Abstract
Nanozymes, in nature, are artificial enzymes. Innovated by Ronald Breslow to mimic enzymes. Nanozymes have widespread applications including targeted cancer therapy, diagnostic medicine and bio-sensing even environmental toxicology. However, these applications are a novel research field in biomedicine, but are growing fast. Enzyme-based applications such as immune-absorbent assay (ELIZA) are expensive because of the complexity of producing enzymes and antibodies. Not only, some nanoparticles can mimic these enzymes such as superoxides, but also they can manipulate biological pathways directly like autophagy. These abilities make them a suitable alternative for both therapy and diagnosis. In this review, we opted on metal nanoparticles and application of this cutting edged technology into modern medicine.
Introduction
Nanomaterials performance as enzymes
Natural enzymes are mostly proteins, made from amino acid sequence and have a different structure. However, most Nanomaterials have different size or structure [Citation1]. Although, proteins are considered as soft materials, nanomaterial is hard with a porphyritic nucleus [Citation2]. Notably, they share a certain likeness, such as overall size, shape and surface charge, which enable nanomaterials to mimic enzyme [Citation3]. In this part, we will look at different nanomaterial that can mimic natural enzymes as the base of texture similarity and flexibility.
Cerium oxide-based nanomaterial
Cerium oxide nanoparticles and magnetic nanoparticles are the most widely used metal oxide. CeO2 nanoparticles, (Ce3+, Ce4+), possess many unique properties that have proven in biomedical and catalytic applications. Nanoceria mimics SOD thanks to catalyzing the dismutation of superoxide anions into hydrogen peroxide and molecular oxygen (2O2− + 2 H+ →H2O2+O2) [Citation1,Citation4]. SOD have an antioxidant ability leading to many biological applications. Some alternative role exists for cerias as SOD (O2−+Ce4+→O2+ Ce3+ and O2−+Ce3++H2+→H2O2+Ce4+). Nanoceria mimics catalase and catalase the decomposition of hydrogen peroxide into molecular oxygen and water (2H2O2→2H2O + O2). Hydrogen peroxide, end product of superoxide radicals’ dismutation (2O2− + 2 H+ → H2O2+O2), plays a dual role in biological systems. It may be a signalling molecule or a non-radical reactive oxygen species. Although hydrogen peroxide itself is stable and less active, but may convert into highly active and detrimental hydroxyl radical through Fenton chemistry. In nature, catalase employed as the most efficient enzyme for the conversion of hydrogen peroxide to less active oxygen. Some studies showed catalase-like activity and SOD-like activity depend on Ce3+/Ce4+ ratio. Oxidase reaction including of oxidizing a substrate by molecular oxygen late, converted into either water or hydrogen peroxide. (Ared+O2+H2O→Aox+H2O2 and Ared+O2→Aox+H2O and Ared+ O2→Aox+O2−). Cerium oxide-based nano materials cerium oxide (ceria) identified as highly catalytic performance in various applications. Thanks to the presence of mixed volume states of Ce3+ and Ce4+, and oxygen vacancies [Citation5]. Cerium (III) nitrate reduces superoxide leading to superoxide oxidase (SOD) mimetic [Citation6]. The initial studies demonstrated that vacancy-engineered nanoceria protected of normal cells from radiation-induced damage but not tumour cells [Citation7]. The protective role of the nanoceria is related to omission of radiation-induced free radicals, which is may be to occur through oxidation process via a Ce3+ to Ce4+ conversion [Citation8]. Nanoceria and enzyme including (SOD, catalase, oxidase) share their properties to some biomedical functions such as omission radicals both in vitro and in vivo [Citation9–12]. For instance, nanoceria introduced as a novel superoxide dismutase (SOD), catalase and oxidase mimetic properties [Citation13]. Recently, revealed that there is a significant similarity between SOD and nanoceria by competitive electron paramagnetic resonance (EPR) analysis [Citation14]. According to the evidence, nanoceria exhibit superoxide dismutase (SOD) activity by using a ferricytochrome [Citation15]. Nanoceria showed antioxidant activity by catalyzing dismutation reaction of superoxide anions into hydrogen peroxide and molecular oxygen [Citation16]. Nanoceria is ROS scavenger and mimics the chemical reactions and antioxidant properties of SOD [Citation17]. However, we do not know yet what physical characteristics exactly, make nanoceria effective at scavenging