeric and drug-loaded stent was set into the goat vessel, which was obtained from the local slaughter house and kept in PBS maintained at 37 °C. The outside diameter of polymeric stent was measured at 37 °C, after fixation at 0, 1, 3, and 5 min.
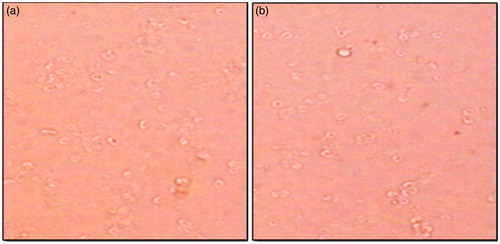
Haemocompatibility (interaction with erythrocytes)
To study interaction between drug-loaded polymeric stent and erythrocytes, hemocompatibility study was performed. In short, blood sample was collected from a pathological laboratory and added into the tubes containing Ethylene Diamine Tetra Acetic Acid (EDTA) solution and centrifuged at 2000 rpm for 10 min. and washed thrice with PBS of pH 7.4 (Kudarha et al. Citation2015). Tacrolimus-loaded polymeric stent was added into erythrocytes suspension and kept for incubation. Interaction of drug-loaded polymeric stent and erythrocytes was observed using Motic microscope (DMWBI-223, Motic digital biological microscope).
Platelet adhesion
Platelet adhesion is a key step for blood coagulation. Platelet adhesion test was conducted by collecting the blood sample from a volunteer. The fresh platelet-rich plasma (PRP) was obtained by centrifuging the blood sample. The control sample (polymeric stent without tacrolimus) and tacrolimus-loaded stent were immersed in PBS for 30 min to equilibrate the surface and then incubated in fresh PRP for 120 min at 37 °C. Finally, both samples were washed with PBS three times to remove the nonadherent platelets. The samples were then soaked in 2% glutaraldehyde for 30 min and 5% glutaraldehyde for 120 min to fix the adhered platelets. After washing again with PBS, the samples were dehydrated in a series of ethanol–water solutions (50, 75, 90, 100% v/v) and kept in a desicator to dry at room temperature (Pan et al. Citation2007). The adhered platelets on sample surfaces were observed by scanning electron microscopy (SEM).
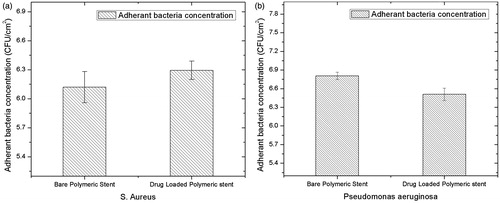
Bacterial adhesion study
To study the interaction of bare polymeric stent and drug-loaded polymeric stent with the bacteria, bacterial adhesion study was performed according to previously reported method (Khandwekar and Doble Citation2011). The two key organisms, Gram-positive (Staphylococcus aureus) and Gram-negative (Pseudomonas aeruginosa) were assessed for quantitative adhesion study. The study was carried out in 100 mL of lysogeny broth inoculated with a single colony of bacteria. The broth was kept at 37 °C overnight in a shaking incubator and then split into two falcon tubes and then centrifuged for 20 min. at 3500 rpm. Cells were suspended in PBS and centrifuged again, same procedure was repeated twice. Finally, cell suspension was re-suspended at a concentration of 1 × 108 cells/mL. In a 24 well plate, a disc of each stent was placed and incubated in 1 mL of cell suspension for 4 h at 37 °C, in incubator and rinsed twice with PBS. The bacterial cells were eluted from the surface in to 2 mL sterile PBS. The procedure involved sonication for 4 min. followed by 1 min. mild vortexing. A known volume of sample was inoculated into lysogeny broth agar and incubated at 37 °C, for 24 h. The colony forming units were counted (which was an indication of the total number of bacteria retained on the surface).
Cell proliferation assay
The 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was performed to assess the cytotoxicity of drug-loaded polymeric stent using human umbilical vein endothelial cells (HUVECs). The HUVECs cells were grown in a 96-well plate and left for seeding for 24 h. Subsequently, cells were cultured in Dulbecco’s modified eagle medium (DMEM) which contains 10% fetal bovine serum (FBS) with 5% CO2 at 37 °C. After 24 h seeding, the old medium was discarded and the cells were incubated with various concentrations of stents (50, 100, 250, 500, 1000 μg/mL) extract liquid. The cells were incubated for further 24 h at 37 °C with 5% CO2. The medium was replaced with serum-free DMEM before addition of 20 μL MTT (5 mg/mL) in PBS. After 4 h, when the MTT fully integrated with cells, 150 μL DMSO was added to the cells. The plates were then oscillated for 10 min to confirm the dissolution of Formazan in DMSO. Finally, the absorbance at 570 nm was measured by using a TRITURUS microplate reader. The values measured were expressed as mean value ± SD (n = 5).
