Abstract
Limitations associated with the storage of red blood cells have motivated the development of novel blood substitutes that are able to withstand long-term storage at elevated temperatures. The hemoglobin of the earthworm Lumbricus terrestris (LtEc) is an attractive blood substitute candidate, since it is resistant to oxidation and aggregation during storage. Several factors were investigated to optimize the thermal and oxidative stability of LtEc during storage, including pH, antioxidant supplements, and deoxygenation. A strategy for the reduction of fully oxidized LtEc with antioxidants was also developed. Overall, LtEc was shown to have the highest thermal stability in Ringer’s Modified Lactate solution with 10 mM HEPES at pH 7.0. Deoxygenation of the LtEc was also shown to significantly reduce oxidation of the ferrous heme iron (e.g., %Fe2+ after 7 d at 37 °C = 75.7%). However, even in cases where oxidation does occur, the addition of 1.8 mM ascorbic acid (AA) was found to reduce 98.3% of the oxidized LtEc (37 μM heme). Most importantly, the oxygen transport properties of LtEc were unaffected by storage at high temperatures or oxidation followed by reduction with AA. These results show that LtEc can be stored at high temperatures (37 °C) without any significant loss of function.
Introduction
Despite decades of research into red blood cell (RBC) preservation, the shelf life of donated blood is currently limited to ∼42 d (Hillyer et al. Citation2006) by a myriad of inevitable chemical and structural changes within RBCs that are collectively called the “storage lesion.” For example, the products of RBC metabolism (e.g., lactic acid and protons) accumulate over time, causing a decrease in pH and significant alterations in glycolysis, including a loss of 2,3-diphosphoglycerate and decreased concentrations of ATP (Zimrin and Hess Citation2009). The decreasing concentrations of ATP lead to irreversible damage to the cell membrane, including an increased rigidity (Zimrin and Hess Citation2009). In addition, the hemoglobin (Hb) within RBCs is also damaged during storage. Specifically, the ferrous iron within each subunit oxidizes (Fe2+ to Fe3+) to produce ferric methemoglobin (metHb) and a superoxide anion (O2-) (Yoshida and Shevkoplyas Citation2010). These superoxide radicals may then react with water to form hydroxyl radicals that damage lipids and proteins within the RBCs (Hess Citation2010).
Methods to prevent Hb oxidation have been an active area of research, since a decrease in Hb oxidation could potentially prolong the shelf life of RBCs. Some methods that have been investigated include cryopreservation, deoxygenation, and antioxidant supplementation. Cryopreservation of red blood cells (i.e., storage at ≤ −150 °C) has been shown to preserve hemoglobin structure and function for long periods of storage, but freeze–thaw cycles cause significant damage to the RBC membrane (Scott et al. Citation2005). Subsequent removal of cryoprotectant agents (e.g., glycerol) is also time consuming (∼1 h) (Lusianti et al. Citation2013). Alternatively, deoxygenation of red blood cells can increase the shelf life of donated blood to 9 weeks at 4 °C by slowing the development of the storage lesion and decreasing oxidative damage (Yoshida and Shevkoplyas Citation2010). However, this process can also be time consuming (∼1–3 h) and there is not yet any conclusive clinical data about the viability and efficacy of deoxygenated RBCs (D’Alessandro et al. Citation2013, Yoshida et al. Citation2007). Finally, the addition of antioxidants can also increase RBC shelf life. The addition of ascorbic acid (AA) at concentrations of 5.86–8.78 mM has been shown to maintain Hb in the ferrous state and increase RBC shelf life to 56 d at 1–6 °C (Raval et al. Citation2013).
Unfortunately, even if Hb oxidation is completely prevented, the cellular nature of RBCs will always limit the shelf life of donated blood. This limitation has motivated the development of acellular blood substitutes that can be stored indefinitely. The most promising blood substitutes thus far have been based on polymerized or PEGylated human or cow Hb derivatives, since O2 transport is the most important function of blood. Unfortunately, while many of these hemoglobin-based oxygen carriers (HBOCs) have shown promise in early clinical trials (e.g., Hemopure (Jahr et al. Citation2008), MP4OX (Olofsson et al. Citation2011), and Polyheme (Moore et al. Citation2009)), they have ultimately failed Phase III clinical trials due to severe adverse effects (heart attack and stroke) that are caused by rapid Hb oxidation and scavenging of the vasodilator nitric oxide by Hb (Chen et al. Citation2009, Natanson et al. Citation2008). These side effects can all be attributed to removing intracellular mammalian Hbs from the protective environment of the RBC and releasing them into the harsh extracellular environment of the bloodstream. Therefore, the naturally extracellular Hbs found in annelids may be a better starting material for blood substitutes. These extracellular Hbs (a.k.a. erythrocruorins, Ecs) have many advantages over mammalian Hbs. For example, the Ec of the earthworm Lumbricus terrestris (LtEc) is an extremely stable complex of 144 globin subunits that is held together with an additional 36 linker chains and a dense network of intermolecular disulfide bonds (Royer et al. Citation2006). Its large size (30 nm diameter, MW = 3.6 MDa) also allows it to be purified easily via ultrafiltration (Elmer et al. Citation2010). In contrast to human hemoglobin (HbA), LtEc also has a positive redox potential (Eo = 112 mV for LtEc, Eo = −50 mV for HbA), meaning that it is more resistant to oxidation and more likely to undergo reduction by antioxidants (Dorman et al. Citation2002). Most importantly, preliminary animal studies have shown that LtEc can be safely transfused into mice and hamsters without any noticeable side effects or immune response (Elmer et al. Citation2012, Hirsch et al. Citation1997). Therefore, LtEc appears to be an effective and safe blood substitute. However, the effects of storage on LtEc have not yet been determined. The purpose of this study is to develop a strategy that maintains the structure and function of LtEc during storage at elevated temperatures.
