Abstract
In this presented work, Syntrichia papillosissima (Copp.) Loeske (S. papillosissima) was used as a natural phytosorbent for IgG purification. These moss species were collected for the natural habitat and prepared for IgG adsorption studies by cleaning, drying, and grinding to uniform size. Syntrichia papillosissima samples were characterized by using FTIR and SEM studies. Functional groups of S. papillosissima were identified by FTIR analysis, while surface characteristics were determined by SEM studies. A batch system was used for the adsorption of IgG onto S. papillosissima surface and physical conditions of the IgG adsorption medium were investigated by modifying the pH, IgG concentration and temperature. Maximum IgG adsorption onto S. papillosissima was found to be 68.01 mg/g moss by using pH 5.0 buffer system. Adsorption kinetic isotherms were also studied and it was found that, Langmuir adsorption model was appropriate for this adsorption study. Reusability profile of S. papillosissima was also investigated and IgG adsorption capacity did not decrease significantly after 5 reuse studies. Results indicated that S. papillosissima species have the capacity to be used as biosorbent for IgG purification, with its low cost, natural and biodegradable structure.
Introduction
There have been increasing attentions to plants as a support material for purification studies. Bryophytes have been used intensively as a phytosorbent because of their surface characteristics and easy to supply properties. There are many examples for usage of bryophytes as an adsorbent such as Cd(II) and Cr(III) removal by Hylocomium splendens (Hedw.) Schimp. [Citation1], 137Cs and 90Sr attachment by Funaria hygrometrica Hedw. [Citation2], Racomitrium lanuginosum (Hedw.) Brid. for Pd separation [Citation3], Co and Sr adsorption by Rhytidiadelphus squarrosus (Hedw.) Warnst. [Citation4], dye effluent removal from waste water by Dicranellavaria (Hedw.) Schimp. [Citation5].
Some bryophytes species have protrusion structures over their leaves in order to adapt themselves to drought conditions. These structures named as papilla and mamilla and they have increased the surface area of leaf considerably. For this reason, wide surface area requisite by affinity chromatography is supplied form these bryophytes species. Despite the chemical structure of bryophytes leafs are not totally explained, they contain significant hydrophobic groups in their structure. Cuticle layer on surface and wax structures increase the hydrophobicity of leafs. It is supposed to increased usage of these bryophytes species by identification of adsorption mechanism of these plants.
Immunoglobulin G (IgG) is an important therapeutic molecule and is one of the major protein components of the human serum. IgG have been intensively used for treatment of immunodeficiencies [Citation6–8], idiopathic purpura, autoimmune and chronic inflammatory disorders and cancers [Citation9–12]. IgG is also very important immunological assay tool and it has been intensively used in various assays such as ELİSA, radioimmunoassay, immunosensor and antibody microarrays [Citation13–15] and these sensitive assays require highly pure form of IgG. Protein A and G are the highly preferred affinity ligands in bioseparation systems and have been widely used for the separation of IgG in order to use in analytical and diagnostic applications [Citation16]. Along with these bioligands, some other pseudo-affinity ligands (metal chelates, immobilized histidine and thiophilic ligands) have been used for the purification studies of IgG [Citation17].
In this presented work, a bryophyte (moss) [Syntrichia papillosissima (Copp.) Loeske] (S. papillosissima) was used as a new and natural support material without any required ligand. For this purposes, moss samples were obtained from natural habitat. These samples were then washed, cleaned and dried; dried samples were grinded and sieved to obtain uniform size for adsorption studies. Characterizations of these natural samples were performed by using Fourier Transform Infrared (FTIR) and Scanning Electron Microscopy (SEM) studies. Adsorption experiments of IgG from its aqueous solutions were carried out in a batch system and different adsorption conditions were investigated. Additionally, reusability profile of the bryophyte for IgG adsorption was tested along with the IgG elution studies.
