Abstract
Panax ginseng berry extract possess remarkable pharmacological effects on skin treatment such as anti-aging, antioxidant, promotor of collagen synthesis and alleviation against atopic dermatitis. In recent years, gold nanoparticles have gained much attention due to their extensive range of applications in particular in the field of drug delivery as a result of their biological compatibility and low toxicity. In a previous study, we designed and developed biocompatible gold and silver nanoparticles based on phytochemical profile and pharmacological efficacy of P. ginseng berry extract, we were able to reduce gold ions to nanoparticles through the process of green synthesis. However, its potential as a cosmetic ingredient is still unexplored. The aim of the present study is to investigate the moisture retention, in-vitro scavenging and whitening properties of gold nanoparticles synthesized from P. ginseng berry in cosmetic applications. Our findings confirm that P. ginseng berry mediated gold nanoparticles exhibited moisture retention capacity. In addition, MTT assay results confirmed that P. ginseng berry mediated gold nanoparticles are non-toxic to human dermal fibroblast and murine melanoma skin cells, possess scavenging activity, protect and provide alleviation against injured caused by H2O2-induced damage. In addition, P. ginseng berry mediated gold nanoparticles, significantly reduced melanin content and suppress tyrosinase activity in α-MSH-stimulated B16BL6 cells. We conclude that P. ginseng berry mediated gold nanoparticles are biocompatible and environmental affable materials and can be a potential novel cosmetic ingredient.
Graphical Abstract
Introduction
Panax ginseng Meyer is a perennial plant of the Araliaceae family commonly known as Korean ginseng [Citation1,Citation2]. Korean ginseng is a well-known medicinal plant around the world due to its multiple pharmacological functions such as anticancer activity, anti-stress, anti-fatigue, antioxidant and anti-aging effects [Citation3,Citation4]. Extensive and thorough research of phytoconstituents in P. ginseng root has been carried out. However, in recent years, interest in phytochemical profile, biological and pharmacological activities of ginseng berry has grown [Citation5].
Nanotechnology researchers are focus on the synthesis of nanoparticles of different shapes, sizes, by chemical or natural compositions and their potential use of human benefits [Citation6]. Engineered nanoparticles are obtained by chemical and physical synthesis but high cost and potential danger are linked to the synthesis procedures. To counteract these disadvantages, the use of plants and plant extracts for the synthesis of functional, environmental affable and biocompatible nanoparticles might be an alternative for the large-scale synthesis of nanoparticles [Citation7]. In our previous study, titled “Ginseng-berry-mediated gold and silver nanoparticle synthesis and evaluation of them in vitro antioxidant, antimicrobial, and cytotoxicity effects on human dermal fibroblast and murine melanoma skin cell lines” [Citation8]. We described the phytochemical activities and components of the P. ginseng berry extract and demonstrated that its phytocomponents (ginsenosides, phenolic compounds, acid polysaccharides and proteins) are capable to reduce and stabilize metal salts into functional nanoparticles. The optimal method of synthesis and characterization of gold and silver nanoparticles are described extensively. In addition, we demonstrated the biological applications of ginseng berry mediated gold and silver nanoparticles such as antimicrobial, antioxidant, inhibition effect on mushroom tyrosinase and biocompatibility.
Recent studies elucidated the advantages of the use of nanoparticles in cosmetics [Citation9,Citation10]. The cosmetic industry is taking great advantages of nanotechnology through the develop of custom-made nanoparticles to enhance the performance and bioavailability of active ingredients in cosmetics products such as sun screams, antiaging creams, moisturizers and perfumes [Citation11]. Gold nanoparticles have been used in cosmetic products such as skin wound disinfection, anti-inflammation, face pack and antiaging cream [Citation9]. Skin is the largest organ of the human body, cosmetic industry is highly competitive and scientists are interested in the development of high quality, efficient and safety ingredients from natural sources to treat skin related problems. Skin acts as a protective barrier that isolates the organism from the surrounding environment. The lipid matrix in the out layer of the skin known as stratum corneum is of remarkable importance. One of its major functions is to prevent incommensurate water loss trough epidermis [Citation12]. Hydration of skin is a key factor to have a healthy skin [Citation13]. On the other hand, skin aging is the growing deterioration of cells and tissues caused by intrinsic and extrinsic or environmental factors [Citation14]. Free radicals are implicated in skin aging, they can react with DNA, proteins and fatty acids originating oxidative damage. Consequently, cause of skin aging is by inducing wrinkling, photo-aging, elastosis, drying, roughness, appearance of fine lines, lack of elasticity and melanogenesis [Citation15,Citation16]. The tyrosinase is an essential enzyme that catalyzes primary and rate-limiting stages in melanogenesis [Citation17]. Hence, tyrosinase activity inhibitors are the most efficient method to inhibit melanin biosynthesis which is one of the causes of freckles and age spots [Citation18].
