?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
In the present study, we have demonstrated receptor for advanced glycation endproducts (RAGE) as a target for delivery of drugs specifically to triple negative breast cancer cells. We have prepared solid lipid nanoparticle formulation of cytotoxic agent di-allyl-disulfide (DADS) to overcome its bioavailability issues. Then, we have surface modified DADS-loaded solid lipid nanoparticles (DADS-SLN) with RAGE antibody to achieve site-specific delivery of DADS to TNBC cells. We found a significant cellular internalization of RAGE surface modified DADS-SLN (DADS-RAGE-SLN) when compared to DADS-SLN. The cytotoxic effect of DADS was also significantly improved with DADS-RAGE-SLN by downregulating anti-apoptotic proteins and upregulating pro-apoptotic proteins as observed by western blot analysis. RAGE-targeted delivery of cytotoxic agents can be, therefore, a promising approach for improving antitumour activity and reducing off-target effects.
Graphical Abstract
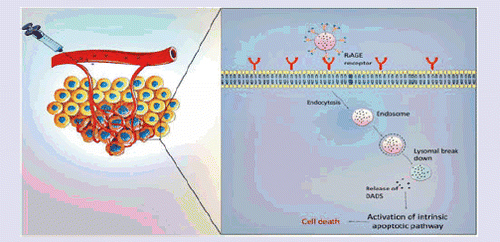
Introduction
Based on the expression of oestrogen receptor (ER), progesterone receptor (PR) and human epidermal growth factor receptor-2 (HER2) receptor, breast cancers can be classified into oestrogen receptor positive (luminal subtype), human epidermal growth factor receptor-2 (HER2), progesterone receptor (PR) and triple-negative breast cancer (TNBC), which lacks ER, PR and HER2 expression [Citation1–3]. TNBC represent around 10–15% of all breast cancers and is considered as an aggressive type of breast cancer with drug resistance, high metastatic and tumour relapse rates [Citation4–6]. Lack of validated molecular targets, prognostic and therapeutic markers in TNBC, unlike other types of breast cancers make TNBC treatment difficult. Present therapeutic armamentarium of this subtype is conventional chemotherapy with cytotoxic agents. However, due to lack of selectivity of chemotherapeutic agents to tumour site and development of drug resistance, chemotherapy ends with poor therapeutic outcomes in TNBC [Citation7,Citation8]. Therefore, there is a need for development of more effective targeted therapies for treatment of TNBC.
Receptor for advanced glycation end-products (RAGE) is a multi-ligand cell-surface receptor belongs to immunoglobulin super family which has inbuilt affinity to interact with advanced glycation end products (AGE) [Citation9,Citation10]. Recently, many studies have demonstrated the role of RAGE in various cancer pathologies, where the upregulation of RAGE was reported to be oncogenic by activating various oncogenic signalling pathways [Citation11,Citation12]. It was reported that, interaction of RAGE with its ligands like, AGE [Citation13], S100/Calgranulin [Citation14], HMGB1 and the S100 family of proteins (S100A4, S100A6, S100A7, S100A9) stimulate P38 and P44/42, MAP kinase Ras-extracellular signal-regulated kinase and NF-κβ to promote tumour progression. Recently, Nasser et al (2015) reported that RAGE-S100A7 signalling axis is associated with metastasis in TNBC. In another study, it was found that there is over expression of RAGE in TNBC cells than MCF-7/SK-Br-3 luminal breast cancer cells [Citation15–17]. We, therefore, hypothesize that RAGE-targeted delivery of cytotoxic drugs might have potential to achieve radical cure by selectively targeting TNBC and also RAGE inhibition can sensitize cancer cells to cytotoxic effects. As a proof of the concept in this study, we propose RAGE receptor targeted delivery of diallyl disulfide (DADS), an organosulfur ingredient of garlic with cytotoxic activity against breast cancer to achieve enhanced antitumour activity.
In our previous study, we have prepared solid lipid nanoparticles of DADS (DADS-SLN) to overcome its bioavailability problems [Citation18]. However, selective targeting of DADS specifically to TNBC cancer cells is questionable. The surface modification of DADS-SLN with anti-RAGE antibody was expected to direct DADS specifically towards TNBC cells due to overexpression of RAGE receptor on TNBC cells, and also minimize off-target cytotoxic effects on healthy cells.