Result and discussion
Thickness
The thickness of bare polymeric stent and drug loaded polymeric stent was determined by using digital vernier caliper (). It showed the thickness of stent in millimeters. In which measurement of thickness was performed at three edges of stent and the mean was calculated. The mean thickness of bare polymeric stent and drug-loaded polymeric stent was found to be 230 μm (0.23 mm), 240 μm (0.24 mm), respectively, as shown in ). During study slight change in thickness of drug-loaded polymeric stent was observed compared with bare polymeric stent (as the thickness in terms of percentage was increased by 4.16%).
Fourier Transform Infrared Specroscopy (FTIR)
The FTIR spectra of pure PLGA, PLA and tacrolimus is shown in , respectively, while shows the FTIR spectrum of tacrolimus-loaded PLA-PLGA polymeric stent. In the spectra of PLA and PLGA peaks near to 1452 cm − 1 and 1473 cm − 1, respectively, correspond to the bending vibration of –CH3. The C–O stretching observed near to 1040 cm − 1. The characteristic peak at 1760 cm − 1 is due to the C = O stretching and peak nearer to 3110 cm − 1 in the both spectra of PLA and PLGA suggest the C–H stretching vibrations (Bartkowiak-Jowsa et al. Citation2011; Pan et al. Citation2007). In the spectrum of tacrolimus, the characteristic strong peak at 1740 cm − 1 and 3600 cm − 1 are due to the C = O stretching and O–H stretching vibrations, respectively, while the peak near to 1640 cm − 1 is due to C = C stretching. The peak near to 1240 cm − 1 suggests the C–O stretching in tacrolimus.
In the spectra of tacrolimus-loaded PLA-PLGA polymeric stent the characteristic, strong and sharp peak of C = O stretching of PLA, PLGA and tacrolimus was observed at 1760 cm − 1, 1755 cm − 1 and 1740 cm − 1, respectively (Bartkowiak-Jowsa et al. Citation2011). The C–O stretching band observed at 1093 cm − 1 and 1046 cm − 1 of PLA and PLGA, respectively. The C–H stretching band at peak 3020 cm − 1, 3017 cm − 1 of PLA and PLGA observed in spectra of physical mixture of PLA, PLGA and tacrolimus at peak 3019 cm − 1. From above discussion it was concluded that no significant interaction was occurred in between drug and polymers.
Differential scanning calorimetric (DSC)
Differential scanning Calorimetry (DSC) is a basic method to investigate the polymorphic state and thermal behavior of the pure drug and drug in the formulation by determining the variation of temperature and energy at phase transition. shows the apparent DSC curves of pure drug (tacrolimus), polymer and tacrolimus-loaded sample. The thermogram of tacrolimus () shows a sharp and intense endothermic peak of drug at around 130 °C which corresponds to its melting temperature. The DSC thermogram of PLA () shows sharp peak around 170 °C. In the thermogram of drug-loaded polymeric sample (), peak of PLA is shifted towards the peak of drug. The DSC heating and cooling curves were recorded as a plot of enthalpy in mW verses the temperature in degree Celsius. On the basis of these findings, it can be concluded that there was no physicochemical interaction between in pure drug tacrolimus and polymer PLA.
Field emission scanning electron microscopy (FESEM)
Low-magnification FESEM images are shown in , it demonstrates the surface morphology of bare polymeric stent and drug-loaded polymeric stent. The surface topography of drug-eluting stents is considered as an important factor in stent performance (Kudarha et al. Citation2015). Bare stents were associated with an increased intimal thickness, persistent intimal fibrin deposition, intraintimal hemorrhage and increased intimal adventitial inflammation (Lee et al. Citation2007). ) shows that bare polymeric stent is having smooth, uniform homogeneous appearance. FESEM image ) of drug-loaded polymeric stent suggest that some flakes and void like structures are present on surface of drug-loaded polymeric stent.