Materials and methods
Purification of LtEc
Earthworms (Lumbricus terrestris) were purchased from Wholesale Bait Supply (Cincinnati, OH). Worms were rinsed in tap water and pureed in a blender until homogenous. The homogenate was centrifuged at 3500g for 30 min at 4 °C. The red supernatant was then centrifuged again at 15,000g for 30 min at 4 °C and sterilized by passing it through a 0.2 μm tangential flow filter (TFF, Spectrum Labs, Rancho Dominguez, CA). The LtEc was then purified via 10 rounds of diafiltration on a 500 kDa MWCO TFF cartridge. In each round of diafiltration, the sample was concentrated to 50 mL, then diluted to 500 mL with 20 mM Tris buffer (pH 7.4). After the last round of diafiltration, the retentate was stored in 1 mL aliquots at −72 °C and centrifuged at 10,000g for 5 min after thawing.
Purification of HbA
Approximately 50 mL of donated human whole blood was purchased from Interstate Blood Supply (Memphis, TN). The blood was centrifuged at 10,000g for 5 min at 4 °C. The serum and white blood cell layers were then aspirated, while the remaining RBC layer was resuspended in 20 mM phosphate buffered saline (PBS, pH 7.4). This wash was repeated three times. The red blood cells were then resuspended in 2 L of 20 mM Tris buffer, pH 7.4 and stored at 4 °C overnight (12–16 h) for lysis. The solution was then centrifuged at 3500g for 15 min at 4 °C to remove the red blood cell debris. The clarified red supernatant was diafiltered (as described above) on a 10 kDa MWCO TFF cartridge. Samples were stored in 1 mL aliquots at −72 °C until needed and centrifuged at 10,000g for 5 min after thawing.
Thermal shift assays
Melting temperatures (Tm) were measured with a thermal shift assay utilizing SYPRO orange dye, which fluoresces when it binds to hydrophobic residues that are exposed as proteins denature at high temperatures (Lavinder et al. Citation2009). For this assay, each sample was diluted to the following optimal concentrations: LtEc(Fe2+) = 0.004 mM heme, metLtEc(Fe3+) = 0.018 mM heme, HbA(Fe2+) = 0.025 mM heme, and metHbA(Fe3+) = 0.055 mM heme. Stocks of 5000× SYPRO orange dye (ThermoFisher Scientific, Waltham, MA, S-6650) were diluted to 200X with Ringer’s solution (115 mM NaCl, 0.3% sodium lactate, 12.25 mM N-acetyl-L-cysteine, 1.4 mM CaCl2, 4.0 mM KCl). About 45 μL of each sample was then mixed with 5 μL of 200× SYPRO orange dye and the thermal shift assay was run on an Applied Biosystems (Waltham, MA) 7300 real-time PCR system that measured sample fluorescence as the temperature was increased from 20 °C to 89 °C in 1 °C increments. The melting temperature (Tm) of each sample was defined as the point of inflection in the fluorescence curve.
Since early experiments revealed that the thermal stability of LtEc steadily decreases over a period of 2 h at acidic pH (from 58 °C to 50 °C at pH = 6.0, data not shown), all the melting temperature measurements shown in were taken after the samples were incubated at room temperature at the corresponding pH for 2–3 h. In contrast, no variation in the thermal stability of HbA over time at acidic pH’s (Tm = 50–53 °C) was observed.
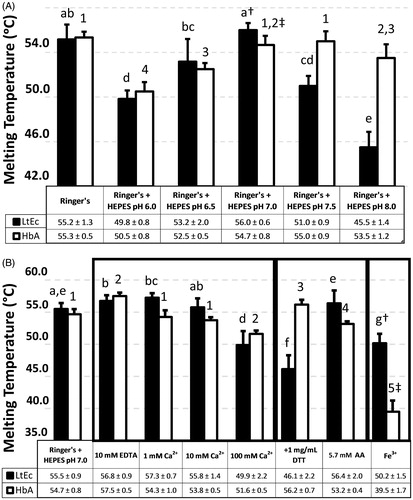
Figure 1. Thermal stability of HbA (0.025 mM heme) and LtEc (0.004 mM heme). Black bars represent LtEc samples, while white bars represent HbA samples. (A) Effects of buffer pH on Tm. pH was maintained with 10 mM HEPES buffer (no HEPES was added to the “Ringer’s” control) and pH values shown indicate the final pH of the mixture. (B) Effects of buffer composition and oxidation state on Tm. All values were compared with the control Ringer’s + HEPES at pH 7.0. Different letters indicate statistical difference amongst LtEc values, and different numbers indicate statistical difference amongst HbA values. Significant differences between LtEc and HbA are denoted with † and ‡.