superoxide anion [Citation18]. Although, some studies suggest that the surface of nanoceria oxidation state plays a perfect role in the SOD mimetic. The ability to scavenge superoxide related to cerium (III) concentrations at the surface particle [Citation19]. On the other hand, SOD plays important role in redox biology and cellular Anti-inflammatory response [Citation20]. Free radicals overproduction such as nitric oxide (NO) by the enzyme inducible nitric oxide synthase (iNOS) is critical to regulate inflammation. NO overproduction leads to some disease and collaborates to tissue destruction, consequently [Citation21]. The ability of nanoceria to scavenge free radical or reactive oxygen species (ROS) and inhibit inflammatory mediator helps to protect the whole cellular architecture against inflammatory disease. Also, it has a potential to manage chronic inflammation conditions thanks to reducing ROS production [Citation22]. In parallel, superoxide dismutase (SOD) enzymes are required for antioxidant defenses, maintaining the steady-state levels of O2−; surprisingly, nanoceria are remarkably efficacious in reducing the inflammation both in vitro and in vivo [Citation23]. SOD have anti-apoptotic properties although the signalling pathway of this anti-apoptosis mechanism is not clear, but maybe related to Ce3+/ Ce4+ redox reactions [Citation24]. Some studies demonstrated that SOD is neuroprotective. In a neurodegenerative disease like Alzheimer, free radicals play an important role [Citation25]. Using a mouse hippocampal brain slice model of cerebral ischemia, showed that ceria nanoparticles reduce ischemic cell death ∼50%. These findings proposed that the scavenging properties of nanoceria to eliminate peroxynitrite. It must be answered, in which mechanism cerium oxide nanoparticles mitigate ischemic brain injury. Peroxynitrite plays a pivotal role in the dissemination of oxidative injury in biological tissues [Citation26]. There is another application of nanoceria-based enzymes such as promotion of stem cell growth. For example, Xiang et al. showed that nanoceria increased vascularization of bone grafts by activating calcium channel through mesenchymal stem cells. [Citation27]. Also, they used cerium oxide nanoparticle as a modified scaffold to improve the blood vessel distribution inside of tissue engineering bone. Because of the unique properties of cerium oxide nanoparticles such as power to proliferate stem cells [Citation28]. It may be used as scaffold/artificial niche. However, ceria-nanoparticles produced as therapeutic agents in some inflammatory disease, they have wide range applications in diagnostics medicine [Citation29]. For example, ceria nanoparticle as oxidase mimic use in the detection of glucose in serum or urea. This method is based on the changes in the physicochemical properties of ceria, as chromogenic indicators, in response to the analyte. Another application of nanoceria is Immunoassay technique thanks to HRP enzyme sensing. Thus, the conjugate nanoceria detect cancer cells [Citation30]. Ceria oxide nanoparticle has a unique application in environmental toxicology for an instant: nanoceria deleted pollutants by oxidation of CO and hydrocarbon from automobile engine lightening [Citation31,Citation32].
Iron oxide-based nanomaterial
Peroxidase, consisting of a large family of enzymes, catalyzes the oxidation of its substrate with peroxide (hydrogen peroxide in most cases) (AH2+ROOH→A + ROH + H2O). Thanks to reaction, peroxidases play many critical roles in biological systems, such as detoxifying reactive oxygen species [Citation33] (e.g. glutathione peroxidase) and defending against pathogens [Citation34] (e.g. myeloperoxidase). Peroxidase (especially HRP) has been widely used in bio-analytical and clinical chemistry, it employed as a conjugate to an antibody for enzymatically catalyzing colorimetric substrates for signalling or imaging [Citation35]. Novel sensing platforms designed for both H2O2 and glucose detection by Fe3O4 nanoparticles as peroxidase [Citation36]. The results of these initial studies stimulated rapidly expanding interests in the use of iron oxide nanomaterial as a peroxidase mimic. Among them, Fe3O4 (magnetite) nanomaterial have studied well. Magneto ferritin (Fe2O3 in ferritin) nanoparticles are employed for targeting and visualizing tumour tissues [Citation37]. GU et al. showed, iron oxide nanoparticles (both Fe3O4 and g-Fe2O3) exhibited dual enzyme mimetic properties including catalase and peroxidase [Citation37].