Textural characterization (mechanical property)
To characterize the mechanical properties of bare polymeric stent and drug-loaded polymeric stent, tensile measurements were performed using Brookfield Texture Analyser. As shown in , 100 to 500 gm load was applied on the bare polymeric stent and drug-loaded polymeric stent, these both stents bear load up to 3–6 s, after that stents were deformed till 14–23 s and afterwards cuts down. These investigations revealed a non-linear curve characteristic of bare polymeric stent and drug-loaded polymeric stent which can bear up to 100 to 500 g load. From the results, it was found that, there was no any major change in mechanical properties of bare polymeric stent and drug-loaded polymeric stent. After insertion of helically coiled polymeric stent into vessel, it can bear maximum blood pressure, so that it cannot disturb from its site of application.
In vitro drug release
PLGA and PLA have several physicochemical properties, which relatively retains entrapped drug for longer period of time. Synthetic class of polymers use for drug delivery are generally biodegradable, which eliminates the possibility of surgery to remove implanted release devices and biocompatibility of the polymer is important so that complications with toxicity and inflammation are avoided (Hines and Kaplan Citation2013). Different types of release mechanism from polymer matrix are reported like diffusion, polymer degradation and erosion, solvent penetration/device swelling to name a few. Based on which, the polymers can control the rate of release of drug. A different component contributes during the release of drug from polymer such as penetration of water and subsequent solubilization of polymer matrix, erosion and diffusion of polymer fragments. Mainly diffusion mechanism is considered during the release of entrapped drug from polymer matrix when stent is efficiently submerged in aqueous environment, Water penetrates from surface to center of release device, which in turn results in hydrolytic cleavage and diffusion of tacrolimus from PLA: PLGA polymer matrix in sustained manner. Hydrophobic part of polymer is lactic acid concentration which substantially controls the release of drug. Hence, due to presence of lactide group polymer matrix has less hydrophilicity, absorb less water and subsequently degrade more slowly (Hines and Kaplan Citation2013; Makadia and Siegel Citation2011).
, shows the in-vitro release pattern of tacrolimus from the polymeric stent. These results indicate that, at 4 day 20.45%, 8 day 38.15%, 12 day 48.08%, 16 day 55.62%, 20 day 67.59% and on 24th day 89.29% release of tacrolimus from polymeric stent. The drug release was analyzed in triplicate by HPLC at 215 nm.
Drug content
The total tacrolimus content on polymeric stent was found to be 626.24 μg. It was determined using HPLC equipped with C18 column, mobile phase was acetonitrile with flow rate of 1.0 mL/min. The detector wavelength was set at 215 nm. Estimated drug content found in tacrolimus-loaded polymeric stent was 83.25%.
In-vitro expansion
The self-expansion experiment denoted that the helically coiled polymeric stent expanded significantly (). Smaller fixing diameter and longer fixing time causes poor expansion. describes the change occurs in shape of polymeric stent with respect to time. shows the original size of polymeric stent and shows the deformed size of original polymeric stent on small diameter metal mandrel. suggests that the shape of polymeric stent was decreased after removing from freezing temperature. The ) confirms that there was significant increase in diameter of deformed polymeric stent, at 37 °C. Polymeric stent regained their original diameter in the 5 min. at in-vitro conditions. The viscoelastic behavior of biodegradable polymers makes the shape memory effect of the material. Self-boosted polymeric stent can be made self-expanding by the credit of shape memory effect of polymers (Meng et al. Citation2006).
Ex- vivo expansion
In ex-vivo study, drug-loaded polymeric stent was inserted into the goat vessel as shown in and kept at 37 °C. The result was in accordance with the result obtained in in-vitro expansion study. suggest that there was significant increase in diameter of deformed tacrolimus-loaded polymeric stent at 37 °C at 1 min, 3 min and 5 min, respectively. There was no minor variation in expansion time between ex-vivo expansion time of polymeric stent and in-vitro expansion time of polymeric stent study.
Haemocompatibility (interaction with erythrocytes)
Hemolysis is the destruction of red blood cells. If hemolysis occurs in-vivo, it may causes anemia, jaundice and other pathological conditions (Kudarha et al. Citation2015). Accordingly, to determine non-toxicity of stent in erythrocytes, hemolysis study was carried out. Result of hemolysis study is shown in which shows the normal structure of erythrocytes. shows the structure of tacrolimus-loaded polymeric stent-treated erythrocytes which reveals that there were no signs of structural changes and erythrocytes retained their normal structure. Hemolysis study confirmed that there was no interaction between drug-loaded polymeric stent and erythrocytes, and no lysis of erythrocytes was observed compared to normal erythrocytes.