Experimental
Materials
DADS and RAGE antibody were purchased from Santa Cruz Biotechnology, India. Palmitic acid was obtained from SD-Fine chemicals limited, Mumbai, India. Acetonitrile used was HPLC grade purchased from Merck, India. Sulforhodamine B (SRB), Poloxamer 188 (F68), Sodium dodecyl sulphate were purchased from Sigma-Aldrich, Bangalore, India. 1-ethyl-3–(3-dimethylaminopropyl) carbodiimide hydrochloride (EDC) and N-hydroxysuccinimide (NHS, 98%) was purchased Sisco Research Laboratories Pvt. Ltd, India. AnnexinV-FITC and propidium iodide were procured from Biolegend Europe BV, Netherlands. ER-Tracker™ Green was purchased from Imperial Life Sciences (P) Ltd, Gurgaon, India. Annexin V, Propidium Iodide (PI), Nile red and DAPI (4′,6-Diamidino-2-Phenylindole, Dihydrochloride) from Thermo Fisher Scientific India Pvt Ltd., Bangalore, India.
Cell and culture conditions
MDA-MB231 triple negative breast cancer cell lines were purchased from National Center for Cell Science (Pune, India). The cell lines were maintained as a continuous culture in Dulbecco’s modified Eagle’s medium (DMEM; Sigma-Aldrich Inc.), supplemented with 10% foetal bovine serum (FBS; Himedia, Mumbai, India), 100 U/mL penicillin and 100 μg/mL streptomycin. Cells were grown in a humidified atmosphere of 5% CO2 at 37 °C. Media was replenished every three days.
Preparation of diallyl disulfide loaded solid lipid nanoparticles (DADS-SLN)
The preparation of DADS-SLN formulation was prepared by solvent diffusion method as reported previously by our group with slight modification [Citation18]. Palmitic acid was used as a lipid, pluronic F-68 and soya lecithin were used as surfactant and co surfactant respectively. The palmitic acid and drug were dissolved in a 5 mL ethanol under the water bath conditions at 70 °C. The resultant organic solution was immediately dispersed into 50 mL of an aqueous phase containing surfactant under continuous mechanical agitation at 400 rpm in a water bath at 70 °C for 5 min. Pre-emulsion (melted lipid droplet) obtained was subsequently transferred into an ice bath to solidify the lipid droplets and then cooled to room temperature till SLN dispersion was obtained. The SLN dispersion was dialyzed against distilled water for 12 h to remove water-soluble impurities (organic solvents and nonadsorbed surfactants) and subsequently centrifuged (7000 rpm, 20 min) to remove large lipid particles and precipitated free DADS. The final dispersion was lyophilized to obtain the solid dispersion form.
Application of box-Behnken design for optimization of DADS-SLN
For the present study, a 17-run, 3-factor, 3-level Box–Behnken design was employed to optimize the DADS-SLN using Design-Expert software (Version 7.1.6. Stat-Ease Inc., 2 Minneapolis, MN). This is a cubic design, characterized by centerpoint replicates and a set of points at the mid-point of each edge of the multidimensional cube that circumscribe the region of interest. Region of interest aids to investigate the main effects, interaction effects and quadratic effects of the formulation ingredients and to optimize the formulation. Design matrix with 17 experimental runs was constructed. The percentage of the amount of lipid (X1), surfactant (X2), and co-surfactant (X3) as the independent variables which were represented by −1, 0 and +1, analogous to the low, middle and high values, respectively, as described in . The model was selected from a quadratic mathematical equation based on significance (p values < .05) from the model and non-significant lack of fit. The optimized DADS-SLN using box-behnken design was used for conjugation with RAGE antibody.
Table 1. Independent and dependent variables applied in experimental design.
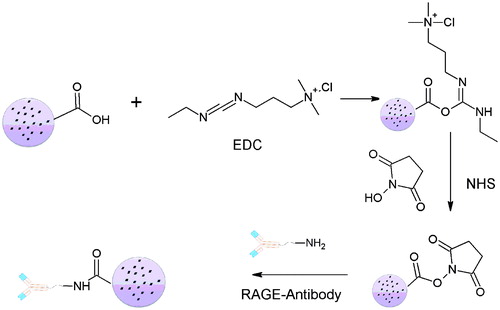
Conjugation of anti-RAGE antibody to DADS-loaded SLN [Citation19]
In all, 10 mg of optimized DADS-SLN were dispersed in 5 ml of PBS (0.02 M, pH 7.4) subsequently 250 μl of EDC (1 mg/ml) and 250 μl of NHS (1 mg/ml) were added to the above suspension. EDC activation occurs with agitation of the above solution for 2 hrs using a magnetic stirrer. Excess unreacted EDC and NHS were dialyzed against millipore water by using cellulose membrane (8000 molecular weight cut-off, HiMedia, India) for 24 h. Activated SLNs were obtained by centrifugation at 7000 rpm for 10 min at 4 °C [Citation20]. Obtained activated SLN suspension was dispersed again in 5 ml of PBS (0.02 M, pH 7.4). RAGE antibody and 0.5 ml pyridine was added and the further reaction was carried out by the magnetic stirring for overnight. For the precipitation of the DADS-RAGE-SLN, 20 ml of Millipore water was added to the mixture. Separation of the unconjugated antibody, EDC and water-soluble impurities in the dispersion was carried out by dialysis against the Millipore water using cellulose membrane (8000 molecular weight cut-off, HiMedia, India) for 48 h. The obtained suspension was lyophilized [Citation21]. The conjugation was confirmed by the FT-IR (Fourier transform infra-red spectroscopy) spectral analysis.
