Abstract
The current study highlights the rapid biosynthesis of gold nanoparticles (Gu–AuNps) and silver chloride nanoparticles (Gu–AgClNps) by aqueous root extract of Glycyrrhiza uralensis, a medicinal plant. G. uralensis has been reported for anticancer and hepatoprotective effects. The reduction of chloroauric acid and silver nitrate by the Glycyrrhiza root extract prompted the formation of Gu–AuNps and Gu–AgClNps within 4 and 40 min at 80 °C, respectively. The complete reaction did not require supplemental reducing and stabilizing agents, which demonstrated green synthesis. Field emission transmission electron microscopy (FE-TEM) revealed the spherical shape of Gu–AuNps and Gu–AgClNps. X-ray diffraction (XRD) showed face-centred cubic structure of Gu–AuNps and Gu–AgClNps with average crystallite size 12.25 nm and 8.01 nm, respectively. The biosynthesized Gu–AgClNps served as competent antimicrobial agent against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa and Salmonella enterica. Additionally, Gu–AuNps and Gu–AgClNps were analyzed for their catalytic ability to reduce methylene blue as model test pollutant. Likewise, both nanoparticles possessed free radical scavenging activity against 2,2-diphenyl-1-picrylhydrzyl (DPPH). Moreover, in vitro cytotoxicity in murine macrophage (RAW264.7) and human breast cancer (MCF7) cells were evaluated. Thus, the study proposes a green synthesis of Gu–AuNps and Gu–AgClNps by G. uralensis extract and in vitro biological applications.
Introduction
Green synthesis of nanoparticles has received a widespread attention as a facile alternative in the development of plasmonic metal nanoparticles. Compared with their bulk counterparts, environmentally friendly nanoparticles may offer effective solutions to current biomedical and environmental challenges in the areas of medicine, catalysis, and water treatment due to their unique physicochemical properties and absence of toxic chemicals [Citation1]. In recent past, physical and chemical methods have been used for the synthesis of gold (AuNps) and silver nanoparticles (AgNps). However, greener methodologies have been found superior than conventional synthesis methods, often offering low-cost, efficient, and benign substitutes of metallic nanoparticle production [Citation2,Citation3]. Various biological sources – micro-organisms, fungi, and plants – have been utilized for the synthesis of nanoparticles, serving dual roles as reducing and stabilizing agents and enhancing biosynthesized nanoparticles with positive biological properties which are beneficial for human applications [Citation4].
To our knowledge, this is the first paper to utilize Chinese Licorice (Glycyrrhiza uralensis) root extract as a potent chemical agent in a one-pot green synthesis of gold (Gu–AuNps) and silver chloride nanoparticles (Gu–AgClNps). The aqueous root extract of G. uralensis was used as reducing and capping agent. The plant has been extensively used as a traditional medicinal herb in China for over many centuries. The medicinal herb has been known to exert anti-viral, anti-oxidant, anti-inflammatory, anti-ulcer, anti-cancer and anti-HIV properties due to the presence of glycyrrhizin and flavonoids as the major ingredients [Citation5].
Antimicrobial activity, biofilm inhibition, anticoagulant, and anti-inflammatory effects of metal nanoparticles have been well studied, which make them ideal candidates for many medical and biological applications [Citation6]. In this present study, we focus on the development of suitable gold and silver chloride nanomaterials with sufficient physicochemical characterization prior to antimicrobial, catalytic, and antioxidant. The formation of nanoparticles was characterized ultraviolet visible (UV–vis) spectrophotometer, field emission transmission electron microscope (FE-TEM), X-ray powder diffraction (XRD), dynamic light scattering (DLS), and Fourier transform infrared spectroscopy (FT-IR). The Gu–AgClNps were further tested for their antimicrobial potential against Escherichia coli, Staphylococcus aureus, Pseudomonas aeruginosa, and Salmonella enterica. Furthermore, Gu–AuNps were evaluated for their catalytic activity toward methylene blue degradation. Both nanoparticles were also evaluated for their antioxidant activity against 2,2-diphenyl-1-picrylhydrzyl (DPPH). Lastly, nanoparticles were explored in murine macrophage (RAW264.7) and breast cancer (MCF7) cell lines to determine the enhanced toxicity responses in normal and cancerous cells.
