Abstract
Haemoglobin site-specifically modified with ferulic acid (FA-Hb), was synthesized. The effects on the characteristic absorption and the oxygen saturation curve of FA-Hb were investigated by UV-Vis spectrophotometry and oxygen analyzer. Furthermore, the autoxidation rates of Hb and FA-Hb were also discussed with or without sodium azide in the system. The results showed that there was no obvious transformation of the heme structure of FA-Hb. It was also demonstrated that the oxygen-carrying capacity of FA-Hb was similar to the natural Hb, but the autoxidation rate of FA-Hb was much lower than Hb in different systems.
Introduction
Due to the limitations of donated blood, such as infectious agents, shortage, and storage issues, the demand for artificial blood substitutes (ABS) has been increasing year by year. As red blood cell substitutes, haemoglobin (Hb)-based O2carriers (HBOCs) have been developed for clinical application in recent years [Citation1,Citation2]. Their usefulness and safety have been demonstrated by many researchers in various fields such as biochemistry, physiology and toxicology [Citation3–5]. Several HBOCs have advanced to phaseII/III clinical trials in the United States [Citation4].
Generally, the starting material of HBOCs is stroma-free Hb (SFH) or chromatographically purified Hb obtained from sources such as human or bovine outdated blood. Various chemical and/or genetic alterations have been employed to produce a stable and functional HBOC including intratetrameric cross-linked Hb, polymers of Hb tetramers [Citation4], and Hb tetramers conjugated to nonprotein macromolecules [Citation6]. Chemical modifications are introduced to stabilize the low oxygen affinity state of the Hb tetramer resulting in a low oxygen affinity, which is a desirable property for HBOCs.
A number of new unresolved problems were found during preclinical and clinical development of current generation HBOCs. These include cardiovascular/hemodynamic effects, immune cell activation, coagulation changes, oxidative stress, and decreased host resistance to overwhelming infection [Citation7]. Among these problems, the autoxidation of Hb is a pressing one to be resolved. Ferrous Hb (Hb(Fe2+)) molecules of HBOCs gradually lose their O2 binding ability, because they react with reactive oxygen species (ROS) such as superoxide or hydrogen peroxide (H2O2) [Citation8,Citation9] to become nonfunctional ferric Hb (Hb(Fe3+), MetHb), in addition to the autoxidation of oxyHb molecules themselves. The oxidation/autoxidation of Hb (Fe2+) to the nonfunctional MetHb form is an important concern in the use of Hb as an oxygen-carrying agent. Uncontrolled and spontaneous oxidation of ferrous iron compromises the function and the safety of the infused HBOCs in some instances [Citation10–12].
The initial oxidative process in Hb involves autoxidation, which is a source for nonfunctional MetHb as well as the ROS superoxide, and then superoxide can both react with Hb and undergo dismutation to produce hydrogen peroxide. Meanwhile, the hydrogen peroxide produced in the former step can react with MetHb, which will produce the ferryl species (Fe4+) [Citation13]. Recent research shows that the formation of the Fe4+form of Hb also induces the formation of heme degradation products [Citation14]. These reactive products of oxidation/autoxidation of HBOCs not only have been known to produce cellular damage, but also can act as potent oxidants capable of oxidatively damaging most biological substrates, including lipids, nucleic acids, and amino acids [Citation15].
In human red blood cells, the concentration of MetHb molecules is usually kept below 1% by reduction systems such as glutathione and ascorbic acid. Furthermore, ROS are eliminated by several mechanisms such as superoxide dismutase (SOD), catalase, and peroxidase that help to prevent the MetHb formation [Citation16,Citation17]. In contrast, MetHb generated during the utilization of HBOCs are not reduced to the ferrous state due to the absence of the reduction systems, because constitutive enzymes and reductants necessary for MetHb reduction are removed during the Hb purification process before the conversion of purified Hb to HBOCs [Citation18].