Finding new cosmetic ingredients from natural sources are of remarkable importance to counteract skin aging, protect skin and enhance its beauty. Ginseng berry materials are gaining popularity as cosmetics ingredients due to its active compounds (ginsenosides, vitamins, folic acid and potassium) and skin therapeutic activities (antioxidant, anti-inflammation, precursor of collagen synthesis, skin whitening effect, moisturizing effect and alleviation for atopic dermatitis related symptoms) [Citation19,Citation20].
In the present study, we are exploring the potential application of gold nanoparticle from P. ginseng berry (Gb-AuNPs) as a cosmetic ingredient. Thus test for moisture retention activity, effect on skin cells morphology and in vitro scavenge assessment of the ginseng berry extract (GBE) and Gb-AuNPs in normal human fibroblast cells. In addition, whitening effect of Gb-AuNPs was confirmed with murine melanoma B16BL6 skin cells through cellular melanin content and inhibition of tyrosinase activity assays.
Materials and methods
Materials
The ginseng berries were supplied by the Ginseng bank, Kyung Hee University, South Korea. Gb-AuNPs were synthetized and characterized in our previous report [Citation8]. Human dermal fibroblast (HDF) and murine melanoma B16BL6 (B16) cell lines were obtained from the Korean Cell Line Bank (Seoul, Korea). Dulbecco’s Modified Eagle Medium (DMEM), foetal bovine serum (FBS) and penicillin–streptomycin solution were purchased from GenDEPOT (Barker, TX), soluble 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyltetrazolium bromide (MTT) was purchased from Life technologies (Eugene, OR), Arbutin and l-3,4-dihydroxyphenylalanin (l-DOPA) was obtained from Abcam (Cambridge, UK), α-melanocyte-stimulating hormone (α-MSH) was purchased from Sigma (St. Louis, IL).
Preparation of aqueous extract of ginseng berry
Aqueous extract of ginseng berry was prepared as previously reported [Citation8]. In brief, 10 g of GB powder was dissolved in 150 mL of DW and autoclave at 100 °C for 30 min and filtered. Further solution was dissolved to a final concentration of 5% (v/v).
Synthesis of Gb-AuNPs
Ginseng berry AuNPs were synthetized at optimal conditions as were stated in our previous report [Citation8]. In brief, gold (III) chloride trihydrate was used as precursor salt for the synthesis of gold nanoparticles. Nanoparticles were synthesized by adding gold salt into 100 mL of 5% of aqueous ginseng berry extract to reach a final concentration of 1 mM at 80 °C.
Evaluation of moisture retention
The in vitro moisture retention activity of Gb-AuNPs was assayed gravimetrically as previous report [Citation21] with some modifications. One millilitre of Gb-AuNPs solution was incubated for 24 h at 40 °C and 55% of relative humidity. The weights before (Wi) and after (Wf) the incubation were measured by an electronic balance. Glycerin was used as moisture retention control, because of it is frequently use in cosmetic preparations. The moisture retention rate (Rr) was evaluated by the weight loss of the sample: Rr (%) = [1 − (Wi,sample −Wf, sample)/(Wi, control−Wf, control)] × 100.
Cell culture
Cell lines were cultured in DMEM and supplemented with 10% of FBS and 1% penicillin–streptomycin at 37 °C in a humidified 95% air and 5% CO2 atmosphere as described previously [Citation22,Citation23].