Materials and methods
Materials
Analytical grade of gold (III) chloride trihydrate (HAuCl4·3H2O) and silver nitrate (AgNO3) were purchased from Sigma-Aldrich Chemicals, St. Louis, MO. Roots of G. uralensis were provided by Hanbang bio lab, South Korea. Standard Neomycin (NEO30) discs used for antimicrobial activity were purchased from Oxoid Ltd., England, UK. The pathogenic strains S. aureus [ATCC 6538], P. aeruginosa [ATCC 27853], S. enterica [ATCC 13076], and E. coli [ATCC 10798] were used. The bacterial strains were cultured on nutrient agar media at 37 °C and preserved at −70 °C in glycerol stock vials for further study. Methylene blue (C16H18CIN3S·nH2O) was purchased from KANTO Chemical Co., Inc., Tokyo, Japan. 2,2-Diphenyl-1-picrylhydrzyl (DPPH) and l-ascorbic acid were purchased from Sigma-Aldrich Chemicals, St. Louis, MO. Other chemicals were procured from Sigma-Aldrich Chemicals and utilized as received.
Preparation of aqueous root extract
About 10 g of G. uralensis roots were grounded into fine powder and extracted for 1 h in 100 mL of distilled water at 100 °C. After boiling, the collected extract was filtered and centrifuged at 8000 rpm for 10 min to remove undissolved particulates. The supernatant was collected, maintained at 100 mL, and stored at 4 °C for nanoparticles synthesis.
Green synthesis of nanoparticles
Root stock filtrate was diluted with sterile distilled water in an equal volume ratio. The precursor salts were added into their corresponding reaction mixtures to reach a final concentration of 1 mM. The reaction mixture was heated in an oil bath at 80 °C to prompt the reduction of Au3+ to Au° and Ag+ ions to Ag atoms. A colour change was observed continuously, which indicated the formation of nanoparticles. After colour change was observed, the nanoparticles were collected by centrifugation at 16,000 rpm for 15 min, followed by repeated washing with sterile water, and kept overnight for air drying.
Characterization of Gu–AuNps and Gu–AgClNps
Characterization of Gu–AuNps and Gu–AgClNps were conducted according to our previous studies [Citation7,Citation8].
In vitro biological studies of Gu–AuNps and Gu–AgClNps
Antimicrobial activity of Gu–AgClNps
The biosynthesized Gu–AgClNps were tested for their antimicrobial activity against pathogenic E. coli, S. aureus, S. enteric, and P. aeruginosa using the disc diffusion method. In this assay, the Mueller–Hinton agar (MHA) plates were spread evenly with 100 μL of overnight log culture of the pathogens. Commercial antibiotic disc Neomycin (NEO30) was maintained as positive control. Sterile paper discs were saturated by 30 μL (500, 1000, and 1500 μg/mL) of freshly prepared Gu–AgClNps solution. The plates were incubated at 37 °C for 24 h. After the incubation period, the zones of inhibition around each disc were measured. The study was done in triplicates [Citation9,Citation10].
Catalytic reduction of methylene blue using Gu–AuNps and Gu–AgClNps
The efficient catalytic reduction of chemical dyes by biosynthesized Gu–AuNps and Gu–AgClNps were confirmed using by the decrease in absorbance value of methylene blue in UV–Vis spectra. About 1 mL of methylene blue (1 × 10−4 M) was mixed with 0.2 mL of aqueous Glycyrrhiza root extract and 1.8 mL of water; this reaction was monitored after 30 and 60 min of incubation in room temperature. Additionally, 1 mL of methylene blue (1 × 10−4 M) was mixed with 0.2 mL Glycyrrhiza root extract and 2 mL 0.1 mg/mL of synthesized Gu–AuNps and Gu–AgClNps and their reaction was monitored after 30 and 60 min of incubation. The absorbance maximum values at 670 nm were compared with that of methylene blue [Citation11].
Antioxidant activity against DPPH free radicals
The antioxidant activity of Gu–AgNPs and Gu–AgClNps were determined through DPPH analysis with minor modifications [Citation12,Citation13]. Six different concentrations of nanoparticles (100, 250, 500, 1000, 1500, and 2000 μg/mL) were mixed with 1 mL of 0.1 mM DPPH and then incubated in the dark for 30 min. Subsequently, the absorbance was measured at 517 nm with ascorbic acid as the positive control. The free radical scavenging activity was determined by the formula, % inhibition = ([absorbance of control – (absorbance of sample − absorbance of blank)]/absorbance of control) × 100, where the absorbance of control was sample replaced by an equivalent volume of distilled water, the absorbance of blank was the same volume of 99.5% ethanol replacing DPPH solution. Three replications were performed to enhance the reliability of the analysis.