Characterization of DADS-SLN and DADS-RAGE-SLN
Determination of particle size, zeta potential and morphology
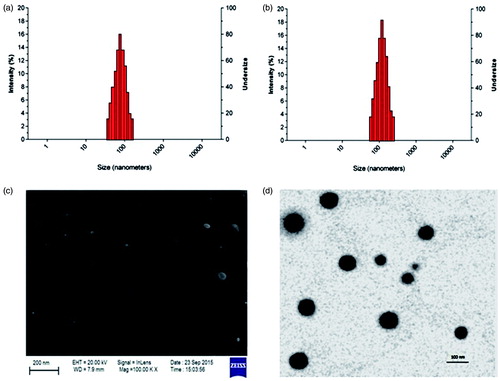
The particle size and zeta potential of DADS-RAGE- SLN and DADS-SLN were measured by dynamic light scattering (DLS) Nanotrac Wave™ (Microtrac, San Diego, CA) before measurement the samples were suspended in deionized water. All measurements were carried out at 25 °C and performed in triplicate. The morphology of DADS-RAGE-SLN was analyzed using scanning electron microscopy(SEM) and transmission electron microscope (TEM) [Citation22,Citation23].
Determination of drug entrapment efficiency and drug loading
The amount of DADS in DADS-RAGE-SLN and DADS-SLN was determined using HPLC. About 10 mg prepared SLN was dissolved in 10 ml of 1:1 mixture of methanol and sodium dodecyl sulphate. Above mixture was placed in a super filter tube, and then centrifuged by a Sigma-3k30 Centrifuges (Sigma-Aldrich, Seelze, Germany) with 14,000 rpm for 10 min at the temperature of 32 °C. The ultrafiltrate was extracted by methanol, and filtered with a 0.45 mm filter. The drug concentration in ultrafiltrate was determined as the content of the free drug [Citation24]. The drug content in the supernatant after centrifugation was measured by HPLC method using a mobile phase delivery pump (LC-20 AD; Shimadzu, Japan) of flow rate 1.0 ml min−1, a photodiode array detector (SPDM20A; Shimadzu, Japan) set at 240 nm, a 20 μL loop (Rheodyne) and Phenomenex Gemini C18 (250 mm × 4.6 mm) was used. The mobile phase consisted of acetonitrile and water (75:25, v/v). The encapsulation efficiency and loading capacity were calculated by the following equations.
Analysis of in vitro drug release
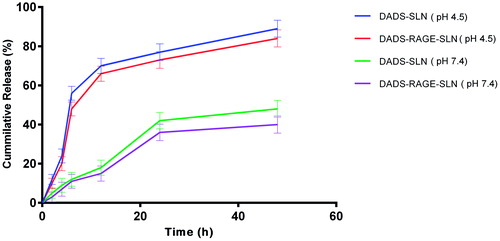
The in vitro release behaviour of DADS-RAGE-SLN and DADS-SLN was analyzed by performing dialysis bag diffusion method. 1.0 mg of DADS-RAGE-SLN and DADS-SLN were dispersed in 10 mL of Phosphate buffer saline (PBS) and then transferred into a pre-swelled dialysis bag with Molecular Weight Cut Off (MWCO) 8–12 kDa (Dialysis membrane-150, HiMedia, Mumbai, India). The bag was individually suspended in 100 ml of phosphate buffer saline (PBS) pH 4.5 and PBS pH 7.4, at 37 °C in water bath at 100 rpm. Sink condition was maintained by periodically removing 2 mL sample and replacing equal volume of fresh buffer medium and monitored throughout the release period. The amount of DADS released was determined by an HPLC. Experiments were carried out in triplicate and data obtained was represented graphically.
Cellular uptake study
MDA-MB231 cells were seeded on 8-well cover glass chamber under standard conditions and left for incubation for overnight. Then the cells were treated with free Nile Red (0.3 μg) and SLN. Cells were washed thrice after incubation for 2 hrs and then fixed and stained with ER-Tracker™ Green and 4, 6-diamidino-2-phenylindole (DAPI) for 1 h. Cell uptake was observed under confocal laser scanning microscopy (Carl Zeiss LSM710, Germany) to determine the cellular uptake of SLN [Citation25,Citation26].