Cell viability assay
Cellular viability was determined by MTT assay method [Citation24]. Cells were seeded at a density of 1 × 105 in 96 well plates and cultured for 24 h. At 90% confluency, cells were treated with various concentration of GBE, Gb-AuNPs or HAuCl4·3H2O (1–200 μg/mL) for 24 h. After incubation period, 10 μL of MTT assay solutions (5 mg/mL in PBS) was added to each well and further incubated at 37 °C for 3 h, then finally 100 μL DMSO was added to dissolve the formazan crystals. The absorbance was measured at 570 nm by an ELISA reader.
Radical scavenging assay against damage to fibroblasts by oxygen free radicals
Human dermal fibroblast cells were used for radical scavenging assay and assay as previously reported by Murrel et al. [Citation25] with minor modifications. Cells were seeded at a density of 1 × 105 cells/well in 96-well plates in DMEM and incubated for 24 h at 37 °C. Cells were treated with increasing concentration of GBE or Gb-AuNPs (25, 50 and 100 μg/ml). Three different experimental assays were performed: (1) co-treatment, addition of GBE or Gb-AuNPs along with H2O2 to cells for 24 h; (2) pre-treatment, cells were treated with GBE or Gb-AuNPs for 24 h prior exposure to the H2O2 for 1 h and (3) post-treatment, HDF cell were treated with H2O2 for 1 h, further washed with 200 μL of PBS and finally treated with GBE or Gb-AuNPs for 24 h. Ascorbic acid (100 μg/mL), a H2O2 scavenger was used as a reference standard. Cell control and cells treated with H2O2 (10 μM) alone were also maintained. The plates were incubated at 37 °C and MTT assay was used to assess the protection offered by Gb-AuNPs. The cells morphology was also examined to check for changes, injuries or microbial contamination.
Effect of Gb-AuNPs on cellular melanin content
B16 melanoma cells were seeded at a density of 2 × 105 cell in 100 mm culture plates and incubated overnight in a humidified atmosphere containing 5% CO2 in air at 37 °C. The cells were then treated with medium containing α-MSH and samples for 24 h. The cellular melanin content of the cultured B16 cells was tested as described previously with slight modifications [Citation24]. In brief, the medium was removed and cells were washed with PBS. Then cells were harvest with trypsin and the cell pellet was solubilized in 500 μL of 1 N NaOH containing 10% DMSO and kept at 80 °C for 1 h. The relative melanin content was determined by measuring the absorbance at 475 nm in an ELISA reader. A standard curve for synthetic melanin (0–500 μg/mL) was prepared for each experiment. Melanin production was expressed as percentage of untreated controls.
Tyrosinase activity assay
Tyrosinase activity was assayed as DOPA oxidase activity [Citation24], B16 cells were cultured in 100 mm plates at a density of 4 × 105 cell/well with 100 nM α-MSH. After 24 h, the cells were treated with various concentrations of Gb-AuNPs (1–100 μg/mL); after 24 h, the medium was removed and cells were washed with iced-cold PBS, and lysed with phosphate buffer (pH 6.9) containing 1% Triton X-100, the mixture was freeze-thawed by incubating at −80 °C for 15 min and then kept at room temperature for 10 min, the samples were clarified by centrifugation at 12 000 g for 15 min. After centrifugation, 10 μL of freshly prepared substrate solution (15 mM L-DOPA in 50 mM sodium phosphate buffer (pH 7.1) was added to 90 μL of lysate supernatant and incubated at a 37 °C for 1 h. The absorbance was then measured at 475 nm using an ELISA reader.
Statistical analysis
All data are presented as mean ± standard deviation (SD). All experiments were independently performed in triplicate. The mean values of the treatment groups were compared with untreated groups using Student’s t-test. Statistical significance was assigned at *p < .05, **p < .01 and ***p < .001.