Materials and methods
Materials
Human immunoglobulin G (IgG) was purchased from Sigma (St. Louis, MO). Ethylene glycol was supplied by Fluka (Switzerland). All other chemicals were of analytical grade and used without any purification or separation process. Deionized water was produced by using Millipore, Simplicity® (18.2 MΩcm) and was used for the preparation of all solutions.
Preparation of S. papillosissima samples
S. papillosissima samples were collected from natural habitat which they grow at Aydın, Paşa yaylası vicinity. Plant materials were cleaned by cutting the dead parts and washing the sample in order remove dust and debris by using distilled water. Cleaned moss samples were dried at 40 °C for 1 week and then grinded in order to separate the samples for particle size.
Characterization of S. papillosissima samples
Functional groups of the S. papillosissima samples were analyzed by using FTIR spectrophotometer (Varian FTS 7000, USA). For this purpose, about 0.1 g of moss samples were mixed with FTIR grade KBr (∼0.1 g) and pressed in a pellet form. The pellet was mounted in the FTIR spectrophotometer and FTIR spectra of moss samples were recorded. Surface morphology and general surface characteristics of moss samples were investigated by using SEM device (Philips XL-30S FEG, the Netherlands). For SEM studies, dried samples were covered by thin layer of gold under vacuum and then mounted in the SEM devices and SEM pictures of the samples were photographed.
Adsorption of IgG from aqueous solutions
All IgG adsorption studies were carried out in batch mode set up. Briefly, moss samples were mixed with 5.0 mL of IgG solution (in a buffer system), and mixed for 2 h at stirring rate of 100 rpm. IgG concentrations was measured spectrophotometrically at 280 nm by using an UV/Vis spectrophotometer (Shimadzu 1601, Japan). Adsorbed amount of IgG calculated (EquationEquation (1))(1) by measuring the initial and final IgG concentration in adsorption solution.
(1) where Q is adsorbed amount of IgG (mg/g); C0 and C are the IgG concentrations in the initial (before mixing with moss samples) and final solutions (after treating with moss samples), respectively (mg/mL); V is the total aqueous volume of adsorption medium (mL); and m is the mass of the moss sample used for experiment (g).
Desorption of IgG and reusability of moss samples
In order to choose the desorption agent, two solutions with different characters were tested; NaCl and ethylene glycol. Higher desorption yields were found by using the solution of ethylene glycol and this demonstrated that hydrophobic interactions were one of the important interactions between IgG and moss surface. In order to determine the reusability profile of the moss samples, IgG adsorption and desorption cycle was repeated for five times. For this, after IgG adsorption step, adsorbed IgG was desorbed from the moss samples by using ethylene glycol solution (50%). Desorbed moss samples washed with distilled water and equilibrated with appropriate buffer solution. Desorption ratio was calculated by following equation (EquationEquation (2))(2) .
(2)
Results and discussion
Characterization of moss samples
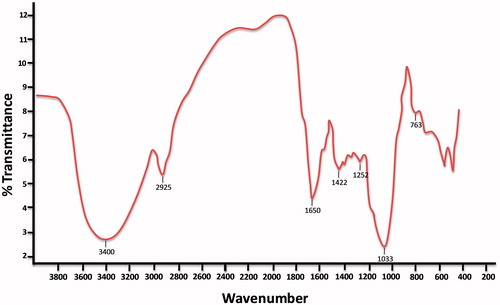
Functional group characteristics of the S. papillosissima were investigated by using FTIR studies. As clearly seen in , the FTIR spectrum of S. papillosissima plant have characteristic stretching vibration bands of aldehyde and/or carboxygroups (around 1650 cm−1), phenol and alcohol groups (3550–3200 cm−1), amine groups (3500–3300 cm−1), ether groups (1070–1150 cm−1); and alkyl chains (2950–2850 cm−1; bending band around 1350–1480 cm−1) and aromatic rings (∼3030 cm−1; and C–H bending band around 760 cm−1) which indicates hydrophobicity. As clearly concluded from these results that, S. papillosissima samples have different type of mixed functional groups along with various characteristics such as hydrophilic and hydrophobic regions.
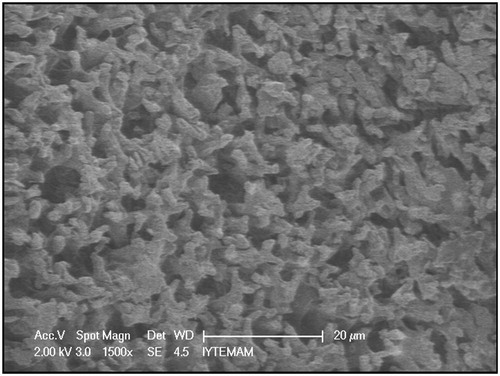
The surface morphology and structure of S. papillosissima samples were investigated by electron microscopy (). As clearly seen here, plant surfaces have porous structures and projections called papilla. These structures increase the surface area of the plant and increased surface area enhance the adsorption capacity.
IgG adsorption studies from aqueous solutions
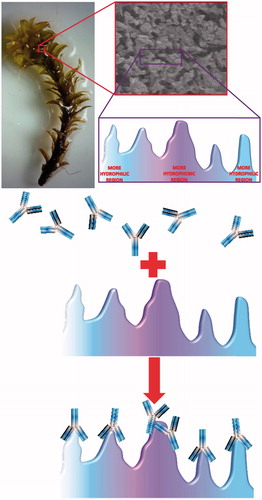
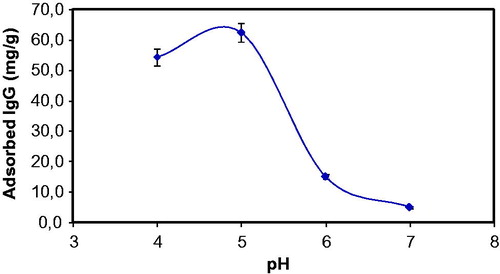
All adsorption studies were carried out in batch mode with different adsorption conditions. One of the most important adsorption conditions is the pH of adsorption medium. In this presented work, effect of medium pH on IgG adsorption onto S. papillosissima sample was studies first. For this, pH of the IgG adsorption medium was changed between 4.0 and 7.0 by using acetate (for pH 4.0–5.0) and phosphate (for pH 6.0–7.0) buffers. Findings from this study were demonstrated in . As seen here, maximum IgG adsorption onto S. papillosissima was observed at pH 5.0. Isoelectric point of IgG is around pH 7.2–8.6, and IgG will be positive charged at pH 5.0. This finding was the evidence showed that one of the driving force interactions between IgG and S. papillosissima is electrostatic interactions. As seen in this figure, IgG adsorption capacity decreased dramatically above pH 5.0. This decrease generally occurs due to the protonation and deprotonation of surface functional groups (alkyl, aldehyde, etc.) of S. papillosissima. However the pH results showed that the interaction between IgG and S. papillosissima is electrostatic, due to the structure of the support material (S. papillosissima) hydrophobic interactions play important role for the adsorption process. There are some works demonstrated that the interactions between IgG and support material are not only electrostatic but also hydrophobic [Citation18]. A schematic presentation for the IgG adsorption onto hydrophobic and hydrophilic region of the S. papillosissima sample was demonstrated in .
Figure 3. Effect of pH on IgG adsorption onto S. papillosissima. IgG concentration 0.5 mg/mL; temperature: 25 °C.
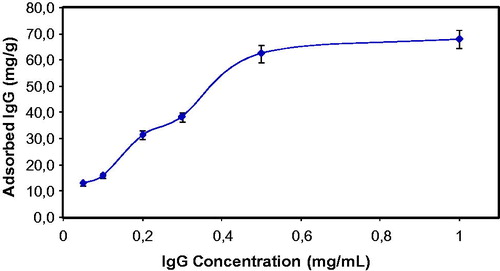
Effect of initial concentration of IgG on the IgG adsorption onto S. papillosissima sample was demonstrated at . As seen here, adsorbed amount of IgG increased with increasing initial IgG concentration, then reached a saturation value at 0.5 mg/mL of IgG amount. This saturation profile generally occurs in a typical adsorption studies, because at this concentration value, all active adsorption sites of the S. papillosissima sample is occupied with adsorbed IgG molecules and after this IgG concentration value, adsorbed amount of IgG do not change anymore.
In order to evaluate the adsorption behavior, adsorption isotherms are used. Two main adsorption models have been used to conclude the adsorption process: Langmuir and Freundlich. These models are expressed as following equations, respectively:
where b and KF are the constants for the Langmuir and the Freundlich isotherms, respectively. n is the exponent of the Freundlich isotherm which expresses the surface heterogeneity, qe the adsorbed amount of IgG and the maximum loading capacity of the adsorption matrix is expressed as qmax.
Adsorption behavior of IgG onto S. papillosissima sample was investigated by using these two model and kinetic parameters are summarized in . When comparing the R2 values of the isotherms, it can be said that Langmuir adsorption model is applicable for this adsorption system by reviewing the experimental data and Langmuir equations results. According to this adsorption model, IgG molecules were adsorbed onto fixed number of the certain adsorption sites of the S. papillosissima, which could hold only one IgG molecule. These adsorption sites were energetically equivalent and placed enough distant to each other, so there were no cross-interaction between adsorbed species and/or adsorption sites.
Table 1. Kinetic constants of Langmuir and Freundlich isotherms.
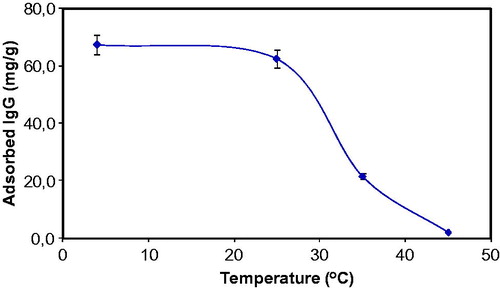
demonstrates the effect of the medium temperature on the IG adsorption efficiency of the S. papillosissima. As clearly seen in this figure that, effect of the medium temperature was investigated in the temperature range of 4–45 °C and IgG adsorption capacity of the S. papillosissima decreased slightly when temperature increased from 4 to 25 °C, and then decreased significantly after 25 °C. Maximum IgG adsorption was achieved at 4 °C. Because S. papillosissima is a biological sample; functional groups, active biological components and biochemical structures of the S. papillosissima are very sensitive for temperature. Due to the biological origin of the sample used in this experiment, adsorption capacity of the S. papillosissima decreased significantly at high temperatures.
Figure 6. Effect of temperature on IgG adsorption onto S. papillosissima. IgG concentration 0.5 mg/mL; pH: 5.0.
Different studies have been reported in the literature for IgG adsorption. Babac et al. [Citation19] reported an adsorption capacity of 6.7 mg of IgG/g by using ConA-modified poly(AAm-AGE) cryogels. Sun et al. [Citation20] used agarose beads embedded agarose-chitosan composite monolithic cryogels as an adsorbent. They reported IgG adsorption capacity of 71.4 mg/g adsorbent. Gondim et al. [Citation21] evaluated the IgG adsorption ability of Cibacron Blue F3GA dye-ligand chitosan/alginate epoxide. They founded IgG adsorption capacity as 110 mg/g adsorbent. Çulha et al. [Citation22] used l-lysine imprinted cryogels for IgG adsorption and adsorption capacity of this cryogels was found to be 55.1 mg/g cryogel. Nakanishi et al. [Citation23] prepared gold nanoparticle-mesoporous silica sheet composites for IgG adsorption. The IgG adsorption capacity obtained as 0.26 mg IgG for per mg carrier, in this study. Ding et al. [Citation18] used carboxylated single-walled carbon nanotubes (SWCNTs) for binding of IgG. The protein-binding capacity of SWCNTs was 0.52 mg IgG per mg of SWCNTs. Bakhshpour et al. [Citation24] used (PHEMA/PEI)-Cu(II) cryogels for IgG adsorption. The maximum IgG adsorption capacity onto (PHEMA/PEI)-Cu(II) cryogel was observed as 72.3 mg/g, in this study. Maximum IgG adsorption capacity of our study which used S. papillosissima as an adsorbent was found to be 68.01 mg/g. This adsorption value is highly comparable with the literature cited and also it was concluded that this phytosorbent have been successfully used for IgG adsorption studies without any ligand modifications or any post functionalizations.
Desorption and repeated use
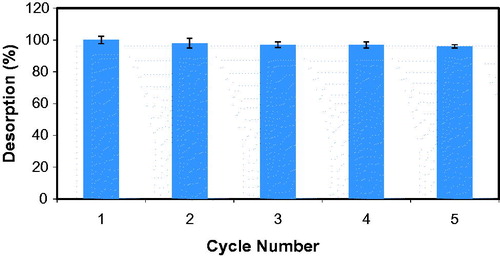
Investigation of reusability profile of the support material is very important because of the large-scale applications of this material. Adsorbed IgG was desorbed from the S. papillosissima by using ethylene glycol solution and desorption efficiency was found to be up to 100%. Reusability of the S. papillosissima based support was studied adsorption-desorption cycle was repeated for 5 times. As clearly seen at , adsorption capacity of the S. papillosissima did not changed significantly at the end of the 5th reuse.
Conclusions
One of the important proteins of the human blood is IgG, which is used by immune system to identify foreign objects. It is a therapeutic agent and has been intensively used for treatment areas as a medicine. Medical usage needs highly purified IgG and typical purification strategy depends on various physicochemical methods. Because of these physicochemical purification steps, unit price of the IgG is very expensive. Due to this monetary disadvantage, development and usage of the new, low cost and effective alternatives for IgG purification have been gained great importance recently. In this study, S. papillosissima is a natural plant and was used as an alternative for IgG purification without any modification. Before the experiments, S. papillosissima samples were grinded and samples which particle size was smaller than 140 μm were collected and used for the IgG adsorption studies. Syntrichia papillosissima is a natural, cheap and biodegradable material and efficiently used for the adsorption of IgG from its aqueous solutions. These biosorbents were used without any post modification and this behavior increased its applicability. Results from this study promise hope and it is expected to pave the way for diverse application for purification or adsorption of other biomolecules.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Sarı A, Mendil D, Tüzen M, et al. Biosorption of Cd(II) and Cr(III) from aqueous solution by moss (Hylocomium splendens) biomass: equilibrium, kinetic and thermodynamic studies. Chem Eng J. 2008;144:1–9.
- Balarama Krishna MV, Rao SV, Arunachalam J, et al. Removal of 137Cs and 90Sr from actual low level radioactive waste solutions using moss as a phyto-sorbent. Sep Purif Technol. 2004;38:149–161.
- Sarı A, Mendil D, Tüzen M, et al. Biosorption of palladium(II) from aqueous solution by moss (Racomitrium lanuginosum) biomass: equilibrium, kinetic and thermodynamic studies. J Hazard Mater. 2009;162:874–879.
- Maresova J, Pipiska M, Rozloznik M, et al. Cobalt and strontium sorption by moss biosorbent: modeling of single and binary metal systems. Desalination. 2011;266:134–141.
- Akkaya G, Özer A. Biosorption of Acid Red 274 (AR 274) on Dicranella varia: determination of equilibrium and kinetic model parameters. Process Biochem. 2005;40:3559–3568.
- Yavuz H, Bereli N, Yılmaz F, et al. Antibody purification from human plasma by metal chelated affinity membranes. In: S. Reichelt, editor. Affinity chromatography methods and protocols. 3rd ed. Mol Biol, 1286, (205), 4. Heidelberg: Humona Press; 2015. p. 43–46.
- Denizli A, Rad AY, Pişkin E. Protein A immobilized polyhydroxyethylmethacrylate beads for affinity sorption of human immunoglobulin G. J Chromatogr B. 1995;668:13–19.
- Wolf HH, Davies SV, Borte M, et al. Efficacy, tolerability, safety and pharmacokinetics of a nanofiltered intravenous immunoglobulin: studies in patients with immune thrombocytopenic purpura and primary immunodeficiencies. Vox Sang. 2003;84:45–53.
- El Alfy MS, Mokhtar GM, El-Laboudy MAM, et al. Randomized trial of anti-D immunoglobulin versus low-dose intravenous immunoglobulin in the treatment of childhood chronic idiopathic thrombocytopenic purpura. Acta Haematol. 2006;115:46–52.
- Gurcan HM, Ahmed AR. Efficacy of various intravenous immunoglobulin therapy protocols in autoimmune and chronic inflammatory disorders. Ann Pharmacother. 2007;41:812–823.
- Chester K, Pedley B, Tolner B, et al. Engineering antibodies for clinical applications in cancer. Tumor Biol. 2004;25:91–98.
- Majidi J, Barar J, Baradaran B, et al. Target therapy of cancer: implementation of monoclonal antibodies and nanobodies. Hum Antibodies. 2009;18:81–100.
- Bischof Delaloye A, Delaloye B. Tumor imaging with monoclonal antibodies. Semin Nucl Med. 1995;25:144–164.
- Miroslav P. Monoclonal and polyclonal antibodies production – preparation of potent biorecognition element. J Appl Biomed. 2009;7:115–121.
- Welbeck K, Leonard P, Gilmartin N, et al. Generation of an anti-NAGase single chain antibody and its application in a biosensor-based assay for the detection of NAGase in milk. J Immunol Methods. 2011;364:14–20.
- Denizli A, Alkan M, Garipcan B, et al. Novel metal-chelate affinity adsorbent for purification of immunoglobulin-G from human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2003;795:93–103.
- Özkara S, Yavuz H, Denizli A. Purification of immunoglobulin G from human plasma by metal-chelate affinity chromatography. J Appl Polym Sci. 2003;89:1567–1572.
- Ding Y, Tian R, Yang Z, et al. Binding of human IgG to single-walled carbon nanotubes accelerated myeloperoxidase-mediated degradation in activated neutrophils. Biophys Chem. 2016;218:36–41.
- Babac C, Yavuz H, Galaev IY, et al. Binding of antibodies to concanavalin A-modified monolithic cryogel. React Funct Polym. 2006;66:1263–1271.
- Sun S, Tang Y, Fu Q, et al. Monolithic cryogels made of agarose-chitosan composite and loaded with agarose beads for purification of immunoglobulin G. Int J Biol Macromol. 2012;50:1002–1007.
- Gondim DR, Dias NA, Bresolin ITL, et al. Human IgG adsorption using dye-ligand epoxy chitosan/alginate as adsorbent: influence of buffer system. Adsorption. 2014;20:925–934.
- Çulha S, Armutçu C, Uzun L, et al. Synthesis of l-lysine imprinted cryogels for immunoglobulin G adsorption. Mater Sci Eng C Mater Biol Appl. 2015;52:315–324.
- Nakanishi K, Tomita M, Masuda Y, et al. Gold nanoparticle–mesoporous silica sheet composites with enhanced antibody adsorption capacity. New J Chem. 2015;39:4070–4077.
- Bakhshpour M, Derazshamshir A, Bereli N, et al. [PHEMA/PEI]–Cu(II) based immobilized metal affinity chromatography cryogels: application on the separation of IgG from human plasma. Mater Sci Eng C. 2016;61:824–831.