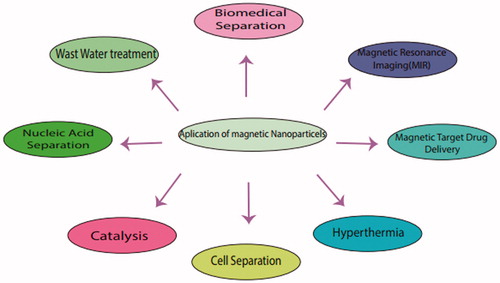
a. (Fe3++ H2O2→FeOOH2++H+, FeOOH2+→Fe2++H2O, Fe2++H2O2→Fe3++OH + H) b. (Fe3++H2O2→FeOOH2++H+, FeOOH2+→Fe2++HO2, HO2→→H++O2−, HO + HO2/O2−→H2O + O2)
Other nanomaterials have been studied for oxidase mimetic properties. Fe2O3 nanowires explored as a sensor for glucose detection using their oxidase-like activity [Citation38]. The non-enzymatic glucose sensor was fabricated with a Fe2O3 nanowire [Citation34]. Also, Iron oxide nanoparticles using magnetically isolate and purify proteins, DNA, viruses and even whole mammalian cells [Citation37]. Autophagy or programmed cell death 2 (PCD2) has attracted a great deal of research interest in tumour biology in recent years. In general, autophagy is eliminating the non-functional bio-molecules even organelles from cellular milieu [Citation35]. Novel iron oxide NPs synthesized that induce autophagy in the lung cancer cell line (A549) and not in normal cells (IMR-90) [Citation36]. Notably, autophagy correlated with ROS production as well as mitochondrial damage. Protection against ROS clearly suggested the implication of ROS in hyper-activation of autophagy and cell death. Pre-treatment of cancer cells with 3-MA (an autophagy inhibitor) also exhibited ROS induction mitochondrial damage and promote cellular death in cancer cells. These results confirmed that iron oxide NPs induce classical mTOR pathway in A549 cells. According to the evidence, iron oxide NPs may trigger cancer cells specifically. One of the most wide nanomaterial via numerous reports in the literature, testify to their potential applications in medical diagnostics, controlled drug release and separation technologies, is superparamagnetic iron oxide (Fe3O4) nanoparticles [Citation39]. These nanoparticles are using in sensing, imaging, separation and capture of analytes [Citation40]. They have used for (bio) analysis, (bio) electrocatalysis, drug delivery, bacteria inactivation [Citation41] and Lithium-Ion Batteries [Citation42,Citation43], Stem cell tracking, which is an emergent field of regenerative medicine, following the stem cells fate after their introduction in the body [Citation44]. Also, upper-paramagnetic iron oxide (SPIO) nanoparticles are very useful for mesenchymal stem cells growth [Citation45]. Ferro-carbon-promoted cell growth thanks to its ability to diminish intracellular H2O2 through intrinsic peroxidase-like activity [Citation46]. Also, ferro-carbon may accelerate cell cycle and proliferation, which may be mediated by the free iron (Fe), released from lysosomal degradation and involves the alteration of Fe on the expression of the protein regulators of the cell cycle [Citation47]. Also, some of the medical applications including Transplant monitoring in a study for therapy of diabetes via transplant pancreas and monitoring by iron oxide nanoparticles for tissue transplantation [Citation48]. Targeted cell death of tumour cells through controlled heating of the damaged tissue via Engineering the magnetic nanoparticles for Hyperthermia is based on magnetic nanoparticle [Citation49]. Notably, Magnetic nanoparticles have promising biomedical applications, especially in drug delivery and tissue engineering [Citation50] (). Also, iron oxide can act as peroxides mimics [Citation51]. There is growing evidence that magnetite nanoparticles, in fact, possess an intrinsic enzyme mimetic activity similar to that found in natural peroxidases, which are widely used to oxidize organic substrates in the treatment of wastewater or as detection tool [Citation52]. Peroxidase enzymes activate as hydrogen peroxide to perform numerous oxidations in nature [Citation53,Citation54]. Also, peroxidases (enzymes that catalyze oxidation reactions) are popular detection tools because they simplify colour changes in the presence of certain dyes. For example, immune-absorbent assay ELIZA tests [Citation55]. They are also useful in the treatment of waste water for oxidizing organic substances [Citation56]. However, the wide potential applications of the peroxidase-like activity of iron oxide nanoparticles in medicine and biotechnology