Results and discussion
Evaluation of moisture retention
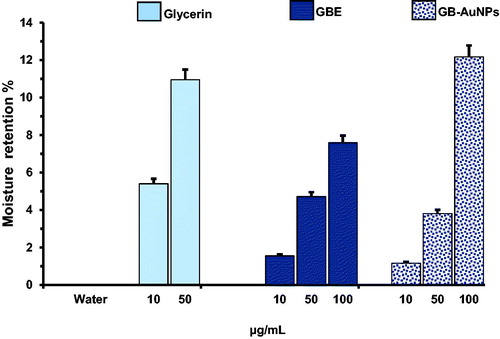
Moistures are often used as medical treatment for healthy and diseased skin [Citation26]. They are able to create a thin film on the outer surface of the skin. The stratum corneum (SC) is the outermost layer of the epidermis, and its function is to prevent the loss of water in skin barrier of mammals [Citation27]. Significant evidence suggest that glycerin exists in the stratum corneum as a natural endogenous humectant [Citation28], glycerin is also a cosmetic ingredient regularly used as a hygroscopic and humectant agent. The in vitro moisture retention property of GBE and Gb-AuNPs was assayed gravimetrically and compared with that of glycerin. shows that moisture retention activities of 10 μg/mL, 50 μg/mL and 100 μg/mL of GBE and Gb-AuNPs. GBE increased percentage of 1.5, 4.71, 7.59% and Gb-AuNPs 1.16, 3.81 and 12.17%. Those values can be compared with the result obtained with glycerin 5.4% and 10.95% at 10 μg/mL and 50 μg/mL, respectively, confirming the moisture retention property of Gb-AuNPs from P. ginseng berry.
Effect of Gb-AuNPs on the cell viability of B16 and HDF cells
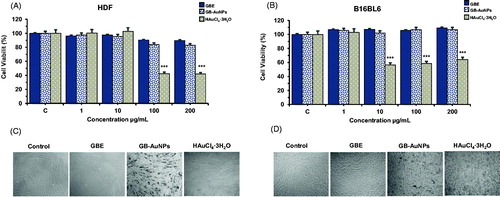
MTT assay was used to evaluate changes in cell viability after mouse B16 and HDF cells were incubated with GBE, Gb-AuNPs and HAuCl4·3H2O at 1, 10, 100 and 200 μg/mL for 24 h. Control wells were incubated with fresh free serum media and experiments were repeated in triplicates. As shown in , HDF and B16 cells treated with GBE and Gb-AuNPs did not have significant cytotoxic effects on the viability of HDF and B16 cells up to 200 μg/mL. Cells treated with HAuCl4·3H2O alone exhibited decrees in cell viability compared with the control group (p < .001). Previous authors have reported that gold nanoparticles were up-taken by cells via the clathrin-mediated endocytosis pathway [Citation29]. This finding suggests that Gb-AuNPs could be taken up by HDF and B16 cells in a similar manner. Taken together, our data suggest that spherical gold nanoparticles are no toxic to HDF and B16 cells.
Figure 2. Cell viability of HDF cells (A), B16BL6 cells (B) and optical microscopy images of HDF cells (C) and B16 cells (D) (40 × Magnification). After treatment with GBE, Gb-AuNPs and HAuCl4·3H2O. Cells (1 × 105 cells/well) were incubated with various concentrations (1–200 μg/mL) of Gb-AuNPs for 24 h. Cell viability was determined by 3-(4,5-dimethyl-thiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. Note that samples did not show toxicity effect up to 200 μg/mL for Gb-AuNPs. Results are expressed as a percentage of sample-treated control and presented as mean ± SD of three separate experiments.
Effect of Gb-AuNPs on morphology of B16 and HDF cells
illustrates the morphology of HDF and B16 treated with 200 μg/mL of GBE, Gb-AuNPs or HAuCl4·3H2O. No visible changes in the cell morphology of HDF and B16 cells were observed between GBE or Gb-AuNPs-treated cells and the control group. Gb-AuNPs-treated HDF and B16 cells could be distinguished by dark purple clusters. Changes in morphology were only evident in HDF and B16 cells treated with HAuCl4·3H2O. Cells were severely injured, shrank and proliferation of autopaghosome were observed.