Platelet adhesion
The platelet adhesion to the stent surfaces is significantly related to the process of protein adsorption and thrombus formation. So, to determine the blood compatibility of bare polymeric stent and drug-loaded polymeric stent, the platelet adhesion test was conducted. In-vitro platelet adhesion was tested by SEM technique. The bare polymeric stent and drug-loaded polymeric stent were treated with platelets and its results are as shown in and . On the bare polymeric stent some round shaped platelets were observed (). After loading of tacrolimus in bare polymeric stent, the platelet adhesion was significantly decreased. Very few platelets were observed adhered to drug-loaded polymeric film () as compared to the bare polymeric stent (), which indicates that the blood compatibility of drug-loaded polymeric film was better than bare polymeric stent.
Figure 10. FESEM images of (a) Bare polymeric stent treated with platelets (b) Drug-loaded polymeric stent treated with platelets.
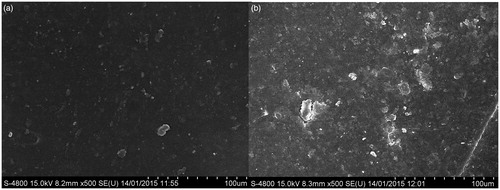
Figure 11. FESEM images of (a) Drug-loaded polymeric stent incubated in PBS solution (b) Drug-loaded polymeric stent incubated in distilled water (c) Drug-loaded polymeric stent (control).

As shown in , some changes were observed on surface of drug-loaded polymeric stent after incubating into the PBS and distilled water at 37 °C with gentle stirring. As initially () there was nonporous surface, but after incubation in PBS and distilled water pore formation occurs. Empty voids can be seen clearly, indicating the passage for drug from the tacrolimus-loaded polymeric stent.
Bacterial adhesion study
Bacterial adhesion is a serious concern related to the formation of biofilm on the surface of stent. Biomaterials are generally made up of biodegradable and biocompatible polymers, on which the bacteria may use it as substrate for proliferation wherein the biofilm formation occurs. Bacterial species adhere to biomaterial surfaces and secrete a layer of polysaccharide, which is basically consisting of extracellular substance and biomass of bacteria. Study specifically designed to observe the bacterial adhesion and attachment to surfaces, which implicates the response and activity of antibiotic activity of polymer and loaded therapeutic molecule (An and Friedman Citation1997; Barros et al. Citation2015; Khandwekar and Doble Citation2011). The ability of bacteria to adhere and proliferate on the surface of bare polymeric stent and drug-loaded polymeric stent was studied and obtained results are presented in . Present study was carried out using S. Aureus and P. Aurigenosa on bare polymeric stent and drug-loaded polymeric stents, which shows reduced bacterial adhesion. Moreover, more bacterial adhesion was observed in case of S. Aureus compared to P. Aurigenosa on drug-loaded stent. Bare polymeric and drug-loaded stent reduces the bacterial adhesion and proved to be effective as antibacterial which is important in case of biofilm formation. Statistical analysis indicated that there was no significant increase in bacterial adhesion as shown in .
Cell proliferation assay
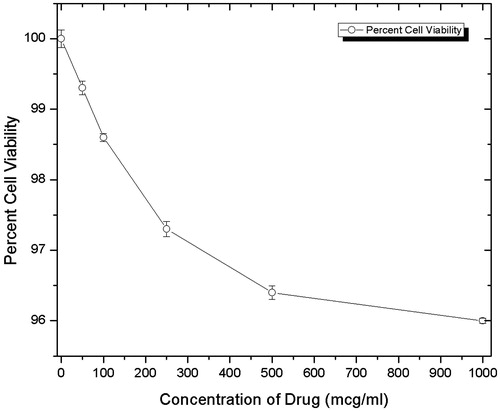
In the cell viability study the 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay was done for determination of cytotoxicity of drug-loaded polymeric stent. In which different concentrations (50, 100, 250, 500, 1000 μg/mL) of stents were used. The obtained results are reported in , from which one can conclude that as concentration increases from 50 μg/mL to 1000 μg/mL, the percent cell viability decreases from 100% to 96.1%. The overall result suggests that the inhibition of cell viability was dose dependent, and the present findings give a clear outline about the less cytototoxicity of drug-loaded polymeric stent.
Conclusion
This study effectively demonstrated that particular ratio of PLA and PLGA can be used as shape memory polymer blend to fabricate self-expandable, bioabsorbable stent. Shape memory effect produced from biodegradable polymers are self-expandable, cost-effective and have good compatibility with blood, hence it may be utilized as an effective platform for biomedical research. Furthermore, it may serve as a better option for existing metallic stent.
Acknowledgements
The authors are thankful to HRPIPER, Shirpur and North Maharashtra University, Jalgaon for providing necessary facilities for carrying out the research effectively.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Lee Y-K, Park JH, Moon HT, Lee DY, Yun JH, Byun Y. 2007. The short-term effects on restenosis and thrombosis of echinomycin-eluting stents topcoated with a hydrophobic heparin-containing polymer. Biomaterials. 28:1523–1530.
- Grabow N, Bünger CM, Schultze C, Schmohl K, Martin DP, Williams SF, et al. 2007. A biodegradable slotted tube stent based on poly (L-lactide) and poly (4-hydroxybutyrate) for rapid balloon-expansion. Ann Biomed Eng. 35:2031–2038.
- Uurto I, Juuti H, Parkkinen J, Kellomäki M, Keski-Nisula L, Nevalainen T, et al. 2003. Requirements for quantitative analysis of intimal reaction in arteries treated with intraluminal stents. J Endovasc Ther. 10:1110–1116.
- Uurto I, Mikkonen J, Parkkinen J, Keski-Nisula L, Nevalainen T, Kellomäki M, et al. 2005. Drug-eluting biodegradable poly-D/L-lactic acid vascular stents: an experimental pilot study. J Endovasc Ther. 12:371–379.
- Tamai H, Igaki K, Tsuji T, Kyo E, Kosuga K, Kawashima A, et al. 1999. A biodegradable poly‐L‐lactic acid coronary stent in the porcine coronary artery. J Interv Cardiol. 12:443–450.
- Su S-H, Chao RY, Landau CL, Nelson KD, Timmons RB, Meidell RS, et al. 2003. Expandable bioresorbable endovascular stent. I. Fabrication and properties. Ann Biomed Eng. 31:667–677.
- Shin H-S, Park K, Kim JH, Kim J-J, Han DK, Moon M-W, et al. 2009. Biocompatible PEG grafting on DLC-coated nitinol alloy for vascular stents. J Bioact Compat Polym. 24:316–328.
- Uurto I, Juuti H, Parkkinen J, Kellomäki M, Keski-Nisula L, Nevalainen T, et al. 2004. Biodegradable self-expanding poly-L/D-lactic acid vascular stent: a pilot study in canine and porcine iliac arteries. J Endovasc Ther. 11:712–718.
- Raval A, Choubey A, Engineer C, Kotadia H, Kothwala D. 2007. Novel biodegradable polymeric matrix coated cardiovascular stent for controlled drug delivery. Trends Biomater Artif Organs. 20:101–110.
- Mangual JO, Li S, Ploehn HJ, Ebner AD, Ritter JA. 2010. Biodegradable nanocomposite magnetite stent for implant-assisted magnetic drug targeting. J Magn Magn Mater. 322:3094–3100.
- Shin YM, Lim KS, Jin JY, Jeong SI, Lee YM, Shin H, et al. 2009. In vitro andin vivo characterization of a coronary stent coated with an elastic biodegradable polymer for the sustained release of paclitaxel. Macromol Res. 17:1039–1042.
- Pendyala LK, Matsumoto D, Shinke T, Iwasaki T, Sugimoto R, Hou D, et al. 2012. Nobori stent shows less vascular inflammation and early recovery of endothelial function compared with Cypher stent. JACC Cardiovasc Interv. 5:436–444.
- Bian H, Zhou S, Liang X, Li Q, Han W. 2012. In vitro study of poly (ethylene carbonate) as a drug-eluting stent coating. Prog Nat Sci. 22:295–302.
- Bartkowiak-Jowsa M, Będziński R, Szaraniec B, Chłopek J. 2011. Mechanical, biological, and microstructural properties of biodegradable models of polymeric stents made of PLLA and alginate fibers. Acta Bioeng Biomech. 13:21–28.
- Meng B, Wang J, Zhu N, Meng Q-Y, Cui F-Z, Xu Y-X. 2006. Study of biodegradable and self-expandable PLLA helical biliary stent in vivo and in vitro. J Mater Sci Mater Med. 17:611–617.
- Hehrlein C, Gollan C, Dönges K, Metz J, Riessen R, Fehsenfeld P, et al. 1995. Low-dose radioactive endovascular stents prevent smooth muscle cell proliferation and neointimal hyperplasia in rabbits. Circulation. 92:1570–1575.
- Pan C, Shao Z, Tang J, Wang J, Huang N. 2007. In vitro studies of platelet adhesion, activation, and protein adsorption on curcumin-eluting biodegradable stent materials. J Biomed Mater Res A. 82:740–746.
- Raval A, Parikh J, Engineer C. 2011. Mechanism and in vitro release kinetic study of sirolimus from a biodegradable polymeric matrix coated cardiovascular stent. Ind Eng Chem Res. 50:9539–9549.
- Lauto A, Ohebshalom M, Esposito M, Mingin J, Li P, Felsen D, et al. 2001. Self-expandable chitosan stent: design and preparation. Biomaterials. 22:1869–1874.
- Paul A, Elias CB, Shum-Tim D, Prakash S. 2013. Bioactive baculovirus nanohybrids for stent based rapid vascular re-endothelialization. Sci Rep. [Epub ahead of print]. DOI: 10.1038/srep02366
- Paul A, Abbasi S, Shum-Tim D, Prakash S. 2010. Nano-and biotechnological approaches in current and future generation of cardiovascular stents. Curr Nanosci. 6:469–478.
- Pandey AP, Singh SS, Patil GB, Patil PO, Bhavsar CJ, Deshmukh PK. 2015. Sonication-assisted drug encapsulation in layer-by-layer self-assembled gelatin-poly (styrenesulfonate) polyelectrolyte nanocapsules: process optimization. Artif Cells Nanomed Biotechnol. 43:413–424.
- Paul A, Shao W, Shum-Tim D, Prakash S. 2012. The attenuation of restenosis following arterial gene transfer using carbon nanotube coated stent incorporating TAT/DNA Ang1+ VEGF nanoparticles. Biomaterials. 33:7655–7664.
- Schmitt L, Grabow N, Lehmann U, Eschenburg C, Sternberg K, Schmitz K-P. 2012. Impact of polymer/drug coatings on the biomechanical performance of self-expanding peripheral drug-eluting stents. Biomed Tech. 57:28–29.
- Amin F, Ali MN, Minhas MA. 2013. An evolutionary appraisal of the efficacy of coronary artery stents relevant to the treatment of coronary heart diseases. Int J Biomed Adv Res. 4:764–775.
- Shen T, Liang L, Lu M. 2011. Novel biodegradable shape memory composites based on PLA and PCL crosslinked by polyisocyanate. Adv Biomed Eng. 12:302–305.
- Small W, I, Singhal P, Wilson TS, Maitland DJ. 2010. Biomedical applications of thermally activated shape memory polymers. J Mater Chem. 20:3356–3366.
- Jaros A, Smola A, Kasperczyk J, Dobrzyński P. 2010. Biodegradowalne polimery z pamięcią kształtu. Chemik. 64:87–96.
- Venkatraman SS, Tan LP, Joso JFD, Boey YCF, Wang X. 2006. Biodegradable stents with elastic memory. Biomaterials. 27:1573–1578.
- Kudarha R, Dhas NL, Pandey A, Belgamwar VS, Ige PP. 2015. Box-Behnken study design for optimization of bicalutamide-loaded nanostructured lipid carrier: stability assessment. Pharm Dev Technol. 20:608–618.
- Khandwekar AP, Doble M. 2011. Physicochemical characterisation and biological evaluation of polyvinylpyrrolidone-iodine engineered polyurethane (Tecoflex®). J Mater Sci Mater Med. 22:1231–1246.
- Hines DJ, Kaplan DL. 2013. Poly (lactic-co-glycolic) acid − controlled-release systems: experimental and modeling insights. Crit Rev Ther Drug Carrier Syst. 30:257–276.
- Makadia HK, Siegel SJ. 2011. Poly lactic-co-glycolic acid (PLGA) as biodegradable controlled drug delivery carrier. Polymers. 3:1377–1397.
- An YH, Friedman RJ. 1997. Laboratory methods for studies of bacterial adhesion. J Microbiol Methods. 30:141–152.
- Barros AA, Rita A, Duarte C, Pires RA, Sampaio‐Marques B, Ludovico P, et al. 2015. Bioresorbable ureteral stents from natural origin polymers. J Biomed Mater Res B Appl Biomater. 103:608–617.