remain to be understood yet. A recent study, performed by Gao et al., found that the Fe3O4 magnetic nanoparticles (MNPs) were intrinsically active catalyst for some oxidation reactions, being similar to natural horseradish peroxidase (HRP) [Citation57]. The peroxidase-like nature of Fe3O4 MNPs was employed to replace the enzyme HRP used in H2O2 detection and immune-absorbent assay and was also explored for the applications in the degradation of phenolic and aniline compounds [Citation58]. Another application of Iron oxide as peroxidase including diagnostic kit for hydrogen peroxide (H2O2) and glucose detection [Citation59]. Experimental studies demonstrated that the H2O2-activating ability of Fe3O4 MNPs is not so close to the removal of hydrogen superoxide, but it may modify by increasing the H2O2-activating ability of Fe3O4 MNPs of refractory organic pollutants from wastewaters [Citation60]. H2O2 detection is useful in many fields such as biology, medicine, environmental protection and the food industry [Citation61]. There is a higher efficient way to Glucose detection via glucose oxidase (GOx) [Citation62]. Fe3O4 MNPs function is on the base of Carboxyl-modified letter oxide (GOCOOH) to possess intrinsic peroxidase-like activity and catalyze the reaction of peroxidase substrate 3,3,5,5-tetramethylbenzidine (TMB) in the presence of H2O2 to produce a blue colour reaction [Citation63]. Highly sensitive and selective colorimetric method for glucose detection has developed in a buffer solution or diluted blood and fruit juice samples [Citation64,Citation65]. Glucose detection is a promising method in clinical and food analysis [Citation66]. Moreover, glucose concentrations in serum samples could be accurately and selectively detected over several other sugars, such as galactose, lactose, mannose, maltose, arabinose, cellobiose, raffinose and xylose [Citation66]. In the recent years, nanoparticle-based techniques for DNA detection has developed [Citation67]. The small labelled size, bio-conjugation ability and the unusual optical and electrical properties of metal nanoparticles make them unique tools for DNA detection [Citation68]. Semiconductor nanoparticles have been extensively used as labels in electrochemical biosensors, especially DNA sensors. For instance, iron nanoparticle used for labelling DNA, DNA probe labelled with gold coated iron core-shell nanoparticles and dissolved the iron-containing nanoparticles following DNA hybridization, the released iron ions were determined by cathode stripping voltammetry in the presence of the 1-nitroso-2-naphthol ligand addition a bromate catalyst [Citation69]. Double-stranded DNA’s shielding against MNPs’ mimicking activity was combined with PCR, a label-free colorimetric platform for DNA sensing developed. Also, aptamers are selected ssDNA or ssRNA that can specifically bind to a target and can be considered as nucleic acid versions of an antibody [Citation70]. Immune-assay is an application via MNPs that help to detect staphylococcal enterotoxin via magnetic nanoparticles [Citation71]. It has performed due to functionalized antibody coated on magnetic nanoparticles captured the enterotoxin antigen from solution [Citation72]. Then, bacterial toxin isolated via magnetic separation, and amplified the surface plasmon resonance (SPR) [Citation73]. In a study by Ibrahim et al., an immunoassay was used for the detection of C-reactive protein (CRP) from rapid whole blood magnetic permeability. The assay employed monoclonal anti-CRP antibody conjugated to dextran iron oxide nanoparticles (70 nm) as superparamagnetic labels and polyclonal anti-CRP antibody conjugated to silica microparticles (to enhance sedimentation of the complex) [Citation74]. Notably, nanoparticle biosensors with modified antibody provide three functions including capture, separation and detection [Citation75]. These novel peroxidase mimic activity of metal nanoparticles can use for immune-staining. Gu et al. demonstrated that ultra-small Fe3O4 MNPs were able to replace expensive enzymes (such as HRP) for immune-histochemistry staining [Citation76]. In