Radical scavenging assay against damage to fibroblasts by oxygen free radicals
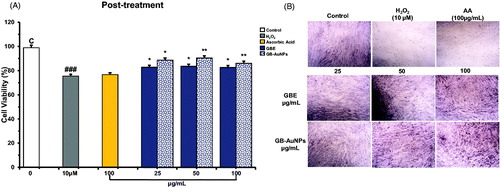
We explore the protective effect of GBE and Gb-AuNPs on HDF cells under oxidative stress. Hydrogen peroxide (H2O2), a strong oxidizing agent that activates the generation of free radicals, was chosen as an oxidant agent in this study. First, we investigate the concentration of H2O2 required to induce recoverable damage on HDF cells at 1 × 105/well by modifying the method developed by Murrel et al. [Citation25] and a dose of 10 μM H2O2 was found to decrease 25–30% of life of HDF cells. The MTT assay was used to investigate the protection activity of GBE and Gb-AuNPs at increasing concentrations. Ascorbic acid (100 μM), as scavenger agent of H2O2, was used as a positive antioxidant control and H2O2-treated cells as negative controls. The HDF cells co-treated with the GBE alone did not show any significant protection or decrease in cell viability at any concentration (25–100 μg/mL) against H2O2 treated cells, similar results were obtained for cells treated with 10–50 μg/mL of Gb-AuNPs (). However when Gb-AuNPs concentration increased to 100 μg/mL, and the cell survival value was 85% ± 5.1 similar to the percentage (84% ± 0.1) of cell treated with ascorbic acid (). The increased antioxidant activity of Gb-AuNPs can be credited to the adsorption of existing bioactive compounds of the P. ginseng berry extract. Pre-treatment of HDF cells with GBE and Gb-AuNPs showed significant protection against H2O2-incuded oxidation HDF cells. On one hand, the cell viability of Gb-AuNPs-treated HDF cells increased in a dose-dependent manner from 81% ± 0.2 to 91% ± 1.43. On the other hand, the cell viability of cell treated with GBE increases only up to 86% ± 1.38, suggesting that Gb-AuNPs protection against H2O2 cell damage is stronger than GBE (). Finally, post-treatment assay shows that ascorbic acid post-treated HDF cells were unable to recover after damage caused by H2O2, while post-treated HDF cell with GBE or Gb-AuNPs shows significant dose-dependent recovery against H2O2-treated cells (). However, GBE shown to be more efficient in the cell recovery than Gb-AuNPs. The possible reason for higher antioxidant activity of GBE and the protection effect of Gb-AuNPs may be correlated to the presence of copious bioactive compounds in the P. ginseng berry extract which enhance Gb-AuNPs phytochemical properties, the most remarkable compounds are ginsenosides, polysaccharides and flavonoids [Citation8]. The effect on morphology of pre-treated and post-treated HDFs can be observed in ; in both cases, control HDFs exhibited normal morphology. However, cells treated with H2O2, turned thin, reduce size, stop growing and died. As expected, pre-treated cells with ascorbic acid were able to resist the damage caused by H2O2 but showed no recovery after post-treatment. HDFs maintained their fibroblastic appearance during pre-treatment and post-treatment with GBE and Gb-AuNPs. All these results obtained showed that pre-treatment with GBE and Gb-AuNPs presented the best protective effect against H2O2-induced oxidative stress. In addition, post-treatment with GBE and Gb-AuNPs might suggest that GBE and Gb-AuNPs might be a potential agent in the recovery of injured skin cells. The interaction of plant metabolites with metal ions during nanoparticle formation may result in improved free radical scavenging compounds. In addition, the electrostatic attractions between negatively charged phytochemicals and positively or neutrally charged Gb-AuNPs act synergistically to improve bioactivity of plants [Citation30,Citation31].
Figure 3. Co-treatment, radical-scavenging effect of Gb-AuNPs on HDF cells against H2O2-induced cell damage. HDF cells were co-treated with various concentrations of Gb-AuNPs and 10 μM of H2O2, incubated for 24 h at 37 °C. Cell viability was determined by MTT assay. Results are expressed as mean ± SD of three separate experiments. Ascorbic acid (100 μg/mL) was used as reference standard.
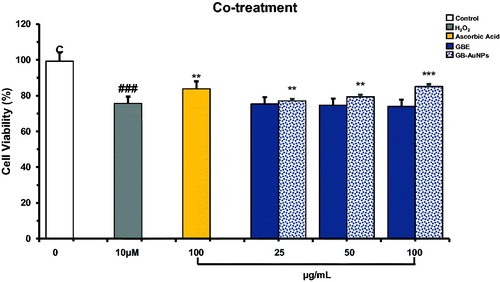
Figure 4. Pre-treatment, radical-scavenging effect of Gb-AuNPs on HDF cells against H2O2-induced cell damage (A) and Optical microscopy images of HDF cells (B) (40 × magnification). HDF cells were pre-treated for 24 h with various concentrations of Gb-AuNPs and then exposed to 10 μM of H2O2 for 1 h at 37 °C. Cell viability was determined by MTT assay. Results are expressed as mean ± SD of three separate experiments. Ascorbic acid (100 μg/mL) was used as a reference standard.