Long-term oxidation assays
Samples were diluted with buffer to concentrations between 0.16 and 0.18 mM heme and a constant pH of 7.4 (unless otherwise noted). The solutions were then sterilized by filtering them with a 0.2 μm syringe filter and split into 200 μl aliquots in autoclaved microcentrifuge tubes that were stored at 4 °C, room temperature (20 °C), or 37 °C. Each day, aliquots from each temperature condition were centrifuged at 10,000g for 5 min to detect aggregation and the absorbance spectra from 500 to 700 nm were recorded to estimate the extent of oxidation in the sample.
Calculation of percent Fe2+
The absorbance spectra of pure ferrous (Fe2+) and ferric (Fe3+) HbA and LtEc were measured at various pH values (6.0, 6.5, 7.0, 7.5, and 8.0). The absorbances of each pure sample at 576 nm (A576, the maxima of the second Q band) and 588 nm (A588, the isosbestic point for LtEc and metLtEc spectra) or 590 nm (A590, the isosbestic point for HbA and metHbA spectra) were then recorded. Using those values as a reference, the level of reduced hemoglobin (%Fe2+) in the stored samples was then estimated with EquationEq. (1)(1) . Asample was calculated as (A576/A588) for an LtEc sample or (A576/A590) for an HbA sample, while AFe2+ and AFe3+ were calculated using (A576/A588) for LtEc or (A576/A590) for HbA from the pure spectra of the fully reduced (Fe2+) or fully oxidized (Fe3+) samples
(1)
Reduction of oxidized Hb with antioxidants
Oxidized LtEc and HbA samples were prepared by adding potassium ferricyanide (K3[Fe(CN)6]) to a final concentration of 1%. The absorbance spectra of these samples were measured and compared with the pure spectra of metHbA and metLtEc to confirm complete oxidation. Excess K3[Fe(CN)6] was then removed from the samples via desalting in columns packed with Sephadex G-25 Medium (GE Healthcare Life Sciences, Pittsburgh, PA). The completely oxidized and desalted samples were then diluted to concentrations of 0.037 mM heme. At this point, 135 μl of metHb/LtEc was mixed with 15 μl of antioxidant stocks and absorbance spectra were recorded for 3 h at room temperature. The oxidation level of the samples was then estimated using EquationEq. (1)(1) .
Deoxygenation
Samples were diluted with 20 mM Tris (pH 7.4) to 0.16–0.18 mM heme and sterilized with 0.2 μm syringe filters, then connected to a Schlenk line. Deoxygenation was achieved by alternately sparging with nitrogen for 10 min and applying vacuum for 5 min for a total of 2 h. The solution was then stored in nitrogen at 37 °C for 1 or 7 d. The sample was then re-oxygenated and used for oxidation and Hemox analysis.
Oxygen affinity (P50)
The oxygen affinity and co-operativity of each sample were measured with a Hemox Analyzer (TCS Scientific, New Hope, PA). This device spectroscopically measures the O2 saturation of Hb samples, while the partial pressure of oxygen (pO2) is decreased from 150 to 2 mm Hg by sparging with pure nitrogen. All samples were run at 25 °C in Tris (20 mM, pH 7.4). The P50 value was estimated as the pO2 at which half of the hemoglobin is bound to O2. The Hill coefficient (n) was calculated using the equation below, in which HbO2 is the fraction of occupied O2 binding sites and [O2] is the free (unbound) oxygen concentration:
(2)
Table 1. Oxygen affinity (P50) and Hill coefficient (n, a measure of co-operativity) of LtEc, LtEc after (t = 7 d) deoxygenation and storage at 37 °C and metLtEc reduced with AA.
Statistical analysis
Statistical analyses were performed with the R studio software (R Studio, Boston, MA). Statistical significance was defined as p < .05. For comparisons of more than two groups, parametric or non-parametric analysis of variance (ANOVA) was performed. For a parametric ANOVA, the assumption of normality (Shapiro–Wilk test) and the assumption of the homogeneity of variances (Bartlett’s test) were found to be valid. Tukey’s honestly significant difference test was used to determine the significant differences for a parametric ANOVA. For a non-parametric ANOVA, either the assumption of normality and/or the assumption of the homogeneity of variances was found to be invalid. A Kruskal–Wallis test determined if there were significant differences among the groups, and a post hoc test established which groups were significantly different. For comparisons among exactly two groups, the appropriate t-test was employed.
Results
Optimizing thermal stability
Buffer optimization experiments were initiated by investigating the thermal stability (i.e., Tm values) of HbA and LtEc at different pH values in Ringer’s modified lactate solution with 10 mM HEPES as a buffer (see ). At pH 7.3, these experiments revealed a Tm of ∼55 °C for HbA and LtEc. The thermal stability of both HbA and LtEc also decreased significantly at acidic pH values (6.0 and 6.5). This effect may be due to dissociation of LtEc, which has been observed at low pH (∼4.2) (Harrington and Hirsch Citation1991). In contrast, alkaline pH had only a slight effect on HbA (Tm = 53.5 °C at pH 8.0), but the thermal stability of LtEc was substantially reduced at pH 7.5 (Tm = 51 °C) and pH 8.0 (Tm = 45.5 °C). Nonetheless, both HbA and LtEc appear to be most stable around neutral pH (Tm = 56 °C for LtEc at pH 7.0, Tm = 55 °C for HbA at pH 7.5). For this reason, all subsequent experiments were run with LtEc and HbA in a Ringer’s solution with HEPES buffer at pH 7.0.