The clinical usefulness of HBOCs requires that the oxygen affinity must be lowered and, in addition, oxidative processes must be suppressed or controlled. Therefore, chemical modifications of Hb, with the anti-oxidation activity, have been proved to be an effective method, which can enhance the safety of HBOCs. Chang and co-workers have carried out a series of researches on HBOCs in order to increase the ability of anti-oxidation [Citation19–21]. For example, they demonstrated the potential utility of acombination of Hb and SOD in a circulating oxygen carrier [Citation22]. The resulting heterogeneous material was shown to decrease the concentration of ROS effectively in circulation through its enzymic activities. This work shows that only a small amount of SOD activity is necessary to protect the system. Alagic et al. also designed novel chemical reagents to cross-link and connect Hb with SOD [Citation23]. The products showed lower oxygenation cooperativity of Hb, but retained the activity of SOD.
Our interest in chemical modification of Hb led us to seek a method by which ROS can be scavenged in order to make HBOCs safer as blood substitutes. Ferulic acid (FA) is an accessible chemical reagent and, as we know, has a known effect on quenching oxygen free radicals and relieving vasospasm [Citation24]. In this study, we report the preparation of HBOC which was chemically modified with bromoacetylethyleneglycolferulate (BAEGF), and then BAEGF was reacted site-specifically with thiol groups on Hb, thereby incorporating the ROS elimination system. We then evaluated the effect of this modification on the suppression of Hb autoxidation under the circumstance of with or without sodium azide.
Experimental
Reagents and instruments
Human umbilical cord blood was a kind gift from Tianjin Stem Cell Gene Engineering Company (Tianjin, China). Other chemicals used were of analytical grade from Aladdin Reagent Co. Ltd. (Shanghai, China) and the solutions were prepared with deionized water. Spectrophotometry analysis was conducted using SHIMADZU UV-2550 (Japan), and the ultrafiltration membrane was used with10 kDa ultrafiltration centrifugal tubes (Nanosep, Omega™, Beijing, China). The NMR test was performed by NanoBay 400 MHz (Avance™III HD, Bruker, Germany); the oxygen affinity was measured on a hemoxanalyzer (TCS Scientific Corporation, PA, USA).
Methods
Purification of Hb from red blood cells
The preparation of purified Hb from human placentas referred to the methods described by Walder [Citation25]. The final product in the retentate was collected and stored at −80 °C.
Synthesis of BAEGF
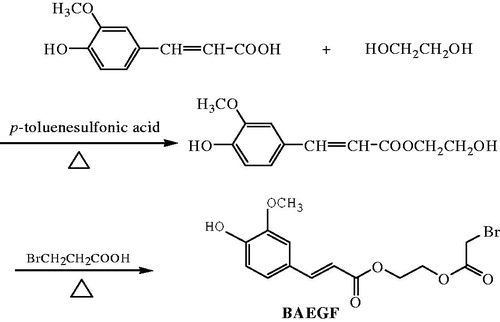
summarizes the route we used to produce BAEGF. p-Toluenesulfonic acid (0.46 g, 0.0024 mol), FA (7.77 g, 0.04 mol) were weighed into 100 mL three-necked flask and stirred with 29.8 g of ethyleneglycol (0.48 mol). Then the flask was heated up to 120 °C under the protection of nitrogen. After 25 min, the mixture was cooled to room temperature and neutralized with 1 M NaOH to pH 7. Then 40 mL of saturated saline solution was added. With ethyl acetate extraction three times, the washings were combined and evaporated to dryness, yielding 11.68 g of monoethyleneglycolferulate.
A mixture of monoethyleneglycolferulate (2.38 g, 0.01 mol), bromoacetic acid (4.17 g, 0.03 mol), p-toluenesulfonic acid (0.19 g, 0.001 mol) in 10 mL dioxane was stirred and heated to reflux under nitrogen for 4 h. The combined extract was washed with brine and dried over anhydrous sodium sulphate. The solvent was removed by rotary evaporation, and then the crude product was purified by column chromatography on silica gel (eluent petroleum ether/ethyl acetate 70/30) to give the product BAEGF as a yellow oil in 43% yield, 1 H NMR (CDCl3, ppm) δ7.65 (d, 1 H, J = 15.9), 7.08 (t, 2 H, J = 8.1, J = 9.9), 6.93(d, 1 H, J = 8.1), 6.31(d, 1 H, J = 15.9), 4.4 6 ∼ 4.35(m, 4 H), 3.94(s, 3 H), 3.90(s,2 H).