In vitro MTT cell cytotoxicity assay
The cytotoxicity of nanoparticles in murine macrophage (RAW264.7) and human breast cancer (MCF7) cell lines were evaluated using 3-(4, 5-dimethyl-2-thiazolyl)-2, 5-diphenyl-2H tetrazolium bromide (MTT) (Life Technologies, Eugene, OR) assay. Both cell lines were cultured in Dulbecco’s Modified Eagles Medium (DMEM) (Gibco-BRL, Grand Island, NY) supplemented with 10% fetal bovine serum (FBS) and 1% penicillin/streptomycin (P/S) (WelGENE Inc., Daegu, Korea) at 37 °C in a humidified atmosphere containing 5% CO2 and 95% air. The cells were seeded at a density of 1 × 104 per well in a 96-well plate (Corning Costar, Lowell, NY). Next, the wells were treated with different concentrations of Gu–AuNps (1, 10, 25, and 50 μg/mL) and Gu–AgClNps (1, 5, 10, and 20 μg/mL) at 37 °C for 48 h at 90% confluency. After incubation, 10 μL of MTT (5 mg/mL, PBS) assay solution was added to each well. The wells were further incubated at 37 °C for 4 h. Then, 100 μL of dimethyl sulphoxide (DMSO) was added to dissolve the insoluble formazan crystals into a coloured solution. Finally, the absorbance of each coloured solution was quantified by an Enzyme-Linked Immunosorbent Assay (ELISA) reader (Bio-Tek Instruments, Inc., Vinooski, VT) at 570 nm. The optical density of formazan formed in untreated cells (negative control) represents 100% cell viability. All experiments were performed in triplicates and means with standard errors were calculated.
Results and discussion
Green synthesis of nanoparticles
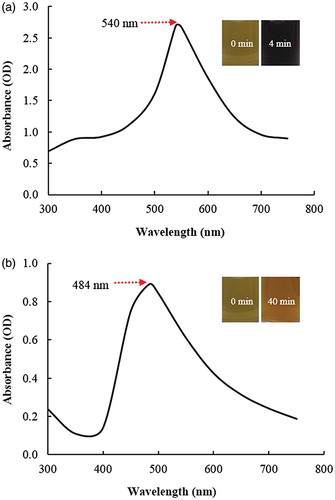
The synthesis of Gu–AuNps and Gu–AgClNps was monitored by change in colour of the reaction mixture with time. The reduction of Au3+ to Au° was completed within 4 min of reaction at 80 °C, and the reaction mixture completely turned to a dark purple which was due to the collective oscillations of electrons in a nanoparticle upon interaction with light [Citation14]. Thus, the appearance of a dark purple colour in the reaction mixture indicated the formation of Gu–AuNps (). For Gu–AgClNps, the reaction mixture completely turned to brown after 40 min of incubation at 80 °C (), whereas no change was observed in a control tube containing only root extract and sterile water in same proportion. Similarly, the brown colour of reaction mixture corresponded to the surface plasmon resonance (SPR) of Gu–AgClNps.
Characterization of nanoparticles
The reduction of gold and silver ions was followed by UV–Vis spectroscopy which showed the highest peaks at 540 nm and 484 nm for gold and silver chloride nanoparticles reaction mixtures, which are characteristic peaks for Gu–AuNps and Gu–AgClNps (). The characteristic absorbance peaks are contributed to SPR phenomenon [Citation14,Citation15]. Similar SPR wavelengths have also been reported in the study of functional D-AuNps and D-AgNps by Dendropanax morbifera leaf extract [Citation16].
The results obtained from the FE-TEM study gives a clear indication regarding the shape and size of the nanoparticles. The Gu–AuNps synthesized in the present study were spherical and 10–15 nm in diameter (). shows a selected are electron diffraction (SAED) pattern of purified spherical Gu–AuNps, with rings corresponding to the [111], [200], [220], and [311] reflections. Furthermore, biosynthesized Gu–AuNps were also characterized by elemental mapping. The elemental mapping results reveal that, in the electron micrograph region of synthesized product, gold is the major element ()). Similarly, Gu–AgClNps were spherical and range from 5 to 15 nm in diameter (). shows a SEAD image of silver nanoparticle, with rings corresponding to the [111], [200], [220], [222], and [420] reflections. The elemental mapping results also indicate the maximum distribution of silver and chloride element in the purified products ( [Citation17]. We suspected that an excess of sodium chloride was present in the Chinese licorice root stock to assist in drying to roots under sunlight. The combination of silver nitrate and silver chloride easily synthesized silver chloride. Silver chloride then was reduced into its atomic form and clustered to form Gu–AgClNps. In EDX analysis, strong signals were observed from the gold and silver atoms in the nanoparticles at 2.1 keV (Au) and 2.8 keV (Cl), 3 keV (Ag), which conform to the characteristic peaks of gold and silver chloride nanocrystallites (). The results were in line with previous reports of EDX spectra of AuNps and AgClNps [Citation14]. Carbon and copper signals originated from the copper grid used for the analysis.
