Abstract
This study aim to synthesize silver nanoparticles (AgNPs) using Anthemis atropatana extract and to evaluate their chemical characteristics and antimicrobial and cytotoxic effects. The biosynthesis of AgNPs is verified using UV–visible spectrum which showing maximum absorption in 430 nm wavelength. Transmission electron microscope (TEM) and scanning electron microscope (SEM) results revealed that AgNPs has a spherical shape with an average size of 38.89 nanometres. The crystalline structure of green synthesized AgNPs in optimal conditions was confirmed by XRD analysis. The pattern of XRD peaks related to face-centred cubic (fcc) (111), (200), (220), (311) and (222) observed. Also, FTIR results verified the AgNPs synthesis using plant extract. In biological tests, the MTT results indicate the dose dependence of cytotoxic effects of AgNPs on colon cancer cell lines (HT29). The AgNPs had maximum cytotoxicity on HT29 cancer cell line at 100 μg/ml concentration, which were statistically significant comparing control cells (p < .001). Moreover, real time PCR and flow cytometry results confirmed the apoptotic effects of AgNPs. According to the results, it seems that the green synthesis of AgNPs is an eco-friendly and cost effective approach. This research provides insight into the development of new anticancer and antibacterial agents.
Introduction
Recently, the development of new methods for synthesis of metallic nanoparticles with appropriate size and morphology is an interesting area in nanotechnology [Citation1]. Among the nanoparticles, silver nanoparticles (AgNPs) have been considered by most researchers with great medical and pharmaceutical applications, because of its remarkable properties, such as high surface to volume ratio, catalytic properties as well as biological effects [Citation2,Citation3]. More studies have indicated good potential of AgNPs as anticancer and antibacterial agent. AgNPs destroy the mitochondrial respiration chain in cancer cells as well as degrading DNA, which ultimately results in cancer cells death. In addition, AgNPs destroy the bacterial cells membrane and consequently the cell is lysed [Citation4,Citation5]. Nowadays, researchers are finding convenient, simple and rapid approaches to synthesize AgNPs with potential for utilization as anti-angiogenic, antimicrobial and anticancer agents [Citation6]. Therefore, the synthesis of AgNPs using easy, low-cost and eco-friendly methods has received increased attention [Citation7]. There are various physical and chemical methods for AgNPs synthesis, including chemical and photochemical reduction, electro-irradiation and laser mediated synthesis, which are expensive and can cause toxicity for the environment [Citation8,Citation9]. In comparison with other methods, biological methods, such as plant extracts and microorganisms are currently used for AgNPs synthesis [Citation10]. Green synthesis of AgNPs using plants extract has been beneficial to microorganisms due to their less biohazard [Citation11]. Plants extract have large number of secondary compounds and biomolecules that act as reducing and capping agents for green synthesis of AgNPs [Citation12]. In recent years, various plants extract, such as Artemisia marschalliana Sprengel [Citation13], Carica Papaya Peel [Citation14], Pongamia pinnata seed [Citation15], Heterotheca inuloides [Citation16] and Borago officinalis [Citation17] have been used for synthesis of AgNPs. Anthemis atropatana is a member of Compositae family, which is native to Iran and various species of Anthemis genus are used as medicinal plants [Citation18]. Many reports suggest that Anthemis genus extract has anticancer, antimicrobial and antioxidant activities and its antioxidant activity is related to the presence of phenolic compounds and flavonoids, which are considered as important factors in reduction of Ag+ to AgNPs [Citation19]. So far, the A. atropatana extract has not been used for the synthesis of AgNPs and the aim of this study was green synthesis of AgNPs using A. atropatana extract and evaluation of its antimicrobial, anticancer and apoptotic effects on cancer cell line. However, based on our knowledge, this is the first study to investigate a rapid and simple approach for the synthesis of AgNPs using A. atropatana extract as a reducing and stabilizing agent.
Experimental
Plant collection and extraction procedure
The plant was obtained from Iranian Biological Resources Centre Herbarium, Tehran, Iran with Voucher specimens No.1342. Fresh aerial parts of A. atropatana were air-dried in the shade for 1–2 weeks. A total of 50 g of dried powder was added to 500 ml of methanol as solvent and then boiled for 20 min. The crude extract was filtered using Whatman No. 1 filter paper (Whatman plc, Kent, UK) and the solvent was eliminated using rotary evaporator (Rv10 digital, Germany). Finally, the prepared extract was kept in 4 °C until further use.