In addition to pH, the effect of calcium (Ca2+) on thermal stability was also investigated, since LtEc has several Ca2+ binding sites (Standley et al. Citation1988). As expected, the addition of 1 mM Ca2+ significantly increased the Tm of LtEc from 55 °C to 57 °C, but had no significant effect on HbA stability. However, the addition of higher amounts of Ca2+ (100 mM) significantly decreased the Tm of both HbA (52 °C) and LtEc (50 °C). To further investigate this phenomenon, the effects of the chelating agent EDTA were investigated, since it can strip Ca2+ from a protein. However, while the addition of EDTA and subsequent removal of Ca2+ might be expected to decrease the stability of LtEc, EDTA instead slightly increased the Tm of HbA (55 °C) and LtEc (57 °C). Since this effect was observed with both LtEc and HbA, the increase in Tm may be due to chelation of trace amounts of trace pro-oxidant metals (e.g., Fe3+) by EDTA. Additionally, previous studies have found that some calcium binding sites in LtEc have a higher affinity than the calcium binding affinity of EDTA (Kuchumov et al. Citation2000). This antioxidant activity could increase thermal stability by preventing the oxidation of the heme iron, which destabilizes both HbA and LtEc.
Since preventing oxidation of any blood substitute during storage is crucial, the effects of two types of reducing agents were also investigated – the thiol-containing dithiothreitol (DTT) and the thiol-free ascorbic acid (AA). Initial experiments showed high-percentage reduction of metLtEc and metHbA when 0.57 mM AA and 1 mg/mL DTT were added. Interestingly, these reducing agents had opposing effects on HbA and LtEc. In the case of HbA, DTT increased the Tm to 56 °C, while AA decreased the Tm to 53 °C. In contrast, the thermal stability of LtEc was significantly decreased by DTT (Tm = 46 °C), while only a slight increase in Tm was observed with AA (55 –56 °C).
The increases in Tm observed with EDTA and the reducing agents suggest that oxidation state influences the thermal stability of both HbA and LtEc. To confirm this hypothesis, the thermal stability of the oxidized (Fe3+) and reduced (Fe2+) forms of HbA and LtEc were directly compared (). As expected, both Hbs were much less stable in the Fe3+ form than in the Fe2+ form. It is also important to note that metHbA is significantly less stable (Tm = 39.5 °C) than metLtEc (Tm = 50.2 °C). Therefore, LtEc appears to be more thermally stable than HbA in both the oxidized and reduced forms (at their corresponding optimum pH).
Minimizing heme oxidation
The effects of buffer type (Tris or Ringer’s) on oxidation of the heme iron in HbA and LtEc were investigated over a period of 9 d at room temperature (∼20 °C) and 37 °C (see ). In the case of HbA, the lowest oxidation rates were observed in Tris buffer (pH 7.4), while the oxidation rate in Ringer’s solution was higher. In contrast, much lower rates of oxidation were observed for LtEc in Ringer’s solution compared with Tris buffer. To determine which component of Ringer’s buffer might be responsible for this phenomenon, this experiment was repeated with Ringer’s solutions that lacked calcium (CaCl2), sodium lactate, or N-acetyl-l-cysteine (NALC), since these components have either an anti-oxidant (lactate and NALC) or structure-stabilizing (CaCl2) effect that reduces oxidation of LtEc (Ibrahim Sallam Citation2007, Polidori et al. Citation1988, Su et al. Citation2014). While the absence of calcium and lactate slightly increased the oxidation rate of LtEc (data not shown), removing NALC significantly increased the oxidation rate to a level similar to Tris buffer (see ). Since these results suggest that NALC had an antioxidant effect on LtEc, higher concentrations of NALC (24.5–100 mM) and AA were also investigated. However, adding more NALC or AA did not reduce LtEc oxidation rates. On the contrary, addition of higher amounts of AA (>5.7 mM) resulted in rapid oxidation.
Figure 2. Effects of buffer composition on LtEc oxidation. (A) Percentage of reduced (Fe2+) LtEc during storage at room temperature or (B) 37 °C in Tris pH 7.4, Ringer’s solution (containing 12.25 mM N-acetyl-l-cysteine) pH 7.0, Ringer’s solution without lactate, and Ringer’s solution without cysteine. (C) Percentage of reduced (Fe2+) HbA in samples kept at room temperature or (D) 37 °C in Tris pH 7.4, Ringer’s solution pH 7.0, Ringer’s solution without lactate, and Ringer’s solution without cysteine.
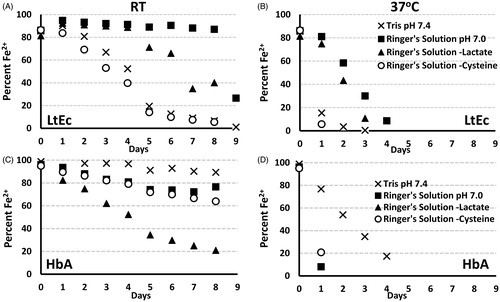
The effects of buffer pH on the oxidation of HbA and LtEc were also investigated (data not shown). While pH significantly influenced thermal stability, long-term storage at different pH values in Ringer’s solution with 10 mM HEPES had no observed effects on the oxidation rate when compared with Ringer’s solution.