Figure 2. TEM micrograph of Gu–AuNps (a) and Gu–AgClNps (b). Selected area electron diffraction pattern of Gu–AuNps (c) and Gu–AgClNps (d). EDX spectrum of gold nanoparticles (e), elemental mapping: electron micrograph region of gold nanoparticles (f), and distribution of gold element, red (g). EDX spectrum of Gu–AgClNps (h), elemental mapping: electron micrograph region of Gu–AgClNps (i), distribution of silver element, green (j) and distribution of chloride element, blue (k). X-ray diffraction spectrum of Gu–AuNps (l), and Gu–AgClNps (m).
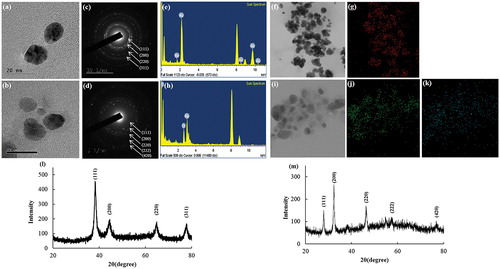
Figure 3. Particle size distribution profile of Gu–AuNps and Gu–AgClNPs with respect to intensity (a, d), number (b, e), and volume (c, f), respectively.
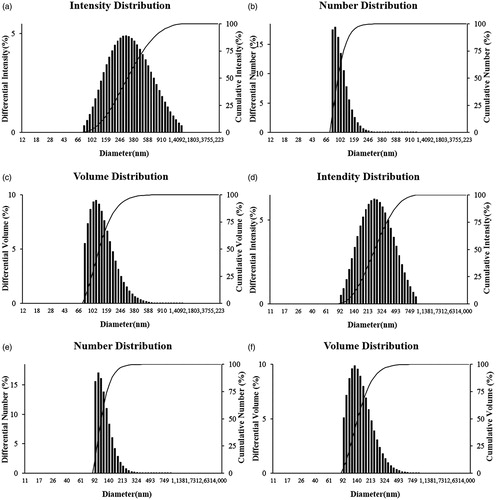
The XRD analyses were performed to confirm the monocrystalline nature of the Gu–AuNps and Gu–AgClNps. demonstrates an XRD pattern of Gu–AuNps. The spectra exhibited intense peaks in the whole spectrum of 2θ value corresponding to [111], [200], [220], and [311]. This pattern was similar to Braggs's reflection of gold nanocrystals indicating that the purified product was composed of crystalline gold. An earlier study showed a similar XRD pattern of gold nanoparticles [Citation17]. m) demonstrates an XRD pattern of Gu–AgClNps. The XRD spectrum of Gu–AgClNps confirmed that the nanoparticles formed were polycrystalline, as evidenced by the peaks at 2θ values corresponding to [111], [200], [220], [222], and [420]. This result was similar to a previously published report [Citation18]. The average particle sizes of Gu–AuNps and Gu–AgClNps were calculated by Debye–Scherrer equation, the average diameters were 12.25 and 8.01 nm, respectively.
The nanoparticles distribution profile of the biosynthesized Gu–AuNps and Gu–AgClNps was studied using a DLS method by a particle size analyzer according to intensity, number, and volume (). Size distribution histogram revealed a wide range of particle size distribution with average particle diameter of 293 and 247.6 nm with PDI 0.286 and 0.181 for Gu–AuNps and Gu–AgClNps, respectively. According to TEM images () and DLS analysis, Gu–AuNps and Gu–AgClNps synthesized by G. uralensis were not entirely monodisperse. The nanoparticles were, in fact, moderately poly-disperse; a common distribution type typically resulted from biological synthesis. The discrepancy in the average sizes of biogenic gold nanoparticles analyzed by XRD (and FE-TEM) and DLS is attributed to the fact that XRD anticipates the crystallite diameter of nanoparticles in dried form whereas DLS measures the hydrodynamic diameter of nanoparticles in aqueous suspension which includes the metallic core and any biological molecules attached or adsorbed on the particle surface.