Green synthesis of AgNPs
For green synthesize of AgNPs, 4 ml of A. atropatana extract added to 100 ml of 0.01 mM silver nitrate (AgNO3) (Merck, Germany) under different temperature and stirring conditions. After 5 min of stirring at room temperature, the colourless solution turned into a brown colour, which reflected the reduction of Ag+ to Ag0 NPs. Then, the precipitate was three times washed with distilled water using centrifuge (all the washing steps was performed for 20 min in 13,000 rpm). Then, the AgNPs were dried at 40 °C for 2 h prior to characterization.
Characterization of AgNps
UV–visible spectroscopic characterization of AgNPs
UV–visible spectroscopy analysis of AgNPs was performed using UV–visible instrument (JASCO V-670 Spectrophotometer) in 200–700 nm wavelength.
X-ray diffraction analysis
X-ray diffraction (XRD) enables the detection of crystallographic structure of AgNPs. In this study, XRD test was performed with Cu Kα radiation in the range of 2θ = 10°–80°.
Transmission electron microscopy (TEM) and scanning electron microscopy (SEM)
AgNPs morphology and size examination were performed using TEM and SEM. Briefly, in TEM, a drop of particles suspension was placed on carbon film grid and photographed after drying at room temperature, a transmission electron microscopy (Leo 906, Zeiss100KV model, Germany) was used. In addition, morphology and size of AgNPs was another evaluated using SEM. SEM images of AgNPs was recorded after coating with gold at a voltage below 30 KV under pressurized vacuum (5–10 Torr) using Philips XL30 model electron microscope.
Fourier transform infrared spectroscopy (FTIR)
Fourier transform infrared (FT-IR) spectroscopy was performed using a spectrum RX1 instrument. FTIR was used to identify the possible functional groups involved in the synthesis of AgNPs. FT-IR spectra were scanned between 4000 and 400 cm−1 at a resolution of 4 cm−1 in the transmittance mode.
Antimicrobial test
Evaluation of minimum inhibitory concentration (MIC)
Minimum inhibitory concentration (MIC) method was used to evaluate the antimicrobial activity of the synthesized AgNPs. In this study, four pathogenic bacteria including Staphylococcus aureus (ATCC 6538), Streptococcus pyogenes (ATCC 19615), Pseudomonas aeruginosa (ATCC 15442) and Escherichia coli (ATCC 25922) were used. For this purpose, 5 × 105 CFU/ml concentration of these bacteria was prepared and each of them was treated with AgNPs (12.5–100 μg/ml). The MIC value was determined as the lowest concentration of AgNPs that inhibited the growth of the tested bacteria. The test was performed in triplicate.
Cytotoxicity assays
Cell lines and culture medium
The human colon cancer (HT29) and mouse fibroblast cell line (L929) were purchased from the Pasteur Institute cell bank, Tehran, Iran. The cells were grown adherently and maintained in Dulbecco’s Modified Eagle’s Medium supplemented with 10% foetal bovine serum, 2 mM glutamine, 1% antibiotic solution (100 U/ml of penicillin, 100 μg/ml of streptomycin) and 1 mM sodium pyruvate (all from Thermo Fisher Scientific, Waltham, MA) in a 5% CO2 incubator at 37 °C. This study was approved by the ethics committee of Islamic Azad University research council and was conducted based on the principles outlined in Declaration of Helsinki.
MTT assay
Cell viability test was performed using 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. Briefly, the cells were enumerated and then seeded into 96-well plates at a density of 104 cells per well and incubated in a CO2 incubator at 37 °C for 24 h. HT29 cancer cells were treated with various concentrations of AgNPs in 96-well plates. Subsequently, 20 μl of MTT solution (5 mg/ml) was added to each AgNP-treated cell followed by incubation at 37 °C and 5% CO2 for 4 h. Finally, the resulting purple-coloured formazan crystals were dissolved in dimethyl sulfoxide and the optical density (OD) of each well was measured. The absorbance was recorded at 570 nm using a microplate reader (Bio-Rad 680; Hercules, CA). The cytotoxic effect of AgNPs on cells was expressed as percentage of cell viability compared to control, which was calculated based on the following formula: percentage of cell viability (%) = mean OD/control OD ×100.