another study, HIV virus was detected by using antibody biosensing on microfluidic magnetic separator chip, which they used to concentrate human immunodeficiency virus (HIV) from plasma [Citation77]. Also, super-paramagnetic nanoparticles conjugated to anti-CD44 to capture the virus, then passed the particles through a packed bed of 25–75-μm-diameter iron oxide particles. An external magnet was used to magnetize the packed bed, which caused the HIV–magnetic nanoparticle conjugated and trapped, thereby separating and concentrating them from the plasma matrix. Off-chip enzyme-linked immune-absorbent assay confirmed that the HIV virions were concentrated ∼80-fold over the original solution [Citation78]. Recently, MNPs were used for colorimetric assay based Fe3O4 magnetic nanoparticle peroxidase mimetic for the rapid detection of organophosphorus pesticide [Citation79], acetylcholine esterase (AChE) and choline oxidase (CHO). H2O2 over-produced in the presence of acetylcholine, then both the enzymes AChE and CHO were catalyzed. H2O2 then activates MNPs to catalyze the oxidation of colorimetric substrates to produce a colour reaction [Citation80]. After incubation with the organophosphorus neurotoxins, the enzymatic activity of AChE was inhibited and produced less H2O2, resulting in a decreased catalytic oxidation of colorimetric substrates over MNP peroxidase mimetic, the companion by decrease colour intensity [Citation81].
Cobalt oxide as catalase and peroxidase
Another metal-0base nanoparticle by peroxidase and catalase enzyme mimic, cobalt oxide, catalyze this reaction as follows: 2O2− + 2 H+ → H2O2+O2 [Citation4]. For example, peroxidase-like activity was used for wastewater treatment by cobalt-doped graphitic carbon nitride (Co-g-C3N4) materials [Citation82], as catalase activity for sensitive and fast sensor of detection H2O2. Another catalytic application of Co3O4 is the calcium detection of milk because of increasing significant presence of calcium ion [Citation82]. Also, it can be in peroxidase and oxidase-like activity that is useful for colorimetric and glucose detection [Citation83]. The high-redox potential of Co3+/Co2+ makes this nanoparticle more stronger than others compared to other metal-based nanomaterial. Thus, the high peroxidase-like activity designed a new immunohistochemical assay [Citation84]. Avastin antibody conjugated onto the surface of Co3O4 nanoparticles. The conjugates obtained were used to detect vascular endothelial growth factor (VEGF) that was overexpressed in tumour tissue. It may be effective for tumour detection [Citation52,Citation84]. Co3O4 nanoparticles (NPs) as peroxidase mimics to catalyze the oxidation of chromophoric substrates by H2O2. For the detection of H2O2 and glucose, substrates surveyed even in real samples. Antioxidant capabilities of Co3O4 compared with three natural antioxidants including, gallic acid (GA), tannic acid (TA) and ascorbic acid (AA) [Citation85] (. Then, enzyme mimics can be used to evaluate antioxidant capabilities and to screen enzymes inhibitors. In addition, Co3O4 by oxidase-like activity can used for the detection of glutathione via TMB-O2-Co3O4 nanotube system [Citation86]. Copper oxide when used as oxidase mimics CuO nanoparticles showing peroxidase-oxidase mimicking activity. Thus, have same activity like other metal oxide nanoparticles such as glucose and L-lactate detection by peroxidase activity [Citation84–86]. Also, another study shows cupric oxide nanoparticle can be used as chemiluminescence cholesterol sensor for detection via peroxidase mimicking [Citation81,Citation85]. Zn–Cuo material is used as a peroxidase to detect three antioxidants tannic acid, tartaric acid and ascorbic acid in addition to glucose detection [Citation58,Citation86], as well as Zn-Cu NPs, when compared to the mentioned antibodies have some potential to act like these antibodies [Citation58]. However, Yan Xo et al. showed Cu2+ as an oxidase mimic is more efficient than Cu/Cuo nanoparticles for histidine detection even in serum or urea detention in presence of O-phenylenediamine (OPD) [Citation59].