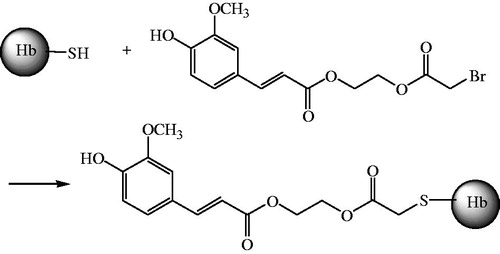
Synthesis of FA-Hb by conjugation of BAEGF to the thiol of Hb
The stroma-free haemoglobin solution (SFH, tetramer, 1 mmol/L) was under the protection of nitrogen in phosphate buffered saline(PBS, pH7.4) for 1 h at 4 ± 2 °C, followed by reaction with BAEGF (36 mg) in 500 μL DMSO, at a 1-in-10 molar excess or 4 h at 4 ± 2 °C with gentle stirring. The BAEGF-modified product (FA-Hb) was purified as the retentate after 10 kDa ultrafiltration centrifugal tube (Nanosep, Omega™) at 4000 r/min for 15 min, and the final product (FA-Hb) was exchanged into 2 mM PBS (pH7.4) ().
Determination for modification degree of FA on Hb
In the process of obtaining FA-Hb, the filtrate was collected after the first centrifugation. UV-Vis spectrum measured the absorption of the filtrate at 327 nm, after the solution was diluted 200 times. The amount of free BAEGF was easy to be known. The BAEGF modified on Hb equals the difference between total and free BAEGF. The molecular number of BAEGF modified on Hb divided by the molecular number of Hb is the degree of modification.
Study of the structure and the oxygen affinity of FA-Hb
UV-Vis full wavelength scanning of the FA-Hb solution (0.1 g/L) was recorded in 200 nm to 600nmrange. Oxygen binding curve of FA-Hb (6.0 g/L) was measured using a Hemoxanalyzer at 37°Cin PBS, pH 7.4. Triplicate measurements for each sample were performed to obtain the average value of P50, which was obtained from the oxygen equilibrium curves.
Autoxidation experiments of Hb/FA-Hb
Autoxidation experiments for Hb/FA-Hb samples (25 μmol/L) in PBS buffer, pH 7.3, containing 0.1 mmol/L EDTA at 37 °C were carried out in the dark. Absorbance changes in the range of 200–600 nm due to the spontaneous oxidation of Hbs were recorded on UV-Vis spectrometer at 1 h intervals. The whole experimental time was 4 h. The blank assays were carried out under the same conditions without Hb/FA-Hb. The time-dependent changes in concentration of the MetHb were calculated based on the above-mentioned method [Citation26].
Autoxidation experiments of Hb/FA-Hb with NaN3
The Hb/FA-Hb solution was diluted to the concentration of 25 μmol/L with PBS buffer, pH7.4, containing 0.3 mmol/L EDTA, and then was mixed with 0.1 mol/L NaN3in sealed tube at 37 °C. The mixture was sampled by UV-Vis spectrometer in the range of 200–600 nm at 0.5 h intervals for 3 h. The blank assays were carried out under the same conditions without Hb/FA-Hb. The time-dependent changes in concentration of the MetHb were calculated based on the above-mentioned method.
Results
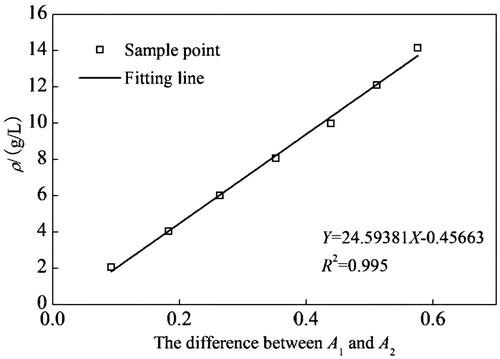
Standard curve of BAEGF
shows the excellent correlation between the absorbance at 327 nm and the concentration of BAEGF. This is in good accordance with Lambert–Beer law in dilute solution, which can act as the basis to calculate the modification degree of BAEGF on Hb.
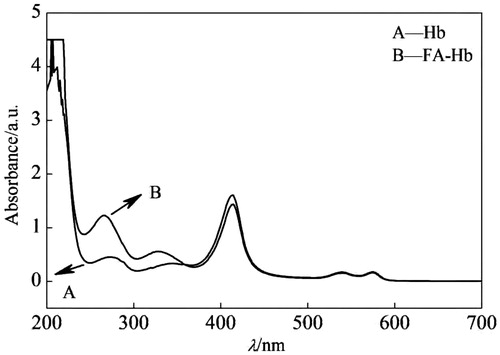
Characteristics of Hb and FA-Hb by UV-Vis
The UV-Vis spectra of Hb and FA-Hb are shown in . In the case of Hb alone, there were three typical peaks, as we know, at 406 nm, 540 nm and 577 nm, respectively (line A). For the spectrum of FA-Hb (line B), besides the former peaks of Hb, there appeared an increasing peak at 327 nm, which was characteristic of BAEGF, indicating the conjugation product (FA-Hb).
Degree of modification
Spectrometric measurements were carried out in a UV-Vis spectrometer. The concentration of BAEGF is 3.995 mmol/L combining the standard curve of BAEGF, and the Hb concentration is 1 mmol/L. Therefore, the degree of BAEGF modification on Hb is 4. This result was verified after oft-repeated experimentation.
Oxygen affinity of Hb and FA-Hb
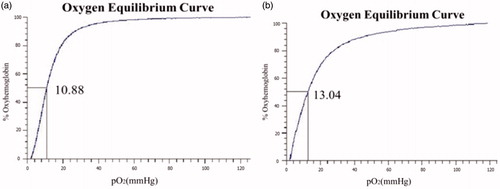
The oxygen affinity (P50) of Hb and FA-Hb are shown in , respectively. After the modification of BAEGF, the P50 value of Hb becomes a little higher (13.04 mmHg) than that before modification (10.88 mmHg), which may be owed to the coordination effect of BAEGF on oxygen release. The consistency in P50value of FA-Hb is more significant than that of Hb.
Autoxidation study of Hb and FA-Hb
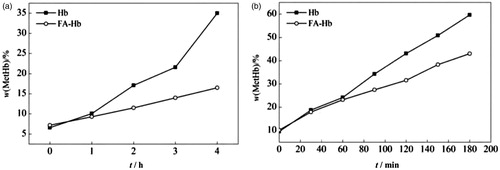
shows the time course of MetHb formation with or without NaN3. In the case of Hb or FA-Hb alone (), there is a gradually time-dependent elevation in the MetHb percentage. After 1 h the MetHb percentage of Hb increases more rapidly than that of FA-Hb. At the end of the experimental time (4 h), the MetHb percentage of Hb and FA-Hb are35% and 16%, respectively. When NaN3 is added to the solution of Hb/FA-Hb, more rapid increases of MetHb percentage are observed in both Hb and FA-Hb system (). The MetHb percentages of Hb and FA-Hb reach up to 60% and 43% at the end of the experimental time (3 h), respectively, and the oxidation rate of FA-Hb is still lower than that of Hb. From these results, it is confirmed that the formation of MetHb is suppressed by the ROS elimination system of FA-Hb.
Discussion
HBOCs can overcome the shortage of blood supply and avoid pathogen-borne diseases through blood transfusion (e.g. AIDS and hepatitis C). In the last few decades, HBOCs of several types have been developed. Nevertheless, none of the HBOCs satisfies all requirements for use as an RBC alternative. An important concern (side effect) of HBOCs is autoxidation during the perfusion and circulation. Autoxidation of Hb involves production of ROS and nonfunctional MetHb, which is a parameter that reflects the in vivo toxicity of Hb. In addition, it is necessary to keep the O2-binding affinity of HBOCs within an appropriate range after chemical modification [Citation27]; the easy regulation of the O2-binding affinity might be useful to meet the requirement of clinical indications such as oxygenation of ischaemic tissues.
Ferulic acid (FA) itself is an acid with high stability and good cost performance, and has a well-known effect on quenching oxygen free radicals, hence preventing the autoxidation of Hb. Thus a kind of FA modified haemoglobin (FA-Hb) is expected to produce the anti-oxidation effect on HBOCs spontaneously, and reduce the toxicity.
In the present study, taking the advantages of FA into consideration, we synthesized a novel chemical reagent, BAEGF. In this reagent we introduced a bromoacetyl group, which reacted site-specifically with thiols on Hb to form a HBOC (FA-Hb). Because of the anti-oxidative ability of phenolic hydroxyl group in FA, we can expected there to be a suppression of MetHb with FA-Hb [Citation28].
UV-Vis wavelength scanning showed that the absorption intensity of Hb before and after modification is not changed, which demonstrated that there is no change in the spatial conformation around heme in FA-Hb. This result indicates that modification has no effects on the function of carrying O2. On the other hand, by UV-Vis spectrum we determined that the degree of modification of BAEGF on Hb is 4. In addition, the detection of oxygen binding curve showed a similar oxygen affinity between Hb and FA-Hb, indicating a good oxygen-carrying ability of FA-Hb.
A primary but important goal of this research is to reduce the autoxidation rates of HBOC via binding FA to Hb. We measured the autoxidation rates of Hb and FA-Hb by calculating their MetHb percentage, respectively. The result showed a much lower autoxidation rate of FA-Hb than Hb. Moreover, as a strong nucleophilic reagent, NaN3is prone to accelerate the oxidation rate of HBOC by interaction with ferric MetHb. Therefore, we determined the autoxidation rates of Hb and FA-Hb in the existence of NaN3. It was found that both autoxidation rates increased after the addition of NaN3, and the oxidation rate of FA-Hb was also more effectively reduced than that of Hb. These results confirmed the suppressive effect of MetHb formation using the FA-Hb as HBOCs.
Conclusion
HBOC site-specifically modified with ferulate (FA-Hb) is prepared in order to enhance the efficacy. The experimental results of our study indicate that FA-Hb, as a kind of novel HBOC, shows not only a good oxygen affinity as native Hb, but an effective suppression of autoxidation in different systems.
Acknowledgements
We appreciate Professor Liu Jiaxin and Wang Hong, for helpful discussions and Li Shen for communicating unpublished results. This report would not have been possible without the support and substantive contributions of the Senior Research Scientists at Institute of Blood Transfusion, Chinese Academy of Medical Sciences, Chengdu, P.R. China.
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Winslow RM. Progress on blood substitutes. Nat Med. 1997;3:474.
- Chang TMS. Blood substitutes: principles, methods, products and clinical trials. Switzerland: S. Karger AG; 1997.
- Tsuchida E, editor. 1998. Blood substitutes: present and Future Perspectives. Amsterdam: Elsevier Science.
- Reiss JG. Oxygen carriers (“blood substitutes”)-raison d’etre, chemistry, and some physiology. Chem Rev. 2001;101:2797–2920.
- D’Agnillo F, Alayash AI. Redox cycling of diaspirincross-liked hemoglobin induced G2/M arrest and apoptosis in cultured endothelial cells. Blood. 2001;98:3315–3323.
- Conover CD, Lejeune L, Shum KL, et al. Physiological effect of polyethylene glycol conjugation on stroma-free bovine hemoglobin in the conscious dog after partial exchange transfusion. Artif Organs. 1997;21:369–378.
- Hess JR. Blood substitutes for surgery and trauma: efficacy and toxicity issues. BioDrugs. 1999;12:81–91.
- Takeoka S, Teramura Y, Atoji T, et al. Effect of Hb-encapsulation with vesicles on H2O2 reaction and lipid peroxidation. Bioconjug Chem. 2002;13:1302–1308.
- Teramura Y, Kanazawa H, Sakai H, et al. Prolonged oxygen-carrying ability of hemoglobin vesicles by coencapsulation of catalase in vivo. Bioconjug Chem. 2003;14:1171–1176.
- Lee R, Neya K, Svizzero TA, et al. Limitations of the efficacy of hemoglobin-based oxygen-carrying solutions. J Appl Physiol 1995;78:236–242.
- Faivre B, Menu B, Labrude P, et al. Hemoglobin autooxidation/oxidation mechanisms and methemoglobin prevention or reduction processes in the bloodstream. Literature review and outline of autooxidation reaction. Artif Cells Blood Subs Immob Biotech. 1998;26:17–26.
- Linberg R, Conover CD, Shum KL, et al. Hemoglobin based oxygen carriers: how much methemoglobin is too much? Artif Cells Blood Substit Immobil Biotechnol. 1998;26:133–148.
- Everse J, Hsia N. The Toxicities of Native and Modified Hemoglobins. Free Radic Biol Med. 1997;22:1075–1099.
- Nagababu E, Rifkind JM. Reaction of Hydrogen Peroxide with ferrylhemoglobin: superoxide production and heme degradation. Biochemistry. 2000;39:12503–12511.
- Cadenas E. Biochemistry of oxygen toxicity. Annu Rev Biochem. 1989;58:79–110.
- Shikama K. A controversy on the mechanism of autoxidation of oxymyoglobin and oxyhaemoglobin: oxidation, dissociation, or displacement? Biochem J. 1984;223:279–280.
- Tomoda A, Yoneyama T, Tsuji A. Changes in intermediate haemoglobins during autoxidation of haemoglobin. Biochem J. 1981;195:485–492.
- Naito Y, Fukutomi I, Masada Y, et al. Virus removal from hemoglobin solution using Planova membrane. Artif Organs. 2002;5:141–145.
- Walter SV, Chang TMS. Chronotropic effects of in vitro perfusion with albumin, stroma-free hemoglobin, and polyhemoglobin solutions. Biomater Artif Cells Artif Organs. 1990;18:283–298.
- Wang MY, Yu YT, Chang TMS. New method for preparing more stable microcapsules for the entrapment of genetically engineered cells. Artif Cells Blood Subs Immob Biotech. 2005;33:257–269.
- Gu J, Chang TMS. Extraction of erythrocyte enzymes for the preparation of polyhemoglobin-catalase-superoxide dismutase. Artif Cells Blood Subs and Biotech. 2009;37:69–77.
- D’Agnillo F, Chang TMS. Polyhemoglobin-superoxide dismutase-catalase as a blood substitute with antioxidant properties. Nat Biotech. 1998;16:667–671.
- Alagic A, Koprianiuk A, Kluger R. Hemoglobin-superoxide dismutase-chemical linkages that create a dual-function protein. J Am Chem Soc. 2005;127:8036–8043.
- Mathew S, Abraham T. Ferulic acid: an antioxidant found naturally in plant cell walls and feruloyl esterases involved in its release and their applications. Crit Rev Biotechnol. 2004;24:59–83.
- Walder RY, Andracki ME, Walder JA. Preparation of intramolecularly cross-linked hemoglobins. Meth Enzymol. 1994;231:274–280.
- Abugo OO, Rifkind JM. Oxidation of hemoglobin and the enhancement produced by nitroblue tetrazolium. J Biol Chem. 1994;269:24845–24853.
- Alayash AI, Summers AG, Wood F, et al. Effects of glutaraldehyde polymerization on oxygen transport and redox properties of bovine hemoglobin. Arch Biochem Biophys. 2001;391:225–234.
- Nicholson SK, Tucker GA, Brameld JM. Effects of dietary polyphenols on gene expression in human vascular endothelial cells. Proc Nutr Soc. 2008;67:42–47.