Analysis of apoptosis genes’ expression level
The expression level of two apoptotic genes including Bax and Bcl2 was measured by real time PCR method. At first, the cells total RNA (treated and untreated) was extracted using RNA extraction kit (Qiagene, Valencia, CA) based on manufacturers procedures and its concentration was measured by Photo nanometre device (IMPLEN GmbH, Germany). Subsequently, cDNA synthesis was done using Revert AidTM cDNA Synthesis Kit (Fermentas, Lithuania) based on manufacturer’s instructions. Subsequently, gene expression level of Bax, Bcl2 genes was evaluated using Bioneer Real Time PCR system. In addition, glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as housekeeping gene. The forward and reverse primers of target genes for real time PCR test are given in . Real Time PCR programme was as following: 95 °C (1 min), 95 °C (15 s), 60 °C (60 s) for 60 cycles. Finally, the target/internal control gene expression ratio was calculated using the formula (ratio = 2−ΔΔCt) using the REST programme (Relative Expression Software Tool).
Table 1. The primers sequences of target genes.
Analysis of apoptosis/necrosis in HT29 cell line
To determination of apoptosis/necrosis in HT29 cells treated with AgNPs, the annexin V/propidium iodide (PI) method (Apoptosis detection kit, Roche, Germany) was used. HT29 cells (1 × 105 cells per well) were treated with IC50 concentration of the AgNPs for 24 h and the cells were subject to flow cytometric analysis for evaluation of apoptosis/necrosis level.
DNA fragmentation test
HT29 cancer cells were incubated with IC50 concentrations of AgNPs for 24 h. Then, the cells were washed using PBS and were then trypsinized. The cell suspension was centrifuged in 4000 rpm and 600 μl lysis buffer (10 mM Tris–HCL, pH = 7.5, 400 mM Nacl, 100 mM EDTA, 0.6% SDS) and 10 μl of RNase solution was added to the precipitate. The resulting solution was incubated for 5 min at 37 °C and 200 μl of 6 M Nacl was added to precipitate the proteins. The resultant solution was incubated on ice for 5 min and centrifuged for 10 min in 16,000 rpm at room temperature. The supernatant was collected, 600 μl isopropanol was added to it and incubated for 15 min on ice and again centrifuged for 20 min in 16,000 rpm and the precipitate was washed with 600 μl of 70% ethanol. The precipitate was dried at room temperature and dissolved in 200 μl of a specific buffer [10 mM Tris–HCL, Tris–EDTA, 1 mM EDTA, (pH 8.0)]. Then, electrophoresis using 2% agarose gel at a voltage of 100 V for 1 h used for observation of DNA.
Statistical analysis
Statistical analysis was performed using SPSS version16 software (SPSS Inc., Chicago, IL) and the results were evaluated by one way ANOVA. Expression level of target genes between control and treated samples was calculated by Tukey’s HSD post-hoc test. Moreover, the data were expressed as mean ± SD and p < .05 was considered statistically significant.
Results and discussion
Synthesis and characterization of AgNPs
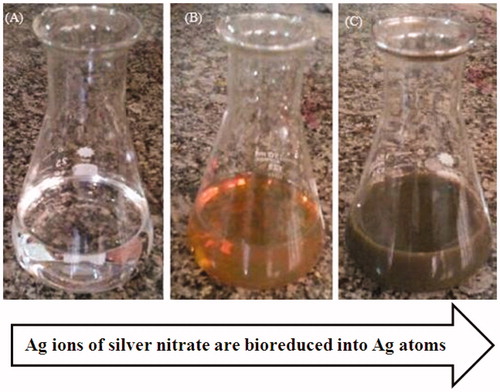
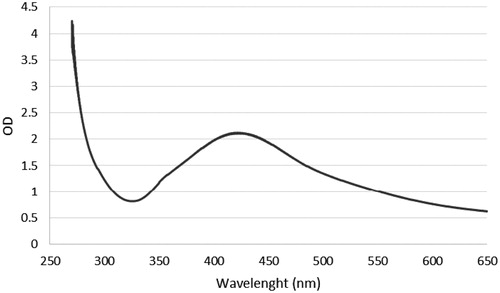
This study focused on synthesis of AgNPs using an eco-friendly and plant mediated synthesis method. AgNPs was synthesized in a continuously heated and stirred mixture of aqueous silver nitrate solution and A. atropatana extract. After several minutes, the colourless reaction mixture slowly turned into a dark-brown suspension (). This indicated that silver ions in the reaction have been converted to elemental silver, which was consistent with other reports of green synthesis using different plant extracts [Citation20,Citation21]. In our study, the rate of synthesis was very high and it was complete in 10 min. Overall, we successfully synthesized AgNPs using the methanolic extract of A. atropatana extract, which act as reducing agent. A. atropatana is cheap and easily available and there is no need to capping and reducing agent. Recently, many plant extracts have been studied for the synthesis of AgNPs [Citation22,Citation23] but, the extract from aerial part of A. atropatana have not been used for green synthesis of AgNPs, so far. For UV–vis analysis, the absorption of NPs was observed in 430 nm wavelength in UV–vis spectrum. Moreover, no additional peaks were observed in the spectrum, which indicates pure biosynthesis of AgNPs (. The biosynthesis mechanism of AgNPs by the plant extract is not fully understood. A combination of amino acids, phenolic acids, amino acids or flavonoids are likely to be the main cause for reduction of metallic silver to AgNPs [Citation24]. Several studies have shown that proteins/enzymes play an important role in the synthesis of nanoparticles [Citation25–27].