Manganese dioxide as oxidase
MnO2 nanomaterials with different morphologies (i.e. nanosheets, nanospheres, nano-sticks, nano-complexes and nanowires) have also been studied to determine their oxidase and peroxidase and catalase mimicking activity [Citation60]. Liu et al. showed that BSA-stabilized Mno+2 can be used instead of HRP enzyme and in the detection of goat anti-human in immunoassay technic as a colorimetric application [Citation60]. In a study, MnO2 nanosheet was used for detection of Glutathione via Electrochemical luminescence [Citation61].
Vanadium pentoxide as peroxidase
Vanadium catalysis showed the following reaction: R-H + Br− + H2O2→R-Br + H2O + OH−. Mono-crystalline VO2 (A) nanoplates were synthesized according to hydrothermal protocol. VO2 (A) nano-plates showed outstanding peroxidase-like activity and mimics peroxidase activity [Citation63]. Also, vanadate has halo peroxidases activity in marine macro-algae due to catalyzing the 2e − oxidation, by H2O2. Vanadium is a Lewis acid, forming an intermediate peroxide/hydroperoxide complex. Additionally, the complex can protect the algae against biofilm formation. Towards the biocidal (anti-bacterial) potential of the peroxidases [Citation62], another application of vanadium peroxidase is neutralization of biofilms by mimic vanadium halo peroxidases [Citation61]. Nanowires had catalytic activity towards peroxidase substrates (such as ABTS and TMB) in the presence of H2O2 as demonstrated in a study from Trammel’s group. Functional materials exerted a strong antibacterial activity against both Gram-negative and Gram-positive bacteria.
Gold nanomaterials
Gold nanoparticles (GNPs) mimic oxidase. Glucose has been detected by GNPs. According to glucose oxidase mechanism (Ared+O2+H2O→Aox+H2O2 and Ared+O2→Aox+H2O and Ared+ O2→Aox+O−2), studies suggested a mechanism, which maybe glucose that firstly adsorb onto the gold nanoparticles. Later, one oxygen reacts with the adsorbed glucose and forms the products which are Gluconic acids and H2O2. Gold nanoparticles have used to successfully detect target DNA or microRNA by the hybridization of the nucleic acids and enzyme activity. Tao et al. use from a synergistic effect of graphene oxide–gold nanocluster (GO-AuNC) hybrid for cancer cell detection by constructed as an enzyme mimic that is able to show high catalytic activity over a broad pH range, especially at neutral pH [Citation87]. Gold nanoparticles with either positive or negative surface charges surprisingly showed peroxidase mimicking activity [Citation88]. Peroxidase-like activity is used for colorimetric detection of urea, urease and urease inhibitor [Citation89]. Gold nanoparticles based on peroxidase function are used to detect kanamycin residue by catalyzingthe reaction between H2O2 and reduced thionine to produce oxidized thionine [Citation67]. A study by Liu et al. showed a great potential of processing gold nanoparticle via PAMAM dendrimer as primary neural protection against oxidative damage via acting like peroxidase [Citation90]. As a common reactive oxygen species, H2O2 is widely used for bacterial inactivation and wound disinfection. Water-soluble Au nanocrystal (NC) micelles with an inserted catalytic Cu (II) centre that act as excellent nanozyme models for imitating ribonuclease were constructed by supramolecular self-assembly. The study of the catalytic behaviour of Au NC micelle catalysis showed that the Au NC micelles exhibited dramatic ribonuclease-like activity [Citation91]. Gold nanoparticles use other material to fabricate the Au-Pt nanorods and Au-M (M = Bi, Pd and Pt) nanostructures to mimic enzyme. Chang et al. demonstrated that bismuth– gold nanoparticles exhibited peroxidase-like activity [Citation92]. Wu, Yin et al. extended the previous studies to bimetal nanoparticles, i.e. Au-Pt nano rods. The Au-Pt nano rods showed multiple enzyme mimetic capabilities. Au-Ag heterogeneous nano rods as nanozyme interfaces with peroxidase-like activity and their application for one-pot analysis of glucose at nearly neutral pH [Citation82]. Also, AgM (M = Au, Pd and Pt) nanostructures have enzyme properties, and a series of silver-based bimetallic nanostructures, i.e. AgM (M = Au, Pd and Pt), oxidized colorimetric substrates to correspond products with H2O2, displaying peroxidase-like activity.