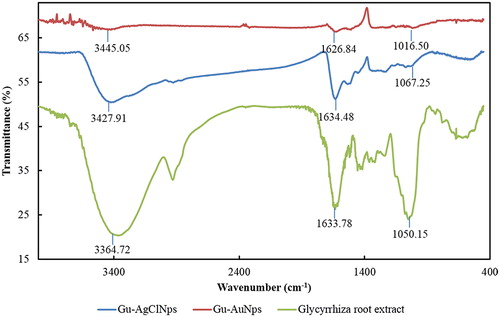
FT-IR analysis was conducted to identify the organic molecules responsible for the bioreduction of gold and silver ions by Glycyrrhiza root extract. FT-IR spectrum of Gu–AuNps was compared against FT-IR spectrum of plant powder (positive control). FT-IR spectra showed multiple characteristic peaks (). The bands shown at 3364–3345, 1626–1634, and 1016–1067 cm−1 are due to the stretching vibrations of alcohol (O–H), aromatic (C = C), and alcohol (C–O) groups, respectively. The peaks appeared at 3364–3445 are attributed to the O–H stretching frequency of hydroxyl group of phenolic compounds. The strong peaks at 1628 cm−1 and 1040 cm−1 represent the carbonyl group stretching vibration of flavonoids and ester bonds in polyphenols [Citation19]. Similar characteristic peaks of Gu–AgNps were also observed. Glycyrrhizin and flavonoids are the major bioactive compounds of Glycyrrhiza uralensis. Similar peaks at 3454.50, 1651.06, and 1041.56 cm−1 also appeared on the FT-IR spectra of glycyrrhizin, indicating that the same functional groups may be present as surface capping layer around Gu–AuNps and Gu–AgClNps [Citation20]. Positive biological activities achieved by Gu–AuNps and Gu–AgClNps were speculated to be due to the presence of various functional groups capping the nanoparticles. From the results, we can clearly see the function groups bonded with Gu–AgClNps were stronger than Gu–AuNps, which maybe influence the nanoparticles functional activity in further study.
Applications of nanoparticles
Antimicrobial activity of Gu–AgClNps
In recent years, AgNps are specifically used as antibacterial/antifungal agents in biotechnology and bioengineering. They can be used for targeted drug delivery which is more effective and having fewer side effects as compared to its bulk counterpart [Citation21]. In our study, Gu–AgClNps were tested for antimicrobial activity against a range of pathogenic micro-organisms, which included S. aureus (), E. coli (), S. enterica (), and P. aeruginosa (). After incubation, the zone of inhibition was determined by measuring the diameter of the zone and antimicrobial activity was observed against S. aureus, E. coli, S. enterica, and P. aeruginosa. shows Gu–AgClNps exhibited zones of inhibition against all of the bacterial strains. The zone of inhibition increased with an increase in nanoparticles concentration. The antimicrobial activities of Gu–AgClNps against four pathogenic microorganisms are disparate; at 30 μg/disc, results indicated that Gu–AgClNps exhibited maximum antimicrobial activity in the following order: S. aureus, S. enterica, E. coli, and P. aeruginosa. Stronger inhibition compared with commercial-grade Neomycin was observed in S. aureus. Many reports suggest that the major mechanism through which AgNps manifest antibacterial properties is either by anchoring or by penetrating the bacterial cell wall and modulating cellular signalling by dephosphorylating putative key peptide substrates on tyrosine residues [Citation22]. AgNPs can easily reach the nuclear content of bacteria, and as they are present in the greatest surface area, therefore, the contact with bacteria is the greatest. This could be the reason why Gu–AgClNps demonstrated high antibacterial activity [Citation23]. Sondi and Salopek-Sondi reported that the antimicrobial activity of AgNps on Gram-negative bacteria was dependent on the concentration of nanoparticles and was closely associated with the formation of pits in the cell wall of bacteria [Citation24]. It has also been reported that AgCl nanoparticle-impregnated bacterial cellulose membranes impose strong antimicrobial activity against E. coli (Gram-negative) and S. aureus (Gram-positive) [Citation25]. On the contrary, Gu–AuNps did not show any zone of inhibition irrespective of the concentration used. Another report has also reported the absence of antimicrobial activity of AuNps [Citation12].
Figure 5. Antimicrobial activity of Gu–AgClNPs against S. aureus (a), E. coli (b), S. enterica (c), and P. aeruginosa (d). Notes: N: Neomycin (NEO30) was maintained as positive control. About 30 μL of different concentrations (500, 1000, and 1500 μg/mL) of Gu–AgClNPs was added into each disc.
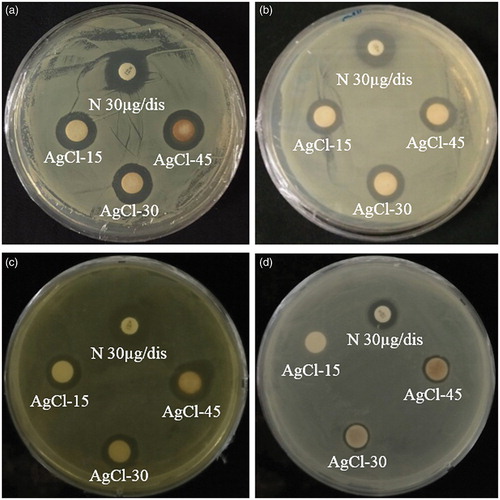
Table 1. Inhibition zone (mm) of Gu–AgClNPs. Neomycin (NEO30) was maintained as positive control. About 30 μL of different concentrations (500, 1000, and 1500 μg/mL) of Gu–AgClNps was added into each disc.
Catalytic activity of Gu–AuNps
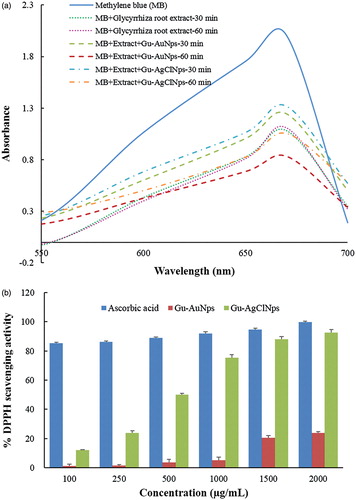
The reduction of methylene blue by plant-mediated nanoparticles has been well documented [Citation11]. Pure methylene blue has a λmax value of 670 nm. Thirty minutes after the addition of Glycyrrhiza root extract to the dye, the absorbance was gradually decreased and shifted to higher wavelength. The decrease of absorbance is indicative of the ability of Glycyrrhiza root extract to degrade methylene blue. Reaction mixture containing dye, Gu–AuNps, and the extract at 30 min time showed higher absorbance value than Glycyrrhiza root extract, but at 60 min reaction mixture showed a marked decrease in the absorbance of methylene blue (). Similarly, the reaction mixture containing dye, Gu–AgClNps, and extract at 30 min also showed higher absorbance value; however a significant decrease was observed at longer incubation (i.e. 60 min). In other words, Gu–AuNps and Gu–AgClNps could synergistically promote the catalytic activity of Glycyrrhizin root extract in the presence of methylene blue with the strongest activity observed with Gu–AuNps. The applications of AuNps and AgNps as catalysts have been reported [Citation26,Citation27], but for AgClNps as catalysts was little reported. In conclusion, Gu–AuNps and Gu–AgClNps possess potentials as effective catalysts for degrading chemical dyes.
Antioxidant activity of Gu–AuNps and Gu–AgClNps
DPPH reducing ability of Gu–AuNps and Gu–AgClNps were assessed by evaluating the colour change spectrophotometrically from purple to yellow at 517 nm [Citation28]. The results of the findings are summarized in . The results on the effect of different concentrations of Gu–AuNps and Gu–AgClNps revealed that the biologically synthesized nanoparticles possessed free radical scavenging activity. We observed that Gu–AgClNps exhibited strong scavenging ability scavenging activities in a dose-dependent manner. The recorded percentage scavenging ability for the lowest concentration of the biosynthesized Gu–AgClNps was 12.06 ± 0.36 and this scavenging ability was increased to 50.80 ± 0.90 when concentration was increased to 500 μg/mL. The IC50 of Gu–AgClNps is 498.93 μg/mL as determined by linear interpolation. The free radical scavenging activity of biosynthesized Gu–AgClNps can be attributed to the antioxidant activity of the main active compounds of the plant, glycyrrhizin [Citation29] and flavonoids [Citation30]. The scavenging ability for Gu–AuNps is lower than Gu–AgClNps. This could be due to the difference in nature of the biosynthesized Gu–AgClNps and Gu–AuNps with respect to the phytochemicals stabilizing the nanoparticles. As seen by FT-IR spectra in , less pronounced peaks were detected in Gu–AuNps than in Gu–AgClNps, indicating the higher quantity of phytochemicals on Gu–AgClNps. Gu–AgClNps combined with the attached functional groups of phytochemicals promote their antioxidant activity against DPPH free radicals.