XRD analysis of AgNPs
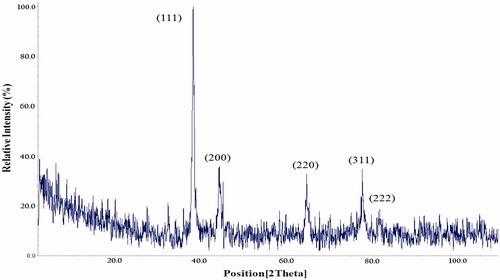
The crystalline structure of green synthesized AgNPs in optimal conditions was confirmed by XRD analysis. The pattern of XRD peaks related to face-centred cubic (fcc) (111), (200), (220), (311) and (222) can be observed in . The results are consistent with other reports that confirm the cubic crystalline structure of synthesized AgNPs [Citation28,Citation29].
SEM and TEM analysis
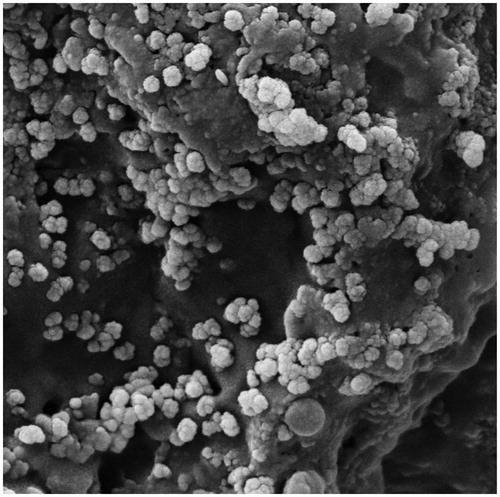
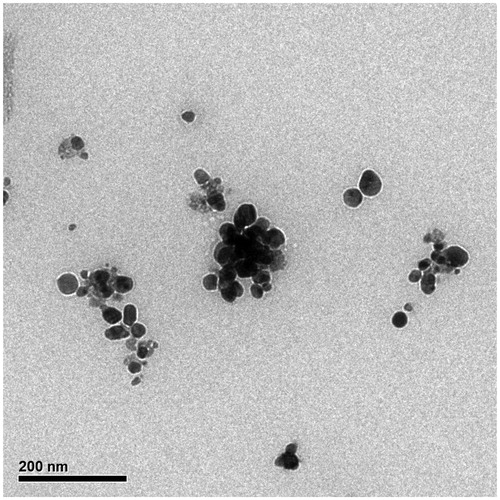
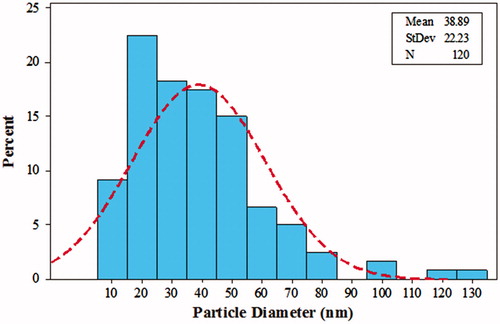
Micrographs of TEM and SEM indicate that the green synthesized AgNPs at optimal conditions had maximum average size of 10–80 nm with mean size of 38.8 nm (). The morphology of AgNPs was assessed through SEM micrographs with a voltage below 30 KV with different magnifications under vacuum pressure (5–10 Torr). As shown in , aggregation and agglomeration occurred in some areas, which can be dispersed by sonication and observed with SEM. In addition, TEM images verified the shape and size of AgNPs ( and ).