Deoxygenation of LtEc
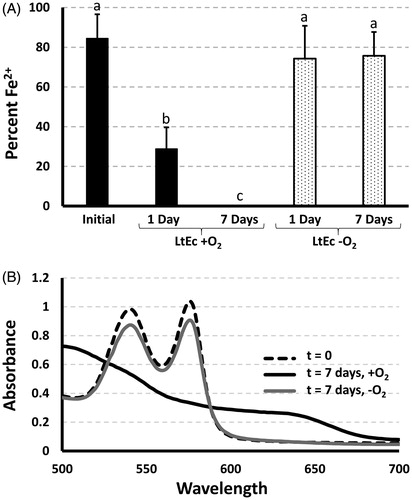
Although storing LtEc in Ringer’s solution at pH 7.0 provided the lowest possible oxidation rate, the LtEc was still oxidized completely within 5 d at 37 °C. In an effort to further reduce LtEc oxidation, LtEc samples were completely deoxygenated and their oxidation rates were monitored at 37 °C. While oxygenated LtEc samples were mostly (70%) oxidized after 1 d and completely oxidized after 7 d, the deoxygenated samples showed no significant increase in oxidation after 1 or 7 d (). A slight decrease in the absorbance spectra was observed () which could be attributed to low amounts of aggregation observed after 7 d, but the percent Fe2+ remained unchanged. It is also important to note that the initial oxidation level of the deoxygenated samples was ∼85%. Therefore, it appears that a small amount of oxidation occurred during the deoxygenation process. Overall, however, deoxygenation appears to be the most effective way to prevent LtEc oxidation.
Figure 3. Effects of deoxygenation on LtEc oxidation. (A) Percent reduced LtEc (Fe2+) stored with and without oxygen at 37 °C for 1 or 7 d. Different letters indicate statistically significant differences among percent Fe2+ values. (B) Normalized representative spectra after 7 d with and without deoxygenation.
Antioxidant screening for reduction of metHb
Even if a Hb sample oxidizes during storage, it may still be a viable blood substitute if it can be fully reduced. To determine the most effective way to reduce metHbA and metLtEc samples, 10 antioxidants were screened for their ability to reduce HbA and LtEc. Two classes of antioxidants were tested – one class with thiol groups (DTT, cysteine, sodium hydrosulfite, glutathione, NALC), and one class without thiols (AA, dextrose, caffeic acid, sodium citrate, sodium lactate). Various concentrations (0.6–60 mM) of these antioxidants were tested over a 3 h period at room temperature, but only the concentration that gave the highest percent reduction of HbA and LtEc are shown in (additional data are included in the Supplementary information). The most effective reducing agents for HbA contained thiols – DTT, cysteine, and sodium hydrosulfite. However, even the optimum concentration of DTT only reduced approximately 52% of the metHbA. Higher concentrations of DTT were also tested (i.e., 10 mg/mL), but caused significant precipitation. In addition to the thiol-containing antioxidants, AA was also able to reduce metHbA somewhat (23%), but the effects of the other antioxidants were negligible (<10% reduction).
Figure 4. Percent reduction of (A) metLtEc and (B) metHbA by optimal concentrations of each antioxidant after 3 h of incubation at room temperature (0.037 mM heme).
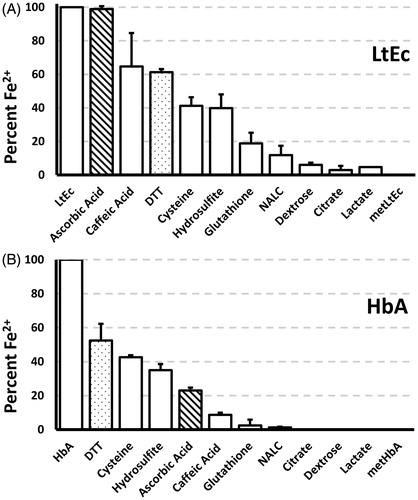
In contrast to HbA, metLtEc (0.037 mM heme) was completely reduced by 1.8 mM AA. Caffeic acid also provided a strong reducing effect, but its relatively lower solubility ultimately limited its percent reduction to 65%. The thiol containing antioxidants were also able to reduce a significant fraction of the metLtEc (40–60%). Unfortunately, all of the thiol-containing antioxidants caused significant precipitation at higher concentrations (> 19 mM). Finally, it is also interesting to note that each of the antioxidants had a more potent reducing effect on LtEc than HbA at the same concentrations.