Effect of Gu–AuNps and Gu–AgClNps on the breast cancer cells
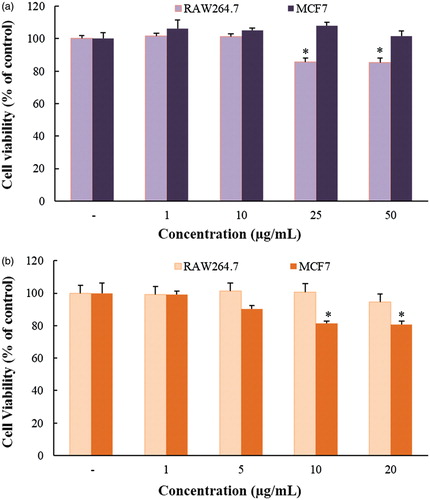
The in vitro cytotoxicity of Gu–AuNps and Gu–AgClNps were examined in murine macrophage (RAW264.7) and breast cancer cell lines (MCF7) by MTT assay. As shown in , as seen by MTT results, Gu–AgClNps showed significant cytotoxicity at 10 μg/mL towards cancerous cells with no apparent cytotoxicity towards normal cells. Several studies have indicated that green synthesis of nanoparticles by plants augments the cytotoxicity of AgNps in cancerous cells [Citation31]. On the contrary, Gu–AuNps were toxic towards RAW264.7 and non-toxic towards MCF7 cell lines, low cytotoxicity of Gu–AuNps may be correlated to the presence of functional groups of phytochemicals provided by the aqueous plant extract. These results suggest that Gu–AgClNps may possess the potential to be applied as an anticancer agent against breast cancer cells.
Figure 7. In vitro cytotoxicity assay of Gu–AuNps (a) and Gu–AgClNPs (b) in murine macrophage (Raw 264.7) and breast cancer (MCF-7) cell lines. Notes: The cells were incubated with the nanoparticles for 48 h, and the experiment was repeated three times. Error bars represent the standard deviation (n = 3).*p < .05 versus control (untreated group).
Conclusion
A green and rapid method of Gu–AuNps and Gu–AgClNps biosynthesis was developed by using G. uralensis root extract. This facile methodology overcomes the limitation of physiochemical methods including cost, chemicals toxicity, and hazardous byproducts. Gu–AgClNps showed maximum zones of inhibition against pathogenic microorganisms in the following order: S. aureus, S. enterica, E. coli, and P. aeruginosa. Gu–AuNps and Gu–AgClNps synergistically catalyzed the reduction of methylene blue with Glycyrrhiza root extract. Additionally, Gu–AgClNps demonstrated excellent scavenging ability against DPPH free radicals. Finally, Gu–AgClNps demonstrated potential anticancer agents against breast cancer cells as evaluated by MTT assays. The results obtained from physicochemical characterization, MTT, antimicrobial, catalytic, and antioxidant activity of nanoparticles support their plausible application in medical field. In the future, selection of such medicinal plants with high therapeutic importance will create a new platform for the nanoparticles synthesis.
Disclosure statement
The authors have no conflicts of interest to report.
Additional information
Funding
References
- Sharma VK, Yngard RA, Lin Y. Silver nanoparticles: green synthesis and their antimicrobial activities. Adv Colloid Interface Sci. 2009;145:83–96.
- Dubey SP, Lahtinen M, Sillanpää M. Green synthesis and characterizations of silver and gold nanoparticles using leaf extract of Rosa rugosa. Colloids Surf A: Physicochem Eng Asp. 2010a;364:34–41.
- Wang C, Mathiyalagan R, Kim YJ, et al. Rapid green synthesis of silver and gold nanoparticles using Dendropanax morbifera leaf extract and their anticancer activities. IJN. 2016a;11:3691–3701.
- Dubey SP, Lahtinen M, Sillanpää M. Tansy fruit mediated greener synthesis of silver and gold nanoparticles. Process Biochem. 2010b;45:1065–1071.
- Shen S, Chang Z, Liu J, et al. Separation of glycyrrhizic acid and liquiritin from Glycyrrhiza uralensis Fisch extract by three-liquid-phase extraction systems. Sep Purif Technol. 2007;53:216–223.
- Kalishwaralal K, Deepak V, Pandian SRK, et al. Biosynthesis of silver and gold nanoparticles using Brevibacterium casei. Colloids Surf B Biointerfaces. 2010;77:257–262.
- Jo JH, Singh P, Kim YJ, et al. Pseudomonas deceptionensis DC5-mediated synthesis of extracellular silver nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:1576–1581.
- Singh P, Kim YJ, Wang C, et al. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. 2016a;44:1150–1157.
- Singh P, Kim YJ, Yang DC. A strategic approach for rapid synthesis of gold and silver nanoparticles by Panax ginseng leaves. Artif Cells Nanomed Biotechnol. 2016b;44:1949–1957.
- Wang C, Singh P, Kim YJ, et al. Characterization and antimicrobial application of biosynthesized gold and silver nanoparticles by using Microbacterium resistens. Artif Cells Nanomed Biotechnol. 2016b;44:1714–1721.
- Edison TJI, Sethuraman MG. Instant green synthesis of silver nanoparticles using Terminalia chebula fruit extract and evaluation of their catalytic activity on reduction of methylene blue. Process Biochem. 2012;47:1351–1357.
- Abbai R, Mathiyalagan R, Markus J, et al. Green synthesis of multifunctional silver and gold nanoparticles from the oriental herbal adaptogen: Siberian ginseng. IJN. 2016;11:3131.
- Song R, Zhang K-q, Wei R-b. In vitro antioxidative activities of squid (Ommastrephes bartrami) viscera autolysates and identification of active peptides. Process Biochem. 2016;51:1674–1682.
- Geethalakshmi R, Sarada D. Gold and silver nanoparticles from Trianthema decandra: synthesis, characterization, and antimicrobial properties. Int J Nanomed. 2012;7:5375–5384.
- Ahmad T, Wani IA, Manzoor N, et al. Biosynthesis, structural characterization and antimicrobial activity of gold and silver nanoparticles. Colloids Surf B Biointerfaces. 2013;107:227–234.
- Wang D, Markus J, Wang C, et al. Green synthesis of gold and silver nanoparticles using aqueous extract of Cibotium barometz root. Artif Cells Nanomed Biotechnol. 2016c. DOI:10.1080/21691401.2016.1260580.
- Verma VC, Kharwar RN, Gange AC. Biosynthesis of antimicrobial silver nanoparticles by the endophytic fungus Aspergillus clavatus. Nanomedicine. 2010;5:33–40.
- Paulkumar K, Rajeshkumar S, Gnanajobitha G, et al. Eco-friendly synthesis of silver chloride nanoparticles using Klebsiella planticola (MTCC 2277). Int J Green Chem Bioprocess. 2013;3:12–16.
- Ahmad A, Syed F, Shah A, et al. Silver and gold nanoparticles from Sargentodoxa cuneata: synthesis, characterization and antileishmanial activity. RSC Adv. 2015;5:73793–73806.
- Rani R, Dilbaghi N, Dhingra D, et al. Optimization and evaluation of bioactive drug-loaded polymeric nanoparticles for drug delivery. Int J Biol Macromol. 2015;78:173–179.
- Omprakash V, Sharada S. Green synthesis and characterization of silver nanoparticles and evaluation of their antibacterial activity using Elettaria Cardamom seeds. J Nanomed Nanotechnol. 2015;6: DOI:10.4172/2157-7439.1000266.
- Shrivastava S, Bera T, Roy A, et al. Characterization of enhanced antibacterial effects of novel silver nanoparticles. Nanotechnology. 2007;18:225103.
- Lok C-N, Ho C-M, Chen R, et al. Proteomic analysis of the mode of antibacterial action of silver nanoparticles. J Proteome Res. 2006;5:916–924.
- Dragieva I, Stoeva S, Stoimenov P, et al. Complex formation in solutions for chemical synthesis of nanoscaled particles prepared by borohydride reduction process. Nanostruct Mater. 1999;12:267–270.
- Hu W, Chen S, Li X, et al. In situ synthesis of silver chloride nanoparticles into bacterial cellulose membranes. Mater Sci Eng: C. 2009;29:1216–1219.
- Jana NR, Sau TK, Pal T. Growing small silver particle as redox catalyst. J Phys Chem B. 1999;103:115–121.
- Sau TK, Pal A, Pal T. Size regime dependent catalysis by gold nanoparticles for the reduction of eosin. J Phys Chem B. 2001;105:9266–9272.
- Markus J, Mathiyalagan R, Kim Y-J, et al. Intracellular synthesis of gold nanoparticles with antioxidant activity by probiotic Lactobacillus kimchicus DCY51 T isolated from Korean kimchi. Enzyme Microb Technol. 2016;95:85–93.
- Li X-L, Zhou A-G, Zhang L, et al. Antioxidant status and immune activity of glycyrrhizin in allergic rhinitis mice. Int J Mol Sci. 2011;12:905–916.
- Rice-Evans CA, Miller NJ, Paganga G. Structure-antioxidant activity relationships of flavonoids and phenolic acids. Free Radic Biol Med. 1996;20:933–956.
- Sankar R, Karthik A, Prabu A, et al. Origanum vulgare mediated biosynthesis of silver nanoparticles for its antibacterial and anticancer activity. Colloids Surf B Biointerfaces. 2013;108:80–84.