Fourier transform infrared spectroscopy (FTIR)
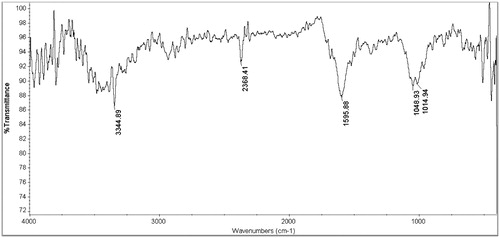
The results of FTIR analysis of AgNPs showed different stretches of bonds at different peaks, 1014, 1048 and 1595, 2368 and 3344 cm−1. The 1014 cm−1 peak is assign to the stretching of C–O ether group. The peak near 1048 cm−1 corresponds to C–O stretching ester group. The band at 1595 cm−1 is related to N–H bending vibration present in the amide linkage of the proteins. These functional groups have role in stability/capping of AgNPs. The band at 2368 cm−1 is assign to cyanide. The peak at 3344 cm−1 corresponds to N–H stretching vibration of the amine and it show that the A. atropatana extract has many functional groups () .
Antibacterial test
In this study, antibacterial activity of green synthesized AgNPs was evaluated against four pathogenic bacteria including S. aureus, S. pyogenes, P. aeruginosa and E. coli. The results show that the effectiveness of AgNPs was variable against selected bacteria (). The highest and lowest MIC values were belonged to P. aeruginosa and S. aureus, respectively. The exact mechanism of antimicrobial activity of AgNPs has not been determined, but more studies show that AgNPs can destroy the bacterial cell membrane, leading to bacterial cell death [Citation30,Citation31]. Furthermore, the silver ions released from AgNPs can react with biomolecules containing nitrogen, sulphur, and oxygen and thus kill the bacteria [Citation32]. Another hypothesis concerning antibacterial activity is related to reaction of silver with DNA of the cells as well as cellular proteins involved in ATP synthesis [Citation33]. Similar studies showed the antibacterial activity of green synthesized AgNPs. Salehi et al. have reported that the green synthesized AgNPs by Artemisia marschalliana Sprengel had antibacterial effects on pathogenic bacteria [Citation13]. Alsalhi et al. indicated the antimicrobial potential of green-synthesized AgNPs against infectious bacteria [Citation34].
Table 2. Minimum inhibitory concentration (MIC) against the four bacteria.
MTT assay
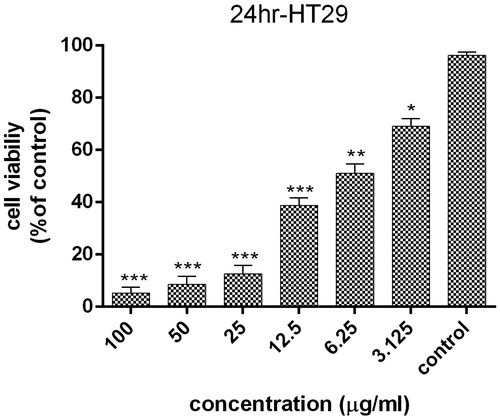
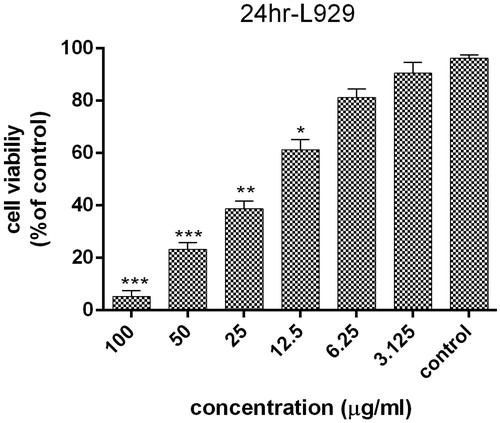
The cytotoxicity of AgNPs on HT29 cell line at 3.125, 6.25, 12.5, 25, 50 and 100 μg/ml concentration of AgNPs after 24 h was determined by the 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyl-tetrazolium bromide (MTT) assay. The results show that the highest cytotoxic effect of AgNPs is observed in 100 μg/ml, which was statistically significant comparing to control cells (p < .001). As indicated in , the cell toxicity of green synthesized AgNPs is dose-dependent. It can be concluded that the AgNPs may enter the cells in different ways, such as phagocytosis, pinocytosis or endocytosis and interact with cellular material [Citation35]. Also, AgNPs might induce the production of toxic oxygen radicals, which leads to DNA fragmentation as well as apoptosis induction [Citation36]. In addition, treatment of L929 cells was done with same concentrations of AgNPs within 24 h. AgNPs showed the highest level of cell proliferation inhibition at 100 μg/ml concentration, which was statistically significant (p < .001), while 6.25 and 3.125 μg/ml concentrations showed no significant differences with the control group (p = .99). According to the results, AgNPs was more effective against HT29 cell line than L929. In addition, IC50 of AgNPs on HT29 and L929 cell line were 4.88 and 15.46 μg/ml at 24 h, respectively. More studies have been showed the cytotoxicity of AgNPs on cancer cell lines. Karlsson et al. indicated that the dose dependent cytotoxicity effect of green synthesized AgNPs on cancer cell line [Citation37] ().