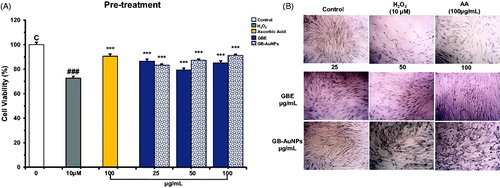
Figure 5. Post-treatment, radical-scavenging effect of Gb-AuNPs on HDF cells against H2O2-induced cell damage (A). and Optical microscopy images of HDF cells (B) (40 × magnification). HDF cells were exposed to 10 μM of H2O2 for 1 h at 37 °C and then treated with various concentrations of Gb-AuNPs for 24 h. Cell viability was determined by MTT assay. Results are expressed as mean ± SD of three separate experiments. Ascorbic acid (100 μg/mL) was used as a reference standard.
Effect of Gb-AuNPs on tyrosinase activity in B16BL6 cells
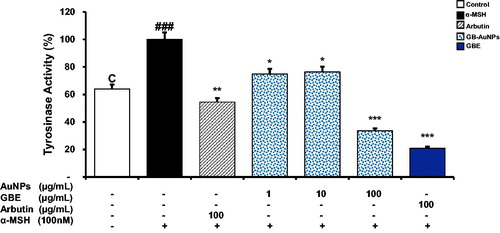
Tyrosinase is a glycoprotein composed by binuclear coppers which catalyzes the conversion of l-tyrosinase into l-Dopa, the rate-limiting point in melanin synthesis [Citation32,Citation33]. Kim et al reported the effect of Korean ginseng berry on skin pigmentation [Citation34]. The aim of our study is to evaluate the anti-tyrosinase activity of Gb-AuNPs on α-MSH-stimulated B16 cells. Cellular tyrosinase activity was significantly increased in B16 cells treated with α-MSH alone compared with control cells (. Gb-AuNPs suppressed significantly tyrosinase activity at 1, 10 and 100 μg/mL. Tyrosinase levels decrease to 21% ± 0.2, 36% ± 0.1 and 54% ± 0.2 when α-MSH-stimulated B16 cells were treated with 100 μg/mL of GBE, Gb-AuNPs or arbutin, respectively. Gb-AuNPs were more effective in reducing tyrosinase levels than arbutin but not higher that GBE treatment. Anti-tyrosinase activity of Gb-AuNPs synthetized from P. ginseng berry extract could be derived from flavonoids absorbed by Gb-AuNPs, the corresponding flavonoids ability to chelate 2 coppers at the active site of tyrosinase enzyme [Citation35].
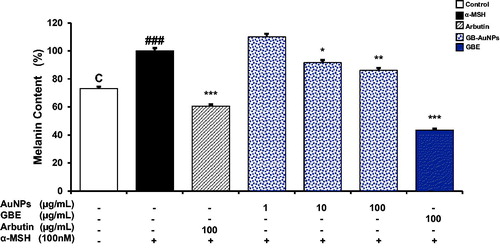
Figure 6. Comparative study of the inhibition effect of GBE and Gb-AuNPs on melanin content in B16BL6 cells. Cells were incubated with different concentrations (1–100 μg/mL) of GBE, Gb-AuNPs or Arbutin (100 μg/mL) in the presence of 100 nM of α-MSH for 24 h. Results are expressed as a percentage of α-MSH-treated control and presented as mean ± SD of three separate experiments. *p < .05 **p < .01, ***p < .001 versus α-MSH treated control by Student’s t-test.
Effect of Gb-AuNPs on cellular melanin content
shows the dose-dependent reduction in melanin content of Gb-AuNPs which led to a dose-dependent decrease in cellular melanin production in α-MSH-stimulated cells. B16 cells treated with 100 μg/mL of Gb-AuNPs show higher inhibition to those treated with the same concentration of arbutin. The antimelanogenic effect of Gb-AuNPs is probable due to syringaresinol which was identified as major tyrosinase inhibitor in ginseng berry extract. Syringaresinol was identified to decrease the accumulation of the age-related pigments in human dermal fibroblast [Citation34]. In addition, phenolic compounds in Gb-AuNPs are capable to inhibit tyrosinase and reduce melanin synthesis, while ginsenosides prevent intracellular increase of reactive oxygen species (ROS) that are responsible to alter redox state of cell membrane proteins resulting in increasing rates of melanin production leading to dark skin [Citation17].
Figure 7. Comparative study of the inhibitory effect of melanogenesis of GBE and Gb-AuNPs through inhibition of tyrosinase activity. B16BL6 cells were incubated with various concentrations of (1–100 μg/mL) GBE, Gb-AuNPs or Arbutin (100 μg/mL) in the presence of 100 nM of α-MSH for 24 h. Tyrosinase activity in cellular lysates was determined as described in “Material and methods” section. Results are expressed as a percentage of α-MSH-treated control and presented as mean ± SD of three separate experiments. *p < .05 **p < .01, ***p < .001 versus α-MSH-treated control by Student’s t-test.
Conclusion
The study highlights the efficacy of Gb-AuNPs as a material for cosmetic applications. Panax ginseng berry extract contains notable bioactive constituents producing gold nanoparticles with multifunctional properties and effects for cosmetic applications. Gb-AuNPs can scavenge effectively and gradually the radical cations have moisture retention properties and are able to suppress cellular tyrosinase and melanin content in α-MSH-stimulated B16 cells. In addition, Gb-AuNPs are non-toxic to HDF and B16 cell and provide protective effect against damage to fibroblasts cells by oxygen-free radicals. These results suggest that Gb-AuNPs could be a promising multifunctional ingredient in cosmetics.
Disclosure statement
All authors have no conflicts of interest to declare.
Additional information
Funding
References
- Kim YJ, Jeon JN, Jang MG, et al. Ginsenoside profiles and related gene expression during foliation in Panax ginseng Meyer. J Ginseng Res. 2014;38:66–72.
- Singh P, Kim YJ, Wang C, et al. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol. 2015;44:811–816.
- Attele AS, Wu JA, Yuan CS. Ginseng pharmacology: multiple constituents and multiple actions. Biochem Pharmacol. 1999;58:1685–1693.
- Singh P, Kim YJ, Wang C, et al. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. 2016;44:1150–1157.
- Lee SY, Kim YK, Park NI, et al. Chemical constituents and biological activities of the berry of Panax ginseng. J Med Plants Res. 2010;4:349–353.
- Wang C, Kim YJ, Singh P, et al. Green synthesis of silver nanoparticles by Bacillus methylotrophicus, and their antimicrobial activity. Artif Cells Nanomed Biotechnol. 2016;44:1127–1132.
- Kumar V, Yadav SK. Plant‐mediated synthesis of silver and gold nanoparticles and their applications. J Chem Technol Biotechnol. 2009;84:151–157.
- Jiménez-Pérez ZE, Mathiyalagan R, Markus J, et al. Ginseng berry mediated gold and silver nanoparticle synthesis and evaluation of their in vitro antioxidant, antimicrobial, and cytotoxicity effect on human dermal fibroblast and murine melanoma skin cell lines. IJN. 2017;12:709.
- Lohani A, Verma A, Joshi H, et al. Nanotechnology-based cosmeceuticals. ISRN Dermatol. 2014;2014: Article ID 843687.
- Wiechers JW, Musee N. Engineered inorganic nanoparticles and cosmetics: facts, issues, knowledge gaps and challenges. J Biomed Nanotechnol. 2010;6:408–431.
- Patel A, Prajapati P, Boghra R. Overview on application of nanoparticles in cosmetics. Asian J Pharm Sci Clin Res. 2011;1:40–55.
- Van Smeden J, Bouwstra J. Stratum Corneum lipids: their role for the skin barrier function in healthy subjects and atopic dermatitis patients. In: Skin barrier function, vol. 49. Basel: Karger Publishers; 2016. p. 8–26.
- Vyumvuhore R, Tfayli A, Biniek K, et al. The relationship between water loss, mechanical stress, and molecular structure of human stratum corneum ex vivo. J Biophoton. 2015;8:217–218.
- Soto ML, Falqué E, Domínguez H. Relevance of natural phenolics from grape and derivative products in the formulation of cosmetics. Cosmetics. 2015;2:259–276.
- Saraf S, Kaur C. Phytoconstituents as photoprotective novel cosmetic formulations. Pharmacogn Rev. 2010;4:1.
- Luft R. The development of mitochondrial medicine. Proc Natl Acad Sci U S A. 1994;91:8731–8738.
- Kim YJ, Uyama H. Tyrosinase inhibitors from natural and synthetic sources: structure, inhibition mechanism and perspective for the future. CMLS, Cell Mol Life Sci. 2005;62:1707–1723.
- Frenk E. Treatment of melasma with depigmenting agents. Melasma: New Approach Treat 1995;9–15.
- Kim J, Cho SY, Kim SH, et al. Ginseng berry and its biological effects as a natural phytochemical. Nat Prod Chem Res. 2016;4:209.
- Zhang W, Cho SY, Xiang G, et al. Ginseng berry extract promotes maturation of mouse dendritic cells. PLoS One. 2015;10:e0130926.
- Tsai CC, Chan CF, Huang WY, et al. Applications of Lactobacillus rhamnosus spent culture supernatant in cosmetic antioxidation, whitening and moisture retention applications. Molecules. 2013;18:14161–14171.
- Lee J, Jung E, Lee J, et al. Panax ginseng induces human Type I collagen synthesis through activation of Smad signaling. J Ethnopharmacol. 2007;109:29–34.
- Yoo DS, Rho H-S, Lee YG, et al. Ginsenoside F1 modulates cellular responses of skin melanoma cells. J Ginseng Res. 2011;35:86–91.
- Wang DD, Jin Y, Wang C, et al. Rare ginsenoside Ia synthesized from F1 by cloning and overexpression of the UDP-glycosyltransferase gene from Bacillus subtilis: synthesis, characterization, and in vitro melanogenesis inhibition activity in BL6B16 cells. J Ginseng Res. 2016. http://dx.doi.org/10.1016/j.jgr.2016.12.009
- Murrell GA, Francis MJ, Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990;265:659–665.
- Ganesan P, Choi DK. Current application of phytocompound-based nanocosmeceuticals for beauty and skin therapy. Int J Nanomed. 2016;11:1987.
- Potts RO, Francoeur ML. The influence of stratum corneum morphology on water permeability. J Invest Dermatol. 1991;96:495–499.
- Verdier‐Sévrain S, Bonte F. Skin hydration: a review on its molecular mechanisms. J Cosmet Dermat. 2007;6:75–82.
- Chithrani BD, Ghazani AA, Chan WC. Determining the size and shape dependence of gold nanoparticle uptake into mammalian cells. Nano Lett. 2006;6:662–668.
- Kumar B, Smita K, Cumbal L, et al. Synthesis of silver nanoparticles using Sacha inchi (Plukenetia volubilis L.) leaf extracts. Saudi J Biol Sci. 2014;21:605–609.
- Swamy MK, Akhtar MS, Mohanty SK, et al. Synthesis and characterization of silver nanoparticles using fruit extract of Momordica cymbalaria and assessment of their in vitro antimicrobial, antioxidant and cytotoxicity activities. Spectrochim Acta Part A: Mol Biomol Spectrosc. 2015;151:939–944.
- Xing R, Wang F, Dong L, et al. Inhibitory effects of Na7PMo11CuO40 on mushroom tyrosinase and melanin formation and its antimicrobial activities. Food Chem. 2016;197:205–211.
- Videira IFdS, Moura DFL, Magina S. Mechanisms regulating melanogenesis. An Bras Dermatol. 2013;88:76–83.
- Kim J, Cho SY, Kim SH, et al. Effects of Korean ginseng berry on skin antipigmentation and antiaging via FoxO3a activation. J Ginseng Res. 2016. http://dx.doi.org/10.1016/j.jgr.2016.05.005
- Chen QX, Kubo I. Kinetics of mushroom tyrosinase inhibition by quercetin. J Agric Food Chem. 2002;50:4108–4112.