Platinum nanomaterial
Platinum nanoparticles encapsulated by apo-ferritin (PtNP-apo-ferritin) and synthesized to detoxify reactive oxygen species. The PtNP-apo-ferritin exhibited SOD-like in vitro [Citation93]. PtNP-apo-ferritin uptake by cells via a ferritin-receptor-mediated process and increased cell viability under stress condition. Compared to ceria nanoparticles, the SOD-like activity of PtNP@apo-ferritin is lower on weight basis [Citation94]. Platinum nanomaterials can mimic catalases and peroxidase [Citation95]. Also, the PtNP-apo-ferritin exhibited dual enzyme mimic behaviours (catalase and peroxidase), both activities dependent on pH and temperature. Catalase activity enhanced by increasing pH and temperature while peroxidases activity have a maximum value at physiological temperature and slightly acidic conditions. The catalytic activities were dependent on Pt content, with higher Pt content having better activities [Citation95]. Recently, Liu et al. revealed that platinum nanoparticles (Pt NPs) exhibit catechol oxidase-like activity, oxidizing polyphenols into the corresponding o-quinones [Citation96].
Carbon-based nanomaterial
Carbon-based nanomaterial, such as fullerene, graphene, carbon nanotubes and their derivatives, mimic various natural enzymes [Citation97]. The peroxidase-like activity of CNTs have reported in another study [Citation98]. The intrinsic peroxidase-like activity of single-walled carbon nanotubes (SWNTs) was investigated by Qu et al. [Citation74]. Similar to HRP, its activity was pH, temperature and H2O2 dependent. Graphene oxide was also found to possess intrinsic peroxidase. Mimicking activity depending on surface-to-volume ratio as well as high affinity towards organic substrates, graphene oxide showed a higher affinity towards TMB than HRP (Km, 0.0237mM for graphene oxide vs. 0.275 mM for HRP), but a lower affinity towards H2O2 than HRP (Km, 3.99 mM for graphene oxide vs. 0.214 mM for HRP). CNTS was used to detect H2O2 and glucose. Other applications include metal ion detection, DNA detection, aptasensors and immunoassay.
Conclusion
Overall, metal nanoparticles surprisingly mimic some bioactive compound. When these common domains share between nanoparticles and enzymes, altered production of bioactive peptide is possible. The point of this cutting-edge technology includes direct or indirect manipulation of some biological pathways, medical diagnostics and environmental toxicology. For these reasons, metal nanoparticles are a good candidate to replace some complicated and cost-effective enzymes or using them as a novel and unique techniques in the medical laboratory. Recent adventures in this field including targeting tumour tissue and molecular imaging expand the molecular therapy horizon. Also, nanozymes have applications in microbial detection strategy. For instance, nanozyme developed to diagnose Ebola. Applications of nanozymes including bioanalytical and electrochemical techniques are growing besides medical uses.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Wei H, Wang E. Nanomaterials with enzyme-like characteristics (enzymes): next-generation artificial enzymes. Chem Soc Rev. 2013;42:6060–6093.
- Breslow R, Overman LE. 'Artificial enzyme' combining a metal catalytic group and a hydrophobic binding cavity. J Am Chem Soc. 1970;92:1075–1077.
- Breslow R. Artificial enzymes. New York: John Wiley & Sons; 2006.
- Abbasi E, Akbarzadeh A, Kouhi M, et al. Graphene: synthesis, bio-applications, and properties. Artif Cells Nanomed Biotechnol. 2016;44:150–156.
- Kirby AJ, Hollfelder F. From enzyme models to model enzymes. Royal Society of Chemistry; 2009.
- Kotov NA. Chemistry. Inorganic nanoparticles as protein mimics. Science. 2010;330:188–189.
- Tarnuzzer RW, Colon J, Patil S, et al. Vacancy engineered ceria nanostructures for protection from radiation-induced cellular damage. Nano Lett. 2005;5:2573–2577.
- Chen J, Patil S, Seal S, et al. Rare earth nanoparticles prevent retinal degeneration induced by intracellular peroxides. Nature Nanotech. 2006;1:142–150.
- Karakoti A, Singh S, Dowding JM, et al. Redox-active radical scavenging nanomaterials. Chem Soc Rev. 2010;39:4422–4432.
- Celardo I, Pedersen JZ, Traversa E, et al. Pharmacological potential of cerium oxide nanoparticles. Nanoscale. 2011;3:1411–1420.
- Silva GA. Nanomedicine: seeing the benefits of ceria. Nat Nanotechnol. 2006;1:92–94.
- Xie J, Zhang X, Wang H, et al. Analytical and environmental applications of nanoparticles as enzyme mimetics. TrAC Trends Anal Chem. 2012;39:114–129.
- Heckert EG, Karakoti AS, Seal S, et al. The role of cerium redox state in the SOD mimetic activity of nanoceria. Biomaterials. 2008;29:2705–2709.
- Hirst SM, Karakoti AS, Tyler RD, et al. Anti-inflammatory properties of cerium oxide nanoparticles. Small. 2009;5:2848–2856.
- Batinic-Haberle I, Tovmasyan A, Roberts ER, et al. SOD therapeutics: latest insights into their structure-activity relationships and impact on the cellular redox-based signaling pathways. Antioxidants Redox Signal. 2014;20:2372–2415.
- Celardo I, De Nicola M, Mandolin C, et al. Ce3+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano. 2011;5:4537–4549.
- Estevez A, Pritchard S, Harper K, et al. Neuroprotective mechanisms of cerium oxide nanoparticles in a mouse hippocampal brain slice model of ischemia. Free Rad Biol Med. 2011;51:1155–1163.
- Das M, Patil S, Bhargava N, et al. Auto-catalytic ceria nanoparticles offer neuroprotection to adult rat spinal cord neurons. Biomaterials. 2007;28:1918–1925.
- Naganuma T, Traversa E. The effect of cerium valence states at cerium oxide nanoparticle surfaces on cell proliferation. Biomaterials. 2014;35:4441–4453.
- Xiang J, Li J, He J, et al. Cerium oxide nanoparticle modified scaffold interface enhances vascularization of bone grafts by activating calcium channel of mesenchymal stem cells. ACS Appl Mater Interfaces. 2016;8:4489–4499.
- Ornatska M, Sharpe E, Andreescu D, et al. Paper bioassay based on ceria nanoparticles as colorimetric probes. Anal Chem. 2011;83:4273–4280.
- Shcherbakov A, Zholobak N, Spivak NY, et al. Advances and prospects of using nanocrystalline ceria in prolongation of lifespan and healthy aging. Russ J Inorg Chem. 2015;60:1595–1625.
- Pautler R, Kelly EY, Huang P-JJ, et al. Attaching DNA to nanoceria: regulating oxidase activity and fluorescence quenching. ACS Appl Mater Interfaces. 2013;5:6820–6825.
- Bi S, Yan Y, Hao S, et al. Colorimetric logic gates based on supramolecular DNAzyme structures. Angewandte Chemie. 2010;122:4540–4544.
- Andreescu D, Bulbul G, Özel RE, et al. Applications and implications of nanoceria reactivity: measurement tools and environmental impact. Environ Sci Nano. 2014;1:445–458.
- Shcherbakov A, Zholobak N, Spivak NY, et al. Advances and prospects of using nanocrystalline ceria in cancer theranostics. Russ J Inorg Chem. 2014;59:1556–1575.
- Xu P, Zeng GM, Huang DL, et al. Use of iron oxide nanomaterials in wastewater treatment: a review. Sci Total Environ. 2012;424:1–10.
- Zhang L, Wu HB, Lou XWD. Iron‐oxide‐based advanced anode materials for lithium‐ion batteries. Adv Energy Mater. 2014. doi: 10.1002/aenm.201300958
- Figuerola A, Di Corato R, Manna L, et al. From iron oxide nanoparticles towards advanced iron-based inorganic materials designed for biomedical applications. Pharmacol Res. 2010;62:126–143.
- Zhou Z, Wang L, Chi X, et al. Engineered iron-oxide-based nanoparticles as enhanced T1 contrast agents for efficient tumor imaging. ACS Nano. 2013;7:3287–3296.
- Goenka S, Sant V, Sant S. Graphene-based nanomaterials for drug delivery and tissue engineering. J Control Release. 2014;173:75–88.
- Ellis WC, Tran CT, Denardo MA, et al. The design of more powerful iron-TAML peroxidase enzyme mimics. J Am Chem Soc. 2009;131:18052–18053.
- Perez JM. Iron oxