?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Multi-epitope vaccines might cause immunity against multiple antigenic targets. Four immunodominant epitopes of HIV-1 genome were used to construct a polytope vaccine, formulated by dendrimer. Two regimens of polytopes mixture with dendrimer were utilized to immunize BALB/c mice. Adjuvants were also used to boost immune responses. The conjugated polytope could arouse significant cellular immune responses (P < 0.05) and Th1 response showed higher intensity compared to Th2 (P < 0.05). Our study depicted that conjugated dendrimer with multi-epitopic rHIVtop4 would efficiently induce cell-mediated immune responses and might be considered as promising delivery system for vaccines formulation.
Introduction
Efficient vaccines against HIV are believed to induce T-lymphocyte responses which are against different target antigens. Vaccine strategies have been evolved from whole organism to recombinant proteins and even further toward the ultimate in minimalist vaccinology, the epitope. The epitope-based approach is highly interesting since just a small but immunologically relevant sequence is almost always capable of inducing protective immunity against a large and complex pathogen (Abdoli et al. Citation2014). Development of polyepitope or polytope vaccines has been a strategy for overcoming the difficulties associated with making large recombinant vaccines containing numerous antigens. Such polytope constructs can be delivered utilizing different vaccine delivery system (Arabi et al. Citation2014).Using nanoparticles as vaccine adjuvants is so appealing because their small size makes the uptake by dendritic cells effectively possible and their biological properties can be altered to fit the desired function. New adjuvants are required to induce proper immune response, but the most vital issue is their safety (Ardestani et al. Citation2015). Moreover, adjuvants must be compatible with various antigens, and they should be inexpensive. The most frequently used adjuvants in human and veterinary vaccines are adjuvants containing aluminum which generally stimulate proper antibody response with long-term safety profile (Ardestani et al. Citation2015). However, aluminum adjuvants are inefficient in arousing cell-mediated immune response; one of the negative points is the issue that they are inactivated through freezing which negatively affects the stability of vaccine antigens; which is related to local adverse vaccine reactions (Cooper et al. Citation2008, Davenport and Petravic Citation2010). CpG ODN is an oligodeoxynucleotide comprising immunostimulatory CpG motifs that triggers human B cells and plasmacytoid dendritic cells via Toll-like receptor 9. The immunostimulatory properties of CPG 7909, express an important strategy in obtaining long-term protection in patients infected with HIV-1 and other hepatitis B vaccine (HBV) hypo-responsive populations. Studies have shown that addition of CPG adjuvant to a commercial HBV vaccine increased the kinetics, magnitude, and longevity of the sero-protective response over 48 weeks (De Rose et al. Citation2005, Dezfuli et al. Citation2014). For efficient HIV vaccines, strong and broad humoral and cellular immune responses are necessary (Davenport and Petravic Citation2010). Therefore, development of a safe and novel antigen delivery system with several mechanisms of immunity enhancement is highly demanded. Several types of HIV vaccine strategies were designed to induce T cell immunity either encode a limited number of the nine HIV-1 genes (often just Env and/or Gag) and/or encode multiple distinct CD8 T cell epitopes. It is now obvious that smaller regulatory genes are vital targets for T cell immunity (Emu et al. Citation2005). Over the past 20 years, the interest in using nanoparticles as vaccine delivery and adjuvants has been increased (Faraji and Wipf Citation2009, Fréchet and Tomalia Citation2001, Heegaard et al. Citation2009). Immuno-stimulatory influences for nanoparticles with different materials, sizes, and surface characteristics have been observed and reported (Heegaard et al. Citation2009). The physical and chemical compositions of nanoparticles affect the cell type to uptake the particles, the trafficking within the cell and the level and mechanism of immuno-stimulation (Fréchet and Tomalia Citation2001, Kaufman and Barouch Citation2009). Dendrimers are well-defined mono-dispersed synthetic globular polymers with a range of interesting chemical and biological characteristics (Kreuter Citation1995). Chemical properties include the existence of multiple accessible surface functional groups that can be utilized for coupling biologically relevant molecules that enable exact hetero-functionalization of surface groups (Kreuter Citation1995). Biologically, dendrimers are highly bio-compatible with predictable bio-distribution and cell membrane interacting properties determined by their size and surface charge (Kreuter Citation1995). Dendrimers have desirable characteristics to fulfill the need for effective immuno-stimulating compounds (adjuvants) that can increase the effectiveness of vaccines, as dendrimers supply molecularly defined multivalent scaffolds to produce completely defined conjugates with small molecules of immuno-stimulators and/or antigens (Kreuter Citation1995, Krieg Citation2006). Moreover, bioactive dendrimer conjugates have so far been known for their capabilities to increase the stability, solubility, and absorption of different types of therapeutics (Kreuter Citation1995, Krieg Citation2006). For example, dendrimer-like alpha-D-glucan nanoparticles could activate dendritic cells as vaccine adjuvants (Kunwar et al. Citation2013).
Herein, we report the influence of anionic PEG-citrate G2 dendrimer containing multi-epitopic HIV-1 vaccine candidate (HIV-1-gag-pol-tat-env) alone and combined with two adjuvants; CpG motif and alum.
Materials and methods
PEG 600, dicyclohexylcarbodiimide (DCC), dimethylsulfoxide (DMSO), and citric acid were obtained from Merck (Darmstadt, Germany). Aluminum hydroxide (Alhydrogel) was purchased from Brenntag Biosector A/S (Frederikssund, Denmark). CpG ODN was purchased from MWG (Eurofins MWG Operon, Ebersberg, Germany).
Synthesis of G2 dendrimer
G1 and G2 dendrimers were prepared according to reported method (Liu et al. Citation2012). G1 dendrimer was synthesized in the reaction of PEG (as the core of dendrimer) with DCC in DMSO. Subsequently, DCC was replaced with citric acid and finally the reaction terminated by adding distilled water. In the process of G2 dendrimer synthesis, DCC and citric acid were added to the G1 solution in DMSO (Liu et al. Citation2012). G2 dendrimer structure was approved by Fourier-transform infrared spectroscopy (FTIR). Zeta potential and size of G1 and G2 dendrimers were determined by dynamic light scattering (DLS) zeta sizer.
The G2 dendrimer conjugation and characterization with polypeptide
The purified polytope (gag/pol/env/tat) was conjugated with G2 dendrimer by mixing equal volumes of dendrimer and polyepitope. Sulfo-NHS was added to the solution to increase the stability of reaction. Following 10-min sonication, conjugation occurred between dendrimer and polyepitope. Zeta potential of conjugated dendrimer with polyepitope was measured by zeta sizer and its structure was determined by FTIR and nuclear magnetic resonance (NMR) spectroscopy.
Animals
Six-to-eight-weeks old female BALB/c mice were provided by Pasteur Institute of Iran (Karaj, Iran). Before the experiments, mice were housed for one week to acclimatize, given free access to food and water, and were maintained in a light/dark cycle (12 h/12 h). All experiments were in accordance with the Animal Care and Use Protocol of Pasteur Institute of Iran.
Experimental groups and immunization
BALB/c mice were divided into six groups (n = 7) and were immunized subcutaneously with 100 μl of each formulation containing 10 μg of candidate vaccine. Group 1 received phosphate-buffered saline (PBS) (control group), Group 2 was injected by conjugated G2 dendrimer with HIV polytope, and Group 3 was immunized by mixture of G2 dendrimer and HIV polytope. Group 4 received conjugated G2 dendrimer with HIV polytope plus alum adjuvants (Sigma-Aldrich, St. Louis, MO). Group 5 was administrated by conjugated G2 dendrimer with HIV polytope plus CpG ODN, group 6 was inoculated by only HIV polytope, and finally group 7 was immunized by HIV polytope + CpG. Experimental groups were immunized three times with 2 weeks interval. shows the immunization regimens for each group.
Table 1. Mice immunization regimen by different formulations.
Monitoring humoral immune response by ELISA
Specific antibodies produced in mice were determined by an optimized indirect enzyme-linked immunosorbent assay (ELISA) method. Briefly, 96-well microtiter plates (Greiner, Germany) were coated with 100 μl of the recombinant HIV-1 tat/pol/gag/env protein (10 μg/ml) diluted in PBS (pH = 7.2) at 4 °C overnight. After washing with PBS containing 0.05% (v/v) Tween 20 (PBS-Tween) and blocking with 1% bovine serum albumin (BSA) in PBS for 2 h at 37 °C, 100 μl of mice sera (diluted 1:50 in 1% BSA/PBS-Tween, Southern Biotech, Birmingham, LA) was added to each well. The plates were incubated at 37 °C for 2 h. After washing, 100 μl of either rat anti-mouse IgG1 or IgG2a conjugated with Biotin (diluted 1:1000 in 1% BSA/PBS-Tween) was added and the plates were incubated at 37 °C for additional 2 h. The plates were then washed and incubated with 1:10000 dilution of streptavidin-horseradish peroxidase at 37 °C for 1 h. Detection was done with 100 μl of O-Phenylenediamine (OPD, Sigma) as the substrate in citrate phosphate buffer (citric acid 0.1M, Na2HPO4 0.2 M, pH 4.5). To assay the specific IgG, wells were washed with wash buffer and incubated for 2 h with 100 μl of 1/5000 dilution of horseradish peroxidase (HRP)-conjugated anti mouse IgG (Sigma). Next, plates were washed and incubated for 30 min with 100 μl of tetramethylbenzidine (TMB) substrate in the dark room. The enzyme reaction was stopped after 30 min by adding 1 M sulfuric acid. The absorbance was measured at 492 nm wavelength (Liu et al. Citation2003).
IFN-γ and IL4 cytokine detection
Two weeks after final immunization, the spleens of three mice from each group were removed. A single cell suspension of splenocytes was seeded in complete Roswell Park Memorial Institute (RPMI) medium 1640 supplemented with 5% FCS, 2 mM glutamine, 5 × 10 −5 mM 2-mercaptoethanol (2-ME), and 10 mM HEPES and 40 μg/ml gentamycin. Cells were incubated in U-bottomed, 96-well microtiter plates (Costar, Cambridge, MA) at a density of 2 × 106 cells/ml in the presence of 10 μg/ml of the recombinant protein (rHIVtop4) and 5 μg/ml of concanavalinA (ConA) as positive control and incubated for 3 days at 37 °C in humidified atmosphere of 5% CO2. Interferon gamma (IFN-γ) and IL4 levels were measured in the collected supernatants by commercially available sandwich-based ELISA kits (R&D Systems Inc., Minneapolis, MN) as described in the kit manual. All tests were performed in duplicate and the mean was recorded for each set of samples (Lu et al. Citation2015).
Lymphoproliferative responses
The spleens of immunized mice were removed and RBCs were lysed with lysis buffer. One hundred microliter of 2 × 106 cell/ml was aliquoted into 96-well flat-bottom culture plates and stimulated with 10 μg/ml of rHIVtop4. Phytohemagglutinin-A (10 μg/ml; Gibco, El Paso, TX) was used as positive control and un-stimulated wells were used as negative controls. Following incubation for 72 h at 37 °C in 5% CO2 humid incubator, 20-μl bromodeoxyuridine (BrdU) was added per well. After incubation for 18 h, the plates were centrifuged and each well was aspirated and dried by hair dryer. Then, the cells were penetrated and 100 μl of anti-BrdU was added for 2 h. Afterwards, the plates were washed and TMB substrate was added in dark. The reaction was stopped by H2SO4 2N. Wavelength absorbance was measured at 450 nm. The stimulation index (SI) was calculated according to the following formula (Nilsson et al. Citation2014):
Cytotoxicity assay
To measure the specific lysis of the target cell, lactate dehydrogenase (LDH) release assay was used. Two weeks after the last immunization, single-cell splenocyte suspensions from the rest of the mice were prepared as effector cells. The effector cells were added to polytope-pulsed P815 target cells at a ratio of 1:100 (Target:Effector), then incubated for 4 h. Lysis of target cells was determined by the LDH assay kit (Takara, Japan) according to the manufacturer’s instructions. Blank PBS buffer and a solution of 0.1% Triton X-100 in PBS were used as controls. The LDH-mediated conversion of the tetrazolium salt into red formazan product was measured at 490 nm after incubation at room temperature for 30 min. The percentage of specific cytolysis was determined by the following formula (Panyam and Labhasetwar Citation2003):
Statistical analysis
All statistical analyses were performed using GraphPad Prism 5 software (GraphPad Software Inc., La Jolla, CA). The differences in the level of cellular and antibody responses were determined by one-way ANOVA. P values less than 0.05 (P < 0.05) was used for statistical significance.
Results
The G2 dendrimer characterization and its conjugation with polypeptide
The size of particles was measured with DLS. Dendrimeric sizes of G2 products were 70–90 nm. Zeta potential measurements improve the reaction between peptide and dendrimer. Addition of peptide to G2 dendrimer increased dendrimer Zeta potential significantly from −2.46 mV to −1.70 mV. Describing FTIR spectrum of G2 and G2-conjugated dendrimers is as shown in . The peak in 1732 cm−1 conforms the presence of C=O groups in ester bond between PEG and citric acid in G2 dendrimer. The peak in 1232 cm−1 refers to C–O groups in ester bond between citric acid end group of G2 dendrimer and new citric acids that were added to G1 dendrimer. This confirms the presence of conjugated G2 dendrimer. The peak in 2500–3430 cm−1 shows the OH of citric acids (). Additionally, as it was indicated above the sharp peaks regarding anionic linear globular dendrimer G2 as well as peptide chemical structure (rising from antigen structure) were clearly shown as illustrated ().
Figure 1. FTIR spectrum of G1 and G2 dendrimers (1a) and NMR characterization of conjugated dendrimer with polytope (1b).
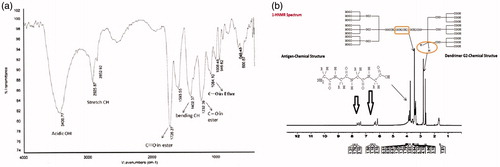
For example, peaks at 2.7–2.9 ppm regarding citric acid of dendrimeric structure and 3.3–3.5 ppm regarding PEG of dendrimer were strongly observed as well as peaks at 6–8 ppm regarding aromatic and amide bonds of peptide structure.
Totally, concurrent observation of hydrogen peaks in HNMR spectrum of conjugate regarding dendrimer and antigen with appropriate hydrogen integral ratio as depicted above lead to confirm conjugation successful.
Evaluating the level of specific total IgG and ratio of IgG2a/IgG1
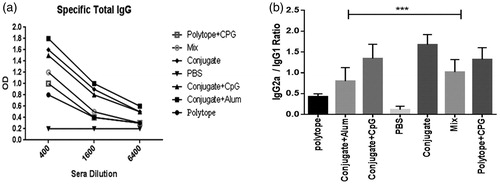
Six groups of mice were considered for immunization as described in . One week after the last booster immunization, the level of specific total IgG, IgG1, and IgG2a antibodies were determined by ELISA assay with respect to rHIVtop4. IgG level was highly enhanced by conjugated candidate vaccine and conjugate adjuvanted with alum and CpG compared to polytope and mix (P < 0.05) as data illustrated in . IgG2a/IgG1 ratios are reported as shown in . The results indicated that injection of the candidate conjugate vaccine caused an increase in the level of IgG1 and IgG2a specific antibody levels with high intensity toward IgG2a which showed significant difference to the control groups (P < 0.05). In addition, group immunized with the mixture of polytope-dendrimer showed approximately the same levels of IgG1 and IgG2a. Conjugate plus alum group induced more IgG1 and less IgG2a compared to conjugate plus alum CpG. The group immunized with polytope raised high level of IgG1 in comparison with IgG2a level.
Figure 2. Analysis of humoral responses, total IgG (2a) and IgG2a/IgG1 ratio (2b) in mice immunized with candidate vaccines in different formulations by ELISA. The asterisks directly above the bars point out statistically significant differences between conjugated immunized groups and the polytope-administrated group of mice.
IFN-γ and IL4 cytokine pattern
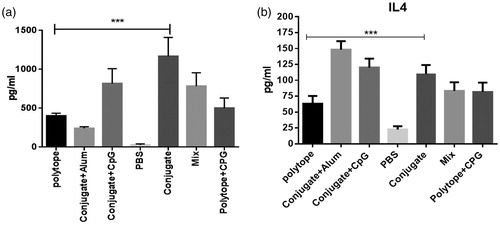
In this study, IFN-γ and IL4 cytokine levels were evaluated by ELISA. Our findings demonstrated that IFN-γ level against rHIVtop4 was significantly higher in groups immunized with the conjugate with or without CpG (G2 and G4) as compared to other groups (P < 0.05). As shown in , the group received conjugate plus alum induced less IFN-γ cytokine level in comparison with the other immunized groups. Result of IL4 cytokine assay demonstrated that immunization with conjugate vaccine adjuvanted with alum elicited the highest IL4 cytokine level. Candidate conjugate vaccine and adjuvanted with CpG elevated IL4 cytokine level as compared to other groups (P < 0.05) ().
Figure 3. IFN-γ and IL4 cytokine production levels. To characterize antigen-specific, cell-mediated responses, splenic mononuclear cells were stimulated in vitro by the recombinant HIVtop4 protein. Data show IFN-γ cytokine levels in independent experiments (mean ± SD) (3a). IL4 (Th2) secretion from splenocytes of administrated mice recalled with polytopes was measured and conjugate adjuvanted with alum indicated higher frequency of IL4-secreting (3b).
Lymphocyte proliferation assay
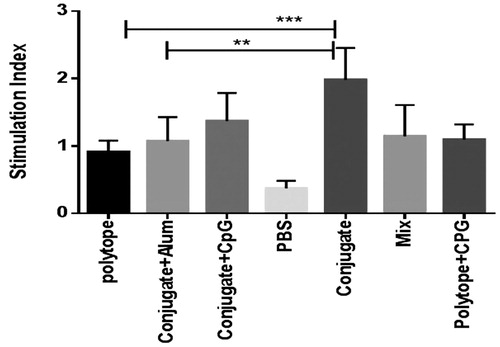
The lymphocyte proliferative response evaluation was carried out by BrdU method. As shown in , immunization of mice by conjugate vaccine significantly increased lymphocyte proliferation in comparison with conjugate vaccine plus CpG and alum (P < 0.05). Immunization of mice with mixture of dendrimer and polytope did not significantly increase lymphocyte proliferation in comparison with polytope-immunized group. Furthermore, there was no significant difference among the groups.
CTL responses
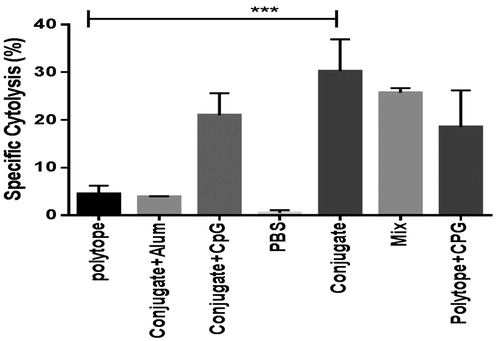
The cytotoxic-T-lymphocyte (CTL) responses specific to polytope were measured by LDH assay. The group of vaccinated mice that received conjugate, developed higher level of Top4 polytope-specific lytic activity compared to those that received conjugate plus CpG or mix of dendrimer and polytope (). In addition, immunization of mice with polytope + CPG significantly increased lymphocyte proliferation in comparison with polytope + alum and polytope-immunized groups.
Discussion
Despite enormous studies over the past three decades to design an effective prophylactic or therapeutic HIV vaccine, they have proved to be unsuccessful and were relinquished in the early stages of development (Peng et al. Citation2013). High antigenic variability in HIV that results from high mutation rates is a critical dilemma in developing efficient vaccines. On the other hand, some domains of HIV proteins are immunosuppressive, therefore, development of the conserved multi-epitope-vaccines derived from HIV-I proteins could be promising approach to elicit a protective cellular and humoral immune responses against HIV (Pion et al. Citation2010). In this study, the potency of gag/pol/env/tat multi-epitope construct mixed or conjugated to dendrimer, and also clarifying the influences of two adjuvants including alum and CpG motif on the immune responses were evaluated.
The mice immunized with conjugated polytope-dendrimer enhanced IgG2a responses against rHIVtop4 dramatically higher than other groups (P < 0.05). Also, co-injection of polytope-dendrimer with CpG motif induced higher IgG2a response compared to other formulations. After the third immunization, the amount of specific total IgG in conjugate adjuvanted with alum and conjugate candidate vaccine immunized groups was significantly higher than those of other groups and negative control groups (P < 0.05). Since, the majority of B cell epitopes are conformational; the result of humoral response also confirms that the conjugation of dendrimer with polytope did not change the conformational structure of polytope. To compare the effects of different formulation strategies on Th cell development, IFN-γ and IL4 secretion were analyzed. Analysis of the IFN-γ levels in the supernatant of splenocytes from mice injected with CpG-adjuvanted and also the conjugated polytope-dendrimer group, were much higher than the other groups (P < 0.05). The IFN-γ cytokine is an indicative of Th1 cellular immune responses. Ullum and colleagues which have measured the amount of IFN-γ in healthy, HIV-infected and AIDS patients, have revealed that decrease in IFN-γ was significantly associated with progression to AIDS (Preis and Langer Citation1979). Emu et al. have also demonstrated that IFN-γ levels were extremely correlated with HIV control in patients with HIV-1 viremia (Qiu et al. Citation2000). The result of IL4 as Th2 indicative, demonstrates the conjugate adjuvanted with alum raised the most level of IL4 in comparison with other immunized mice. However, the conjugate candidate vaccine is able to elicit the IL4 level as the same level of conjugate adjuvanted with CpG.
Results of the lymphoproliferative and CTLs assays suggested that the conjugate vaccine candidate has been able to induce the strongest proliferative responses and CTLs in accordance with the IgG2a/IgG1 ratio response. Considering proliferative responses which are indicators for activation of cellular immune responses and also the importance of the cellular immune responses in controlling viral infections, this could be propounded as one of the advantages of this candidate vaccine. CTLs play key role in clearance of viral infection. Analysis of the immune responses profile of the HIV-infected patients showed that antiviral CTL activity is associated with elimination of virus particles during the acute phase of infection and a drop in the CTL activity is correlated with the disease progression (Reynell and Trkola Citation2012, Romagnani et al. Citation1994). Sundaram et al. reported a novel multivalent human CTL peptide construct which effectively elicited multi-specific CTL responses against HTLV (Suhrbier Citation1997).
CpG oligodeoxynucleotides (CpG ODN) are short single-stranded synthetic DNA motifs which are considered as pathogen-associated molecular patterns recognized by the pattern recognition receptor, Toll-like Receptor 9, which results in mounting an innate immune response characterized by the production of Th1 and pro-inflammatory cytokines (Suhrbier Citation2002). When CpG ODN was used as a vaccine adjuvant with conjugated polytope-dendrimer, increase in Th1 responses was not addressed. Unlike our results, Reece and et al. (Sundaram et al. Citation2003) showed that immunization with HIV Gag protein and TLR9 ligand CpG ODN had significantly augmented Gag-specific Th 1 and antibody responses compared to animals immunized with HIV Gag protein alone.
All together, these data verify that PEG-citrate G2 dendrimer containing multi-epitopic HIV-1 vaccine candidate elicits T-cell responses toward Th1-type.
Dendrimers are extremely branched molecules with homogeneous and controllable size (Ullum et al. Citation1997). In addition, dendrimers with many peripheral groups and interior cavities are potential vectors for drugs, peptides, and genes for HIV inhibition (Wang et al. Citation2006). In this study, when dendrimer was conjugated with multi-epitopic rHIVtop4, it led to raise high level of IgG2a and IFN-γ. Furthermore, the strongest proliferative responses and cytotoxic-T-lymphocytes responses were induced. Our results suggest that conjugated dendrimers may have potential adjuvant properties to shift immune responses from Th2 to Th1 immune responses. Th1 cells are critical for promoting cell-mediated immunity and play a vital role in host defense systems against viruses (Wille-Reece et al. Citation2005). Pion et al. reported that loading of 2G-NN16 carbosilane dendrimer with HIV-derived peptide (nef, gp160, and p24) showed higher cellular uptake in comparison with derived peptide alone and caused dendritic cells to express TNF-β, IL-5, INFγ, and IL-2 (Xiao et al. Citation2001). Consequently, inducing these cytokines could elicit the differentiation of Th1 cells in vivo. Interestingly, unmodified polycationic PAMAM dendrimers have been applied to prevent binding of Tat protein to trans-acting responsive element (TAR) RNA of HIV (Zhao et al. Citation2004, Citation2014).
Taken together, our results indicate that dendrimers could be used as premising delivery system and as a Th1 cells eliciting adjuvant in multi-epitope candidate vaccine formulation. The future studies will reveal the details of immune shifting in new dendrimer macromolecules complexes.
Acknowledgments
This work was supported by Iran National Science Foundation (INSF) to Pasteur Institute of Iran, Tehran, Iran under [grant number 808].
Disclosure statement
Authors do not have any conflict of interest.
Additional information
Funding
References
- Abdoli A, Soleimanjahi H, Kheiri MT, Jamali A, Mazaheri V, Alitappeh AM. 2014. An H1-H3 chimeric influenza virosome confers complete protection against lethal challenge with PR8 (H1N1) and X47 (H3N2) viruses in mice. Pathog Dis. 72:197–207.
- Arabi S, Aghasadeghi MR, Memarnejadian A, Kohram F, Aghababa H, Khoramabadi N, et al. 2014. Cloning, expression and purification of a novel multi-epitopic HIV-1 vaccine candidate: a preliminary study on immunoreactivity. Vacc Res. 1:10–15.
- Ardestani MS, Fordoei AS, Abdoli A, Cohan AR, Bahramali G, Sadat SM, et al. 2015. Nanosilver based anionic linear globular dendrimer with a special significant antiretroviral activity. J Mater Sci Mater Med. 26:1–8.
- Cooper CL, Angel JB, Seguin I, Davis HL, Cameron DW. 2008. CPG 7909 adjuvant plus hepatitis B virus vaccination in HIV-infected adults achieves long-term seroprotection for up to 5 years. Clin Infect Dis. 46:1310–1314.
- Davenport MP, Petravic J. 2010. CD8+ T cell control of HIV–a known unknown. PLoS Pathog. 6:e1000728.
- De Rose R, Chea S, Dale CJ, Reece J, Fernandez CS, Wilson KM, et al. 2005. Subtype AE HIV-1 DNA and recombinant Fowlpox virus vaccines encoding five shared HIV-1 genes: safety and T cell immunogenicity in macaques. Vaccine. 23:1949–1956.
- Dezfuli HT, Shahbazzadeh D, Eidi A, Bagheri KP, Pakravan N, Amini S, et al. 2014. Induction of IFN-γ cytokine response against hepatitis B surface antigen using melittin. Gastroenterol Hepatol Bed Bench. 7:108–117.
- Emu B, Sinclair E, Favre D, Moretto WJ, Hsue P, Hoh R, et al. 2005. Phenotypic, functional, and kinetic parameters associated with apparent T-cell control of human immunodeficiency virus replication in individuals with and without antiretroviral treatment. J Virol. 79:14169–14178.
- Faraji AH, Wipf P. 2009. Nanoparticles in cellular drug delivery. Bioorg Med Chem Bioorgan Med Chem. 17:2950–2962.
- Fréchet JMJ, Tomalia DA. 2001. Dendrimers and Other Dendritic Polymers. In: Scheirs J, Ed. West Sussex: Wiley.
- Heegaard PMH, Boas U, Sorensen NS. 2009. Dendrimers for vaccine and immunostimulatory uses. A review. Bioconjug Chem Bioconjugate Chem. 21:405–418.
- Kaufman DR, Barouch DH. 2009. Translational mini review series on vaccines for HIV: T lymphocyte trafficking and vaccine‐elicited mucosal immunity. Clin Exp Immunol. 157:165–173.
- Kreuter J. 1995. Nanoparticles as Adjuvants for Vaccines, in Vaccine Design. New York (NY): Springer; pp. 463–472.
- Krieg AM. 2006. Therapeutic potential of Toll-like receptor 9 activation. Nat Rev Drug Discov. 5:471–484.
- Kunwar P, Hawkins N, Dinges WL, Liu Y, Gabriel EE, Swan DA, et al. 2013. Superior control of HIV-1 replication by CD8+ T cells targeting conserved epitopes: implications for HIV vaccine design. PLoS One. 8:e64405.
- Liu J, Gray WD, Davis ME, Luo Y. 2012. Peptide- and saccharide-conjugated dendrimers for targeted drug delivery: a concise review. Interf Focus. 2:307–324.
- Liu Z, Xiao Y, Chen Y-H. 2003. Epitope-vaccine strategy against HIV-1: today and tomorrow. Immunobiology. 208:423–428.
- Lu F, Mencia A, Bi L, Taylor A, Yao Y, HogenEsch H. 2015. Dendrimer-like alpha-d-glucan nanoparticles activate dendritic cells and are effective vaccine adjuvants. J Control Release. 204:51–59.
- Nilsson C, Godoy-Ramirez K, Hejdeman B, Bråve A, Gudmundsdotter L, Hallengärd D, et al. 2014. Broad and potent cellular and humoral immune responses after a second late HIV-modified vaccinia virus ankara vaccination in HIV-DNA-primed and HIV-modified vaccinia virus Ankara-boosted Swedish vaccinees. AIDS Res Human Retrov. 30:299–311.
- Panyam J, Labhasetwar V. 2003. Biodegradable nanoparticles for drug and gene delivery to cells and tissue. Adv Drug Deliver Rev. 55:329–347.
- Peng J, Wu Z, Qi X, Chen Y, Li X. 2013. Dendrimers as potential therapeutic tools in HIV inhibition. Molecules. 18:7912–7929.
- Pion M, Serramia MJ, Diaz L, Bryszewska M, Gallart T, García F, et al. 2010. Phenotype and functional analysis of human monocytes-derived dendritic cells loaded with a carbosilane dendrimer. Biomaterials. 31:8749–8758.
- Preis I, Langer RS. 1979. A single-step immunization by sustained antigen release. J Immunol Methods. 28:193–197.
- Qiu J-T, Liu B, Tian C, Pavlakis GN, Yu XF. 2000. Enhancement of primary and secondary cellular immune responses against human immunodeficiency virus type 1 gag by using DNA expression vectors that target Gag antigen to the secretory pathway. J Virol. 74:5997–6005.
- Reynell L, Trkola A. 2012. HIV vaccines: an attainable goal? Swiss Med Wkly. 142:w13535.
- Romagnani S, Prete G, Manetti R, Ravina A, Annunziato F, De Carli M, et al. 1994. Role of TH1/TH2 cytokines in HIV infection. Immunol Rev. 140:73–92.
- Suhrbier A. 1997. Multi-epitope DNA vaccines. Immunol Cell Biol. 75:402–408.
- Suhrbier A. 2002. Polytope vaccines for the codelivery of multiple CD8 T-cell epitopes. Expert Rev Vaccines. 1:207–213.
- Sundaram R, Sun Y, Walker CM, Lemonnier FA, Jacobson S, Kaumaya PT. 2003. A novel multivalent human CTL peptide construct elicits robust cellular immune responses in HLA-A*0201 transgenic mice: implications for HTLV-1 vaccine design. Vaccine. 21:2767–2781.
- Ullum H, Lepri AC, Bendtzen K, Victor J, Gøtzsche PC, Phillips AN, et al. 1997. Low production of interferon γ is related to disease progression in HIV infection: evidence from a cohort of 347 HIV-infected individuals. AIDS Res Human Retrov. 13:1039–1046.
- Wang W, Guo Z, Chen Y, Liu T, Jiang L. 2006. Influence of generation 2-5 of PAMAM dendrimer on the inhibition of Tat peptide/ TAR RNA binding in HIV-1 transcription. Chem Biol Drug Des. 68:314–318.
- Wille-Reece U, Flynn BJ, Loré K, Koup RA, Kedl RM, Mattapallil JJ, et al. 2005. HIV Gag protein conjugated to a Toll-like receptor 7/8 agonist improves the magnitude and quality of Th1 and CD8+ T cell responses in nonhuman primates. P Natl Acad Sci USA. 102:15190–15194.
- Xiao Y, Lu Y, Chen YH. 2001. Epitope-vaccine as a new strategy against HIV-1 mutation. Immunol Lett. 77:3–6.
- Zhao H, Li J, Xi F, Jiang L. 2004. Polyamidoamine dendrimers inhibit binding of Tat peptide to TAR RNA. FEBS Lett. 563:241–245.
- Zhao L, Seth A, Wibowo N, Zhao CX, Mitter N, Yu C, Middelberg AP. 2014. Nanoparticle vaccines. Vaccine. 32:327–337.