Oxygen transport properties of deoxygenated LtEc and reduced metLtEc
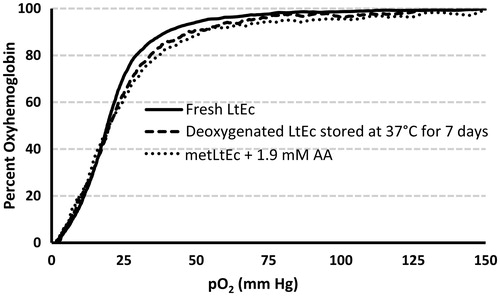
To determine if storage at high temperatures or reduction of metLtEc affect the O2 transport function of LtEc, a Hemox analyzer was used to measure the oxygen affinity (P50) and Hill coefficient (n) of LtEc samples after (1) deoxygenation and storage at 37 °C for 7 d and (2) after LtEc was fully oxidized and subsequently reduced with 1.9 mM AA. As shown in , the oxygen equilibrium curves of LtEc before and after storage were nearly identical, with no significant change in oxygen affinity (P50 =18.80–20.62 mm Hg), see . The Hill coefficients of the deoxygenated samples (n = 2.18) and AA-reduced samples (n = 2.16) were significantly lower than the Hill coefficient of the LtEc control (n = 2.91). This slight decrease in co-operativity might be attributed to low levels of residual metLtEc in the samples or modifications of amino acids that are involved in allostery and cooperativity. Overall, however, the O2 transport capacity of both samples is almost identical to fresh LtEc.
Figure 5. Representative oxygen equilibrium curves of LtEc, LtEc after (t = 7 d) deoxygenation and storage at 37 °C, and after metLtEc was reduced with 0.34 mM AA. Samples were diluted in Tris buffer (pH 7.4) and fully oxygenated (pO2 = 150 mm Hg) at 25 °C, then slowly deoxygenated to a pO2 of 2 mm Hg by bubbling the sample with pure N2. Meanwhile, the percent oxygenated hemoglobin (y-axis) was simultaneously measured with UV–Vis spectroscopy.
Discussion
The optimum storage buffer for HbA appears to be Tris buffer at a pH of 7–7.5. This formulation provided the highest thermal stability (Tm = 55 °C) and lowest oxidation rates. Significantly higher oxidation rates were observed for HbA in Ringer’s solution, while significant decreases in thermal stability were observed at acidic pH values. DTT appears to be the best reducing agent for HbA, since it provided the highest percent reduction of pure metHbA (see ) and it slightly increased the thermal stability of HbA (Tm = 56.2 °C). Conversely, AA seems to promote the oxidation of HbA during storage (data not shown) and decreases thermal stability (see , Tm = 53.2 °C). These effects may be explained by the conversion of AA to dehydroascorbate (DHA), which is a strong pro-oxidant (Deutsch Citation1998).
In contrast to HbA, the optimum storage buffer for LtEc was Ringer’s solution with a pH of 7.0. Ringer’s solution is already commonly used for traumatic resuscitation without any significant side effects, so it may be a viable storage buffer for blood substitutes (Lorenzo et al. Citation1998). The thermal stability of LtEc is maximized at pH 7.0 (Tm = 56 °C), but if LtEc must be stored at physiological pH (∼7.4) for transfusions, its thermal stability at pH 7.5 is still relatively high (Tm = 51 °C). However, LtEc should not be stored at higher pH levels, since its thermal stability significantly decreases at alkaline pH (Tm = 45 °C at pH 8.0). This effect has also been observed with other Ec’s, including Glossoscolex paulistus, Planorbis corneus, and Arenicola cristata (Bispo et al. Citation2005, Vinogradov et al. Citation1980, Wood and Mosby Citation1975).
Further investigation of the composition of Ringer’s solution revealed that the most active antioxidant is NALC (see ), while lactate also provides a lesser antioxidant effect. One of the other ingredients in Ringer’s solution, Ca2+, also provides a slight increase in the thermal stability of LtEc (ΔTm = +1.8 °C) at low concentrations. LtEc is known to have several Ca2+ binding sites, which contribute to its stability and oxygen binding capability (Kuchumov et al. Citation2000). Unfortunately, higher concentrations of these key ingredients (NALC and Ca2+) did not further enhance the stability of LtEc or decrease oxidation rates. Addition of other reducing agents (e.g., AA and DTT) also failed to prevent LtEc oxidation and DTT decreased the thermal stability of LtEc. This decrease in thermal stability can be attributed to the reduction of the numerous disulfide bonds within LtEc (Kao et al. Citation2006). Alternatively, deoxygenation of the LtEc allowed it to be stored at 37 °C for 7 d without significant oxidation or loss of function. This is not surprising, since O2 is responsible for the auto-oxidation of hemoglobin (Dickerson et al. Citation1993).
In the undesirable event that LtEc does oxidize completely during storage, our results show that it might still be useful as a viable blood substitute. First of all, shows that oxidation does decrease the thermal stability of LtEc (ΔTm = −5.3 °C), but it is important to note that the Tm of metLtEc (Tm = 50.2 °C) is still higher than most conceivable storage temperatures. This is in contrast to metHbA, which has a much lower Tm of 39.5 °C. Second, shows that metLtEc can be completely reduced by AA at a molar ratio of 8192:1 (AA:LtEc). This complete reduction is due to the redox potential of LtEc (112 mV) (Harrington et al. Citation2007), which is higher than the oxidation potential of ascorbic acid (51.2 mV at pH 7.2) (Ball Citation1937). Unfortunately, the feasibility of this strategy is limited by the fact that the amount of ascorbic acid required to fully reduce a full unit of LtEc (500 mL of 14 g/dL LtEc) under our reported conditions would be approximately 16 g of AA, which is significantly higher than the typical concentration of AA in blood (∼0.34 mM). Finally, it is also important to mention that our experiments were conducted at concentrations (∼0.18 mM) that are lower than physiological Hb concentrations (∼18 mM) and it has been shown that the oxidation rate of HbA increases at higher concentrations (Mansouri and Perry Citation1987). Nonetheless, our results show that deoxygenated LtEc has a virtually negligible oxidation rate and ascorbic acid could be used to effectively reduce a partially oxidized LtEc solution.
In conclusion, this work shows that deoxygenated LtEc in Ringer’s solution can be stored at high temperatures (37 °C) for long periods (≥ 1 week) without significant denaturation or oxidation. Even if partial oxidation of the LtEc does occur, the metLtEc is still highly stable and can be reduced by AA to restore O2 transport. Altogether, these results show that LtEc is a highly stable blood substitute that does not require refrigeration. Future animal studies involving transfusion of LtEc that has been stored at 37 °C for 1 week or longer will need to be conducted to confirm the safety of LtEc after storage, but these results suggest that LtEc may be particularly useful for treating hemorrhagic shock in remote areas or battlefields where storage facilities for donated blood are unavailable.
Christine_Muzzelo_et-al_supplemental_content.pdf
Download PDF (306.2 KB)Acknowledgements
The authors would like to thank Dr. Charles Coe for his advice and help with our deoxygenation experiments. The authors would also like to thank Devon Zimmerman for assistance with protein purification.
Disclosure statement
The authors have no conflict of interest to declare.
Additional information
Funding
References
- Ball EG. 1937. Studies on oxidation–reduction: XXIII. Ascorbic Acid. J Biol Chem. 118:219–239.
- Bispo JAC, Landini GF, Santos JLR, Norberto DR, Bonafe CFS. 2005. Tendency for oxidation of annelid hemoglobin at alkaline pH and dissociated states probed by redox titration. Comp Biochem Physiol Part B: Biochem Mol Biol. 141:498–504. doi: 10.1016/j.cbpc.2005.06.002
- Chen J-Y, Scerbo M, Kramer G. 2009. A review of blood substitutes: examining the history, clinical trial results, and ethics of hemoglobin-based oxygen carriers. Clinics (Sao Paulo). 64:803–813. doi: 10.1590/S1807-59322009000800016
- D’Alessandro A, Gevi F, Zolla L. 2013. Red blood cell metabolism under prolonged anaerobic storage. Mol Biosyst. 9:1196–1209. doi: 10.1039/c3mb25575a
- Deutsch JC. 1998. Oxygen-accepting antioxidants which arise during ascorbate oxidation. Anal Biochem. 265:238–245. doi: 10.1006/abio.1998.2940
- Dickerson LD, Sauermasarwa A, Herron N, Fendrick CM, Busch DH. 1993. The electron-transfer mechanism of autoxidation for hemoglobin, myoglobin, and their iron(II) cyclidene models. J Am Chem Soc. 115:3623–3626.
- Dorman SC, Kenny CF, Miller L, Hirsch RE, Harrington JP. 2002. Role of redox potential of hemoglobin-based oxygen carriers on methemoglobin reduction by plasma components. Artif Cells Blood Substit Immobil Biotechnol. 30:39–51.
- Elmer J, Harris DR, Sun G, Palmer AF. 2010. Purification of hemoglobin by tangential flow filtration with diafiltration. Biotechnol Prog. 25:1402–1410. doi: 10.1002/btpr.217
- Elmer J, Zorc K, Rameez S, Zhou Y, Cabrales P, Palmer AF. 2012. Hypervolemic infusion of Lumbricus terrestris erythrocruorin purified by tangential-flow filtration. Transfusion. 52:1729–1740. doi: 10.1111/j.1537-2995.2011.03523.x
- Harrington JP, Hirsch RE. 1991. Analysis of the acid and alkaline dissociation of earthworm hemoglobin, Lumbricus terrestris, by front-face fluorescence spectroscopy. Biochim Biophys Acta. 1076:351–358.
- Harrington JP, Kobayashi S, Dorman SC, Zito SL, Hirsch RE. 2007. Acellular invertebrate hemoglobins as model therapeutic oxygen carriers: unique redox potentials. Artif Cells Blood Substit Immobil Biotechnol. 35:53–67. doi: 10.1080/10731190600974491
- Hess JR. 2010. Red cell storage. J Proteomics. 73:368–373. doi: 10.1016/j.jprot.2009.11.005
- Hillyer CD, Silberstein LE, Ness PM, Anderson KC, Roback JD. 2006. Blood Banking and Transfusion Medicine: Basic Principles & Practice, 2nd ed. Philadelphia, PA: Churchill Livingstone.
- Hirsch RE, Jelicks LA, Wittenberg BA, Kaul DK, Shear HL, Harrington JP. 1997. A first evaluation of the natural high molecular weight polymeric Lumbricus terrestris hemoglobin as an oxygen carrier. Artif Cells Blood Substit Immobil Biotechnol. 25:429–444. doi: 10.3109/10731199709118932
- Ibrahim Sallam K. 2007. Antimicrobial and antioxidant effects of sodium acetate, sodium lactate, and sodium citrate in refrigerated sliced salmon. Food Control. 18:566–575. doi: 10.1016/j.foodcont.2006.02.002
- Jahr JS, Mackenzie C, Pearce LB, Pitman A, Greenburg AG. 2008. HBOC-201 as an alternative to blood transfusion: efficacy and safety evaluation in a multicenter phase III trial in elective orthopedic surgery. J Trauma. 64:1484–1497. doi: 10.1097/TA.0b013e318173a93f
- Kao WY, Qin J, Fushitani K, Smith SS, Gorr TA, Riggs CK, et al. 2006. Linker chains of the gigantic hemoglobin of the earthworm Lumbricus terrestris: primary structures of linkers L2, L3, and L4 and analysis of the connectivity of the disulfide bonds in linker L1. Proteins Struct Funct Genet. 63:174–187. doi: 10.1002/prot.20852
- Kuchumov AR, Loo JA, Vinogradov SN. 2000. Subunit distribution of calcium-binding sites in Lumbricus terrestris hemoglobin. J Protein Chem. 19:139–149. doi: 10.1023/A:1007086717412
- Lavinder JJ, Hari SB, Sullivan BJ, Magliery TJ. 2009. High-throughput thermal scanning: a general, rapid dye-binding thermal shift screen for protein engineering. J Am Chem Soc. 131:3794–3795. doi: 10.1021/ja8049063
- Lorenzo M, Davis JW, Negin S, Kaups K, Parks S, Brubaker D, et al. 1998. Can Ringer’s lactate be used safely with blood transfusions? Am J Surg. 175:308–310.
- Lusianti RE, Benson JD, Acker JP, Higgins AZ. 2013. Rapid removal of glycerol from frozen-thawed red blood cells. Biotechnol Prog. 29:609–620. doi: 10.1002/btpr.1710
- Mansouri A, Perry CA. 1987. Hemoglobin autoxidation at physiological concentrations. Hemoglobin. 11:353–371. doi: 10.3109/03630268709042854
- Moore EE, Moore FA, Fabian TC, Bernard AC, Fulda GJ, Hoyt DB, et al. 2009. Human polymerized hemoglobin for the treatment of hemorrhagic shock when blood is unavailable: the USA Multicenter Trial. J Am Coll Surg. 208:1–13. doi: 10.1016/j.jamcollsurg.2008.09.023
- Natanson C, Kern SJ, Lurie P, Banks SM, Wolfe SM. 2008. Cell-free hemoglobin-based blood substitutes and risk of myocardial infarction and death: a meta-analysis. JAMA. 299:2304–2312. doi: 10.1001/jama.299.19.jrv80007
- Olofsson CI, Górecki AZ, Dirksen R, Kofranek I, Majewski JA, Mazurkiewicz T, et al. 2011. Evaluation of MP4OX for prevention of perioperative hypotension in patients undergoing primary hip arthroplasty with spinal anesthesia: a randomized, double-blind, multicenter study. Anesthesiology. 114:1048–1063. doi: 10.1097/ALN.0b013e318215e198
- Polidori G, Mainwaring MG, Vinogradov SN. 1988. The effect of alkaline earth cations and of ionic strength on the dissociation of earthworm hemoglobin at alkaline pH. Comp Biochem Physiol: A Comp Physiol. 89:541–545.
- Raval JS, Fontes J, Banerjee U, Yazer MH, Mank E, Palmer AF. 2013. Ascorbic acid improves membrane fragility and decreases haemolysis during red blood cell storage. Transfus Med. 23:87–93. doi: 10.1111/tme.12013
- Royer WE, Sharma H, Strand K, Knapp JE, Bhyravbhatla B. 2006. Lumbricus erythrocruorin at 3.5 ?? resolution: architecture of a mega Dalton respiratory complex. Structure 14:1167–1177. doi: 10.1016/j.str.2006.05.011
- Scott KL, Lecak J, Acker JP. 2005. Biopreservation of red blood cells: past, present, and future. Transfus Med Rev. 19:127–142. doi: 10.1016/j.tmrv.2004.11.004
- Standley PR, Mainwaring MG, Gotoh T, Vinogradov SN. 1988. The calcium, copper and zinc content of some annelid extracellular haemoglobins. Biochem J. 249:915–916.
- Su X, Guo S, Huang X, Wang X, Qi D, Yang C. 2014. Control of oxidative reactions of hemoglobin in the design of blood substitutes: role of the Vc, NAC, TEMPO and their reductant system. Artif Cells Nanomed Biotechnol. 42:222–228. doi: 10.3109/21691401.2013.834907
- Vinogradov SN, Shlom JM, Doyle M. 1980. Dissociation of the extracellular hemoglobin of Arenicola cristata. Comp Biochem Physiol: Part B Biochem. 65:145–150. doi: 10.1016/0305-0491(80)90123-6
- Wood EJ, Mosby LJ. 1975. Physicochemical properties of Planorbis corneus erythrocruorin. Biochem J. 149:437–445.
- Yoshida T, AuBuchon JP, Tryzelaar L, Foster KY, Bitensky MW. 2007. Extended storage of red blood cells under anaerobic conditions. Vox Sang. 92:22–31. doi: 10.1111/j.1423-0410.2006.00860.x
- Yoshida T, Shevkoplyas SS. 2010. Anaerobic storage of red blood cells. Blood Transfus. 8:220–236. doi: 10.2450/2010.0022-10
- Zimrin AB, Hess JR. 2009. Current issues relating to the transfusion of stored red blood cells. Vox Sang. 96:93–103. doi: 10.1111/j.1423-0410.2008.01117.x