Analysis of apoptosis-related gene expression
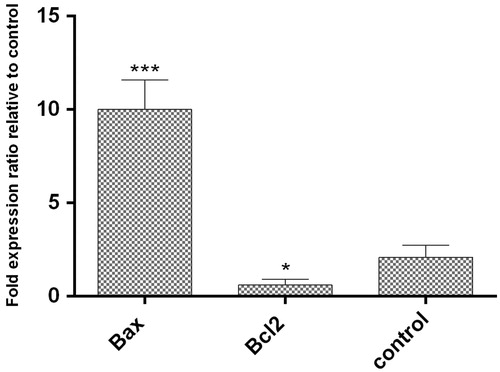
Gene expression levels of pro-apoptotic and anti-apoptotic genes at mRNA level in AgNPs treated HT29 cells were evaluated by Real Time PCR after 24 h. Our results show that the expression ratio of Bax and Bcl2 genes to reference gene (GAPDH) in HT29 cells after treatment with AgNPs was up-regulated (10.33 ± 0.76% (p < .05) and down-regulated (0.43 ± 0.53% (p < .05), respectively (. Previous studies also have shown that AgNPs can induce apoptosis genes in other cancer cell lines [Citation38].
Figure 10. The expression of Bax and Bcl2 genes level comparing to reference gene (GAPDH). The expression ratio of Bcl2 and Bax genes in HT29 cancer cell line treated with AgNPs was reduced by 0.43 ± 0.53% and increased by 10.33 ± 0.76% within 24 h, respectively. (p < .05)*, p < .01**, p < .001***, n = 3).
Apoptosis and necrosis test
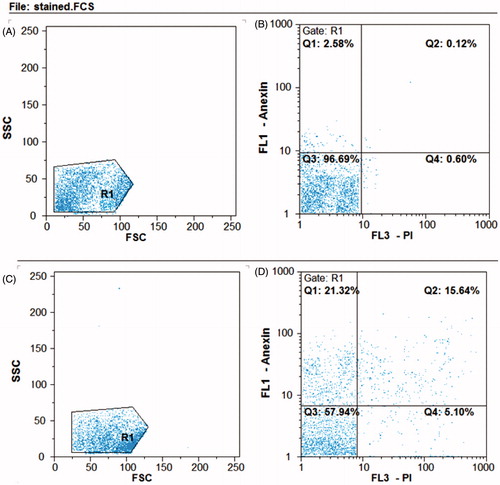
To determine apoptosis and necrosis in HT29 cell line treated with IC50 concentrations of AgNPs, the cells were stained with FITC Annexin V and PI and studied by flow cytometry () . During the early phase of apoptosis, phosphatidylinositol serine is translocate to cell membrane and is stained by annexin V, whereas, PI binds to nucleus in necrotic cells [Citation39]. Flow cytometry results are shown in , in which the upper left square (Q1) represents the percentage of late apoptotic cells and top left square (Q2) early apoptotic cells. As the results show, the AgNPs have directed the cell into early and delayed apoptosis by 15.64 and 21.32%, respectively. Therefore, it can be concluded that the synthesized AgNPs conduct the cells towards apoptosis, which is important in cancer therapy.
Figure 11. Flow cytometry analysis of HT29 cell line. B) Untreated control samples D) AgNPs treated samples. A and C represent the entire content of the cells. The bottom left square: live cells, top left square: early apoptosis, bottom right square: necrosis, upper right square: delayed apoptosis. In the samples treated with AgNPs, the cells enter to early apoptosis (15.64%) and delayed apoptosis (21.32%).
DNA fragmentation assay
The results of DNA fragmentation test demonstrated that the AgNPs treated HT29 cells exhibited extensive double strand breaks, thereby yielding a ladder appearance (Lane 3), while the DNA of control HT29 cells exhibited minimum breakage (Lane 2) (. Fragmented DNA is one the reason for induction of apoptosis in HT29 cells.
Conclusion
In this study, A. atropatana extract was used for the first time as a reducing and capping agent to synthesize AgNPs without production of any harmful chemicals. In addition, this method is facile, rapid and eco-friendly. There are several compounds within the extract, which can reduce the silver ions to AgNPs. Our results also show that the synthesized AgNPs had good physical properties as well as biological effects. Moreover, the AgNPs had antibacterial and anticancer effects and it can induce the apoptosis more intensely than necrosis in cancer cell line. According to significant biological effects of the synthesized AgNPs, it suggested that further studies were performed for AgNPs pharmaceutical importance.