In vitro cytotoxicity studies
The cytotoxicity of DADS, DADS-SLN and DADS-RAGE-SLN against MDA-MB231 was measured by SRB assay [Citation27]. Briefly, MDA-MB231 cells with a density of (1 × 104/well) were seeded in a 96-well plate and left for incubation for 24 h under a humidified atmosphere of 95% air and 5% CO2 at 37 °C. Then, the cells were treated with serial concentrations (1.562, 3.125, 6.25, 12.5, 25, 50, 100 μM) of DADS, DADS-SLN and DADS-RAGE-SLN at 37 °C. The cells were rinsed with cell medium three times and further incubated at 37 °C for 72 h, followed by fixing of cells with cold trichloroacetic acid, washing and drying in the air. Subsequently, the fixed cells were then stained with 0.4% SRB dye for 30 min and the excess dye was washed by 1% acetic acid. After bound dye dissolved in 10 mM Tris base solution, the absorbance was determined using a Thermo scientific multiscan FC microplate photometer at the wavelength of 540 nm. The data were expressed as the percentages of viable cells compared to the survival of control group (cells treated with medium) and presented as mean ± SD.
Annexin V/propidium iodide double staining assay
Annexin V/Propidium Iodide (PI) dual staining assay is a sensitive method implemented for detection of apoptotic cells. MDA-MB231 cells were seeded in 6-well plate at a density of 2 × 104 cells/well and left for incubation for 24 h for adhesion. Then, old culture media was replaced with fresh culture media and cells were treated with DADS (5 μM), DADS-SLN (5 μM) and DADS-RAGE-SLNs (5 μM) for 24 h. Later, the cells were washed thrice by PBS and stained with annexin V-FITC (2.5 μl) and PI (2.5 μl) at 37 °C for 30 min. The cells were then analyzed by flow cytometry (BD FACS Canto TM, BD Biosciences) was carried out to identify and quantitate apoptotic cells [Citation28]. Annexin V has affinity towards phosphatidylserine located on the outer surface of apoptotic cells which gives direct apoptotic cell count.
Western blotting
RIPA buffer (comprising 20 mM NaH2PO4, 50 mM NaF, 2 mM EDTA, 150 mM NaCl, 1% Deoxycholic Acid, 0.1% SDS, pH 7.2) was used to prepare whole cell lysates for western blot analysis (Lin et al., 2005). Protease inhibitor cocktail (Sigma Aldrich, Mumbai) was used for western blot analysis. The protein (40 mg) was taken and resolved using 10% sodium dodecyl sulphate-polyacrylamide gel electrophoresis (SDS-PAGE). Antibodies (anti-β-actin, anti-Bcl-2, anti-survivin, anti-caspase-9) were selected and obtained from Santacruz biotech and horseradish peroxidase-conjugated goat anti-rabbit IgG was used as secondary antibody. This was later detected using chemiluminescence reagents. Hitachi Gene tools CCD-BIO software with Hitachi scanning densitometry was used for determination of relative protein expression.
Statistical analysis
Statistical analysis was done by performing Student’s t test. The differences were considered significant for p values of <.05. Data were subjected to statistical analysis using Graphpad prism software (version 6) (Graph Pad Software Inc., La Jolla, CA).
Results and discussion
Optimization of DADS-SLNs
We have optimized the formula of DADS-SLN using the Box-Behnken experimental design, using different ratios of lipid, surfactant and co surfactant (predetermined factors). The optimization was done based on the responses observed for all 17 formulations (). The effects of pre-determined factors on particle size (PS) and (EE) was observed using perturbation plots and response surface plots (). The model was validated by applying Analysis of variance (ANOVA) (). The optimized formulation was selected using desirability criterion of PS and EE. It was predicted according to the design that 50.57 mg of lipid, 1.30% w/v of surfactant and 1.98%w/v of Co surfactant will give PS of 113.011 nm and EE of 80.0.77%. Based on the predicted composition of lipid surfactant and co-surfactant a new batch of SLN was prepared. The actual values we observed using the same ratios are PS of 116.20 nm and EE of 79.23%. The observed results are in close agreement with predicted values of the design. Therefore, the same concentrations of lipid, surfactant and cosurfactant was used for preparation of DADS-SLN. Regression formula generated for the design in terms of coded factors is
Figure 1. Response surface plots showing the effects of lipid, surfactant and co-surfactant on particle size (a) and entrapment efficiency (b). Perturbation plot showing the effect of lipid, surfactant and co-surfactant on particle size (c) and entrapment efficiency (d).
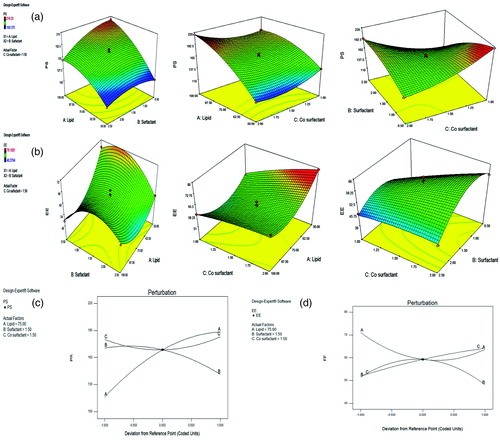
Table 2. Experimental batches and responses obtained for desired parameters.
Table 3. Analysis of variance (ANOVA) for response surface quadratic model (for PS).
Table 4. Analysis of variance (ANOVA) for response surface quadratic model (for EE).
Where, PS and EE are Particle size and Entrapment efficiency, respectively.
Conformation of surface modification of DADS-SLN using FT-IR
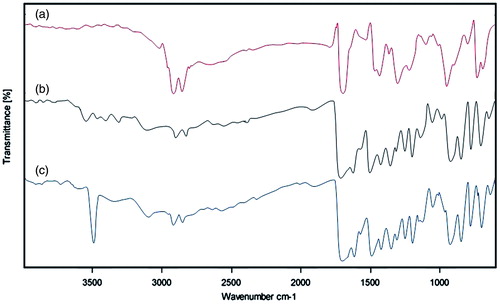
The optimized formulation of DADS-SLN was used for surface modification with RAGE antibody using EDC as cross linker (). The surface modification was expected to takes place by the reaction between (−COOH) groups of palmitic acid and (−NH2) group of RAGE antibody. Surface modification of DADS-SLN with RAGE antibody was confirmed by the FTIR analysis (). RAGE antibody exhibit two bands at 3320.94 cm−1 and 3408.54 cm−1 which indicates the presence of the (–NH2) groups. The FTIR spectra of DADS-RAGE-SLN showed a stronger band at 3507.47 cm−1 which indicates the presence of aromatic secondary amine group that indicates the successful conjugation by the (–NH2) groups of RAGE antibody with (–COOH) groups of palmitic acid.
Characterization of DADS-SLN and DADS-RAGE-SLN
The result of characterization of nanoformulation were given in . By dynamic light scattering it was observed that particle size of DADS-RAGE-SLN is 148.18 ± 1.73 nm. When compared to DADS-SLN (116.20 ± 1.54 nm), the particle size of DADS-RAGE-SLN was high which may be due to the surface modification of antibody. Zeta potential of DADS-RAGE-SLN and DADS-SLN was 3.7mv and −7.8mv, respectively. The zeta potential shift may be due to the negatively charged carboxyl group of palmitic acid conjugation with RAGE antibody by amide bond formation. This suggests the proper conjugation of the amine group of RAGE antibody with carboxyl group of palmitic acid. SEM and TEM studies reported that DADS-RAGE-SLN was mono-dispersed and spherical shaped. The entrapment efficiency of DADS-RAGE-SLN and DADS-SLN was found to be 69.76 ± 0.11% and 79.23 ± 0.55%, respectively. The drug loading of DADS-RAGE-SLN and DADS-SLN was found to be 34.72333 ± 0.115% and 29.75 ± 0.44658%.
In vitro release of DADS from DADS-SLN and DADS-RAGE-SLN
There was no burst release of DADS observed with both DADS-SLN and DADS-RAGE-SLN (). Lipids enhance the rigidity of the encapsulation layers. Surface modification of the lipid can, therefore, alter the flexibility of the lipid layer and delay the rate of drug release. In our study, there is sustained release of drug in DADS-RAGE-SLN when compared to DADS-SLNs this may be due to surface modification. Abnormal glycolytic rate, high lactic acid generation and inappropriate drainage by convective transport, H + ions stack up can be seen in the tumour tissue. So, there will be acidic pH at tumour site [Citation29]. To mimic the pH variation between cellular exterior (pH 7.4) and intracellular lysosome (pH 4.5), drug release was carried out at different pH environment. DADS is a weak acid and alkaline in nature is due to the pair of sulphide groups and it shows higher solubility at lower pH. Thereby, the encapsulated DADS in the SLN has tendency to enter into the release medium of lower pH. Due to this reason, we observe higher amount DADS release was observed at lower pH.
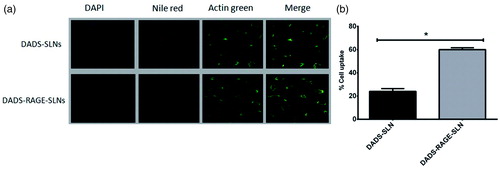
DADS-RAGE-SLN formulation has greater cellular uptake in MDA-MB231 cells
Cellular uptake of the DADS-RAGE-SLNs and DADS-SLN was evaluated in MDA-MB231 cells by triple fluorescence staining method. This method employs three stains where, the red fluorescence from nile red labels nanoparticles, green fluorescence from Actin-Tracker Green labels the actin, and blue fluorescence from DAPI labels the nucleus. The lipophilic stain nile red is poorly water soluble stain (<1 μg/mL), it is labels the SLNs. The cellular uptake of DADS-RAGE-SLN and DADS-SLN in MDA-MB231 cells was shown in .
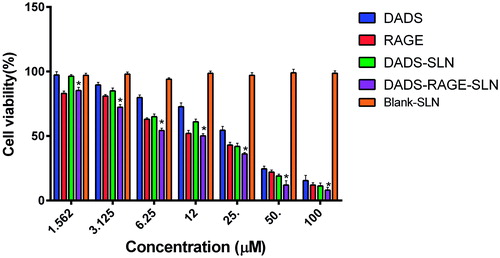
DADS-RAGE-SLN potentiated the cytotoxic effects of DADS
The in vitro cytotoxic activity of DADS, DADS-SLN and DADS-RAGE-SLN were evaluated by the SRB assay in MDA-MB231, TNBC cell line, the cell viability are shown in . Blank SLNs didn’t exhibit significant cytotoxicity in MDA-MB231 cells which confirm the safety of the SLNs as carriers. MDA-MB231 cells treated with DADS-SLN and DADS-RAGE-SLN exhibited cytotoxicity at various concentrations (1.562, 3.125, 6.25, 12.5, 25, 50, 100 μM). It is evident that DADS, DADS-SLN and DADS-RAGE-SLN exhibited dose-dependent cytotoxic action. DADS-SLN had exhibited lower cytotoxic action when compared with DADS-RAGE-SLN which might be due to the efflux of the diffused drug in the cytoplasm by P-glycoprotein (P-gp) pumps. DADS-RAGE-SLN might be internalized into cells via receptor-mediated endocytosis that can bypass p-gp efflux proteins which leads to enhanced internalization of drug inside cells [Citation30,Citation31].
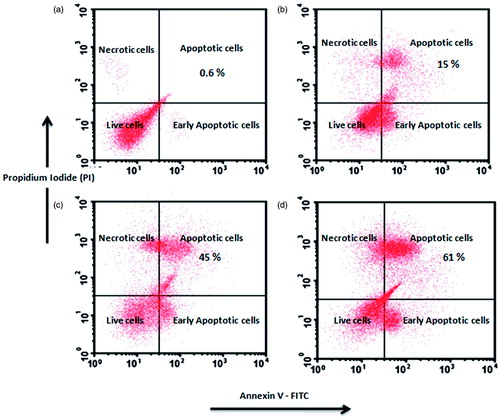
Treatment with DADS-RAGE-SLN increased the percentage of apoptotic cells
Annexin V dye labels the extrinsic phosphatidyl serine (PS) and differentiates apoptotic cells from live cells. Another fluorescent dye PI permeates into necrotic cells and could not internalize into live cells. These two dyes can, therefore, differentiate the early and late apoptotic cells in the flow cytometer. (), the amount of early apoptotic and late apoptosis cells in the control were 0.6% and 0.9%, respectively. It was observed that the percentage of apoptotic cells was higher in DADS-RAGE-SLN (61.8%) when compared to DADS-SLN (45%) and DADS (15%). From this experiment, it was observed that DADS-RAGE-SLN improve the apoptotic activity of DADS in MDA-MB231 cells.
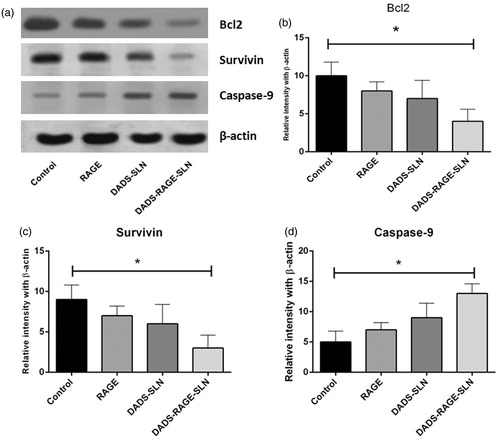
DADS-RAGE-SLN augmented the expression of proapoptotic proteins and inhibited antiapoptotic protein expression
Apoptosis is generally regulated by the various proapoptotic and antiapoptotic proteins. Generally apoptotic response is regulated by intrinsic apoptotic pathway (mitochondrial mediated) and extrinsic apoptotic pathway. It was reported that DADS induce apoptosis by increasing ROS levels, which is associated with intrinsic apoptotic pathway. We, therefore, observed the expression of various anti-apoptotic and pro-apoptotic proteins of intrinsic apoptotic pathway in MDA-MB231 (TNBC) cells. It was reported that, cancer cells resist apoptosis due to chemotherapeutic agents by upregulation of anti-apoptotic proteins and down regulation of apoptotic proteins. The Bcl2 family of proteins (Bcl-2, Bcl-xL and Mcl-1) and survivin are antiapoptotic proteins, which are responsible for chemoresistance. Bax, Caspase-3, Caspase-9 are some of the examples of pro-apoptotic proteins [Citation32,Citation33]. DADS-RAGE-SLN exhibited upregulation of pro-protein expressions of caspase-9, and downregulation of antiapoptotic proteins such as Bcl2 and survivin, when compared with the DADS and DADS-SLN (). This suggests synergy between DADS and RAGE to induce intrinsic apoptosis in MDA-MB231 cells [Citation34].
Conclusions
DADS-SLN formulation which was optimized using Box-Behnken experimental design was prepared to overcome the bioavailability problems of DADS and then we have surface modified DADS-SLN with RAGE antibody to form DADS-RAGE-SLNs. Surface modification of DADS-SLN with RAGE antibody significantly increased the uptake of drug in RAGE over expressing MDA-MB231 cells. RAGE-targeted therapy can, therefore, overcome off-target effects of DADS. DADS-RAGE-SLN exhibited greater cytotoxic effect when compared to DADS and DADS-SLN, which might be attributed to the ability of RAGE antibody to interfere with intrinsic apoptotic signalling pathways. It was observed that DADS-RAGE-SLN significantly downregulated anti-apoptotic proteins (Bcl2 and survivin), and upregulated pro-apoptotic caspase-9. Thereby we have assumed that the increased apoptotic ability of DADS-RAGE-SLN may be by interfering intrinsic apoptotic pathway. In conclusion, RAGE is a promising molecular target in TNBC. RAGE-targeted delivery of cytotoxic drugs reduces the off-target effects to healthy cells as RAGE is overexpressed in TNBC cells when compared to healthy cells. In addition, RAGE-mediated delivery of cytotoxic agents will have improved antitumour activity when compared to either RAGE or cytotoxic agent alone. However, this study is a preliminary report of in vitro results, further RAGE-targeted delivery to improve antitumour activity need to confirm in vivo in animal models.
| Abbreviations | ||
| DADS | = | diallyl disulfide |
| DADS-SLN | = | diallyl disulfide loaded solid lipid nanoparticles |
| DADS-RAGE-SLN RAGE | = | antibody modified DADS-SLN |
| DLS | = | dynamic light scattering |
| SLN | = | solid lipid nanoparticles |
| PS | = | particle size |
| EE | = | entrapment efficiency |
| DOE | = | design of experiment |
| RSM | = | response surface methodology |
| SRB | = | sulforhodamine B |
| DCFHDA | = | dichlorodihydrofluorescein diacetate |
| FTIR | = | fourier Transform Infra-Red |
| ROS | = | reactive oxygen species |
| P-gp | = | P-glycoprotein |
| PI | = | propidium Iodide |
| MDR | = | multidrug resistance |
Acknowledgements
The authors thank Gattefosse foundation, USA for kindly providing lipid for formulation.
Disclosure statement
The authors have no competing interests to disclose.
References
- Perou CM, Sørlie T, Eisen MB, et al. Molecular portraits of human breast tumours. Nature. 2000;406:747–752.
- Brenton JD, Carey LA, Ahmed AA, et al. Molecular classification and molecular forecasting of breast cancer: ready for clinical application? J Clin Oncol. 2005;23:7350–7360.
- Smolarek AK, Suh N. Chemopreventive activity of vitamin E in breast cancer: a focus on γ- and δ-tocopherol. Nutrients. 2011;3:962–986.
- Badve S, Dabbs DJ, Schnitt SJ, et al. Basal-like and triple-negative breast cancers: a critical review with an emphasis on the implications for pathologists and oncologists. Mod Pathol. 2011;24:157–167.
- Linn SC, Van’t Veer LJ. Clinical relevance of the triple-negative breast cancer concept: genetic basis and clinical utility of the concept. Eur J Cancer. 2009;45:11–26.
- Peppercorn J, Perou CM, Carey LA. Molecular subtypes in breast cancer evaluation and management: divide and conquer. Cancer Invest. 2008;26:1–10.
- Radia A-M, Yaser A-M, Ma X, et al. Specific siRNA targeting receptor for advanced glycation end products (RAGE) decreases proliferation in human breast cancer cell lines. Int J Mol Sci. 2013;14:7959–7978.
- You S, Li W. Administration of nanodrugs in proper menstrual stage for maximal drug retention in breast cancer. Med Hypotheses. 2008;71:141–147.
- Lehmann BD, Pietenpol JA. Identification and use of biomarkers in treatment strategies for triple-negative breast cancer subtypes. J Pathol. 2014;232:142–150.
- Neeper M, Schmidt A, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992;267:14998–15004.
- Hsieh H-L, Schäfer BW, Sasaki N, et al. Expression analysis of S100 proteins and RAGE in human tumors using tissue microarrays. Biochem Biophys Res Commun. 2003;307:375–381.
- Leclerc E, Fritz G, Vetter SW, et al. Binding of S100 proteins to RAGE: an update. Biochim Biophys Acta. 2009;1793:993–1007.
- Yan SF, Ramasamy R, Schmidt AM. Mechanisms of disease: advanced glycation end-products and their receptor in inflammation and diabetes complications. Nat Clin Pract Endocrinol Metab. 2008;4:285–293.
- Wu X, Mi Y, Yang H, et al. The activation of HMGB1 as a progression factor on inflammation response in normal human bronchial epithelial cells through RAGE/JNK/NF-κB pathway. Mol Cell Biochem. 2013;380:249–257.
- Nasser MW, Wani NA, Ahirwar DK, et al. RAGE mediates S100A7-induced breast cancer growth and metastasis by modulating the tumor microenvironment. Cancer Res. 2015;75:974–985.
- Yeh C-H, Sturgis L, Haidacher J, et al. Requirement for p38 and p44/p42 mitogen-activated protein kinases in RAGE-mediated nuclear factor-κB transcriptional activation and cytokine secretion. Diabetes. 2001;50:1495–1504.
- Lata K, Mukherjee TK. Knockdown of receptor for advanced glycation end products attenuate 17α-ethinyl-estradiol dependent proliferation and survival of MCF-7 breast cancer cells. Biochim Biophys Acta. 2014;1840:1083–1091.
- Talluri SV, Kuppusamy G, Karri VVSR, et al. Application of quality-by-design approach to optimize diallyl disulfide-loaded solid lipid nanoparticles. Artif Cells Nanomed Biotechnol. 2017;45:474–488.
- Tummala S, Kumar MS, Pindiprolu SK. Improved anti-tumor activity of oxaliplatin by encapsulating in anti-DR5 targeted gold nanoparticles. Drug Deliv. 2016;23:3505–3519.
- Das M, Sahoo SK. Folate decorated dual drug loaded nanoparticle: role of curcumin in enhancing therapeutic potential of nutlin-3a by reversing multidrug resistance. PLoS One. 2012;7:e32920.
- Zhang X-G, Miao J, Dai Y-Q, et al. Reversal activity of nanostructured lipid carriers loading cytotoxic drug in multi-drug resistant cancer cells. Int J Pharm. 2008;361:239–244.
- Venkata Siddhartha T, Senthil V, Sai Kishan I, et al. Design and development of oral nanoparticulated insulin in multiple emulsion. Curr Drug Deliv. 2014;11:472–485.
- Talluri S, Pindiprolu S, Janarthanam R, Kuppusamy G, editors. Development and efficacy evaluation of smart nanocarriers for targeting breast cancers. Eur J Cancer. 2016;57:S122.
- Hu F-Q, Jiang S-P, Du Y-Z, et al. Preparation and characterization of stearic acid nanostructured lipid carriers by solvent diffusion method in an aqueous system. Colloids Surf B Biointerfaces. 2005;45:167–173.
- Zhang L, Zhu D, Dong X, Sun H, Song C, Wang C, et al. Folate-modified lipid–polymer hybrid nanoparticles for targeted paclitaxel delivery. Int J Nanomedicine. 2015;10:2101.
- Sharma G, Park J, Sharma AR, et al. Methoxy Poly (ethylene glycol)-Poly (lactide) nanoparticles encapsulating quercetin act as an effective anticancer agent by inducing apoptosis in breast cancer. Pharm Res. 2015;32:723–735.
- Vichai V, Kirtikara K. Sulforhodamine B colorimetric assay for cytotoxicity screening. Nat Protoc. 2006;1:1112–1116.
- Chen R, Wang S, Zhang J, et al. Aloe-emodin loaded solid lipid nanoparticles: formulation design and in vitro anti-cancer study. Drug Deliv. 2015;22:666–674.
- Vaupel P, Kallinowski F, Okunieff P. Blood flow, oxygen and nutrient supply, and metabolic microenvironment of human tumors: a review. Cancer Res. 1989;49:6449–6465.
- Panyam J, ZHOU W-Z, PRABHA S, et al. Rapid endo-lysosomal escape of poly (DL-lactide-co-glycolide) nanoparticles: implications for drug and gene delivery. FASEB J. 2002;16:1217–1226.
- Gabizon AA. Selective tumor localization and improved therapeutic index of anthracyclines encapsulated in long-circulating liposomes. Cancer Res. 1992;52:891–896.
- Elumalai P, Gunadharini D, Senthilkumar K, et al. Induction of apoptosis in human breast cancer cells by nimbolide through extrinsic and intrinsic pathway. Toxicol Lett. 2012;215:131–142.
- Gillings AS, Balmanno K, Wiggins CM, et al. Apoptosis and autophagy: BIM as a mediator of tumour cell death in response to oncogene-targeted therapeutics. FEBS J. 2009;276:6050–6062.
- Lee J-E, Lee R-A, Kim K-H, et al. Induction of apoptosis with diallyl disulfide in AGS gastric cancer cell line. J Korean Surg Soc. 2011;81:85–95.