Disclosure statement
The authors report no conflicts of interest.
References
- Swamy MK, Akhtar MS, Mohanty SK, et al. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochim Acta A Mol Biomol Spectrosc. 2015;151:939–944.
- Gopinath V, Mubarak AD, Priyadarshini S, et al. Biosynthesis of silver nanoparticles from Tribulus terrestris and its antimicrobial activity: a novel biological approach. Colloids Surf B Biointerfaces. 2012;96:69–74.
- Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145:83–96.
- He Y, Du Z, Ma S, et al. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus Longan Lour. J Mol Recognit. 2016;11:300–309.
- Nayak D, Pradhan S, Ashe S, et al. Biologically synthesised silver nanoparticles from three diverse family of plant extracts and their anticancer activity against epidermoid A431 carcinoma. J Colloid Interface Sci. 2015;457:329–338.
- Ramar M, Manikandan B, Marimuthu PN, et al. Synthesis of silver nanoparticles using Solanum trilobatum fruits extract and its antibacterial, cytotoxic activity against human breast cancer cell line MCF 7. Spectrochim Acta A Mol Biomol Spectrosc. 2015;140:223–228.
- Nadagouda MN, Speth TF, Varma RS. Microwave-assisted green synthesis of silver nanostructures. Acc Chem Res. 2011;44:469–478.
- Tao A, Sinsermsuksaku P, Yang P. Polyhedral silver nanocrystals with distinct scattering signatures. Angew Chem Int Ed Engl. 2006;45:4597–4601.
- Li K, Zhang FS. A novel approach for preparing silver nanoparticles under electron beam irradiation. J Nanopart Res. 2010;12:1423–1428.
- Rafique M, Sadaf I, Rafique MS, et al. A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol. 2016;8:1–20.
- Hema JA, Malaka R, Muthukumarasamy NP, et al. Green synthesis of silver nanoparticles using Zea mays and exploration of its biological applications. IET Nanobiotechnol. 2016;10:288–294.
- Krishnaraj C, Muthukumaran P, Ramachandran R, et al. Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDAMB-231, human breast cancer cells. Biotechnol Rep. 2014;4:42–49.
- Salehi S, Shandiz SA, Ghanbar F, et al. Phytosynthesis of silver nanoparticles using Artemisia marschalliana Sprengel aerial part extract and assessment of their antioxidant, anticancer, and antibacterial properties. Int J Nanomedicine. 2016;11:1835–1846.
- Kokila T, Ramesh PS, Geetha D. Biosynthesis of AgNPs using Carica Papaya peel extract and evaluation of its antioxidant and antimicrobial activities. Ecotoxicol Environ Saf. 2016;134:467–473.
- Beg M, Maji A, Mandal AK, et al. Green synthesis of silver nanoparticles using Pongamia pinnata seed: characterization, antibacterial property, and spectroscopic investigation of interaction with human serum albumin. J Mol Recognit. 2016;30:e2565. doi: 10.1002/jmr.2565
- Morales-Luckie RA, Lopezfuentes-Ruiz AA, Olea-Mejía OF, et al. Synthesis of silver nanoparticles using aqueous extracts of Heterotheca inuloides as reducing agent and natural fibers as templates: Agave lechuguilla and silk. Mater Sci Eng C Mater Biol Appl. 2016;69:429–436
- Singh H, Du J, Yi TH. Green and rapid synthesis of silver nanoparticles using Borago officinalis leaf extract: anticancer and antibacterial activities. Artif Cells Nanomed Biotechnol. 2016;6:1–7.
- Rustaiyan A, Aberomand Azar P, Moradalizadeh M, et al. Volatile Constituents of Three Compositae Herbs: Anthemis altissima L. var. altissima Conyza canadensis (L.) Cronq. and Grantina aucheri Boiss. Growing Wild in Iran. J Essent Oil Res. 2004;16:579–581.
- Bardaweel SK, Tawaha KA, Hudaib MM. Antioxidant, antimicrobial and antiproliferative activities of Anthemis palestina essential oil. BMC Complement Altern Med. 2014;14:297.
- Arunachalam K, Shanmuganathan B, Sreeja PS, et al. Phytosynthesis of silver nanoparticles using the leaves extract of Ficus talboti king and evaluation of antioxidant and antibacterial activities. Environ Sci Pollut Res Int. 2015;22:18066–18075.
- Ramesh PS, Kokila T, Geetha D. Plant mediated green synthesis and antibacterial activity of silver nanoparticles using Emblica officinalis fruit extract. Spectrochim Acta A Mol Biomol Spectrosc. 2015;142:339–343.
- Godipurge SS, Yallappa S, Biradar NJ, et al. A facile and green strategy for the synthesis of Au, Ag and Au-Ag alloy nanoparticles using aerial parts of R. hypocrateriformis extract and their biological evaluation. Enzyme Microb Technol. 2016;95:174–184.
- Yee MS, Khiew PS, Chiu WS, et al. Green synthesis of graphene-silver nanocomposites and its application as a potent marine antifouling agent. Colloids Surf B Biointerfaces. 2016;148:392–401.
- Mandal D, Kumar Dash S, Das B, et al. Bio-fabricated silver nanoparticles preferentially targets Gram positive depending on cell surface charge. Biomed Pharmacother. 2016;83:548–558.
- Priya RS, Geetha D, Ramesh PS. Antioxidant activity of chemically synthesized AgNPs and biosynthesized Pongamia pinnata leaf extract mediated AgNPs – a comparative study. Ecotoxicol Environ Saf. 2016;134:308–318.
- Gopinath K, Kumaraguru S, Bhakyaraj K, et al. Green synthesis of silver, gold and silver/gold bimetallic nanoparticles using the Gloriosa superba leaf extract and their antibacterial and antibiofilm activities. Microb Pathog. 2016;101:1–11.
- Soman S, Ray JG. Silver nanoparticles synthesized using aqueous leaf extract of Ziziphus oenoplia (L.) Mill: characterization and assessment of antibacterial activity. J Photochem Photobiol B. 2016;163:391–402.
- Patra JK, Baek KH. Biosynthesis of silver nanoparticles using aqueous extract of silky hairs of corn and investigation of its antibacterial and anticandidal synergistic activity and antioxidant potential. IET Nanobiotechnol. 2016;10:326–333.
- Rodríguez-González C, Velázquez-Villalba P, Salas P, et al. Green synthesis of nanosilver-decorated graphene oxide sheets. IET Nanobiotechnol. 2016;10:301–307.
- Syed B, M N NP, B L D, et al. Synthesis of silver nanoparticles by endosymbiont Pseudomonas fluorescens CA 417 and their bactericidal activity. Enzyme Microb Technol. 2016;95:128–136.
- Fouad H, Hongjie L, Yanmei D, et al. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif Cells Nanomed Biotechnol. 2016;18:1–10.
- Składanowski M, Golinska P, Rudnicka K, et al. Evaluation of cytotoxicity, immune compatibility and antibacterial activity of biogenic silver nanoparticles. Med Microbiol Immunol. 2016;205:603–613.
- Magalhães AP, Moreira FC, Alves DR, et al. Silver nanoparticles in resin luting cements: antibacterial and physiochemical properties. J Clin Exp Dent. 2016;8:e415–e422.
- Alsalhi MS, Devanesan S, Alfuraydi AA, et al. Green synthesis of silver nanoparticles using Pimpinella anisum seeds: antimicrobial activity and cytotoxicity on human neonatal skin stromal cells and colon cancer cells. IJN. 2016;11:4439–4449.
- Chan EL, Zhang C, Cheung GS. Cytotoxicity of a novel nano-silver particle endodontic irrigant. Clin Cosmet Investig Dent. 2015;7:65–74.
- Wang Z, Liu S, Ma J, et al. Silver nanoparticles induced RNA polymerase-silver binding and RNA transcription inhibition in erythroid progenitor cells. ACS Nano. 2013;7:4171–4186.
- Karlsson HL, Gliga AR, Calléja FM, et al. Mechanism-based genotoxicity screening of metal oxide nanoparticles using the ToxTracker panel of reporter cell lines. Part Fibre Toxicol. 2014;11:41.
- Baharara J, Namvar F, Ramezani T, et al. Silver nanoparticles biosynthesized using Achillea biebersteinii flower extract: apoptosis induction in MCF-7 cells via caspase activation and regulation of Bax and Bcl-2 gene expression. Molecules. 2015;20:2693–2706.
- Sadat Shandiz SA, Farasati S, Saeedi B, et al. Up regulation of KAI1 gene expression and apoptosis effect of imatinib mesylate in gastric adenocarcinoma (AGS) cell line. Asian Pac J Trop Dis. 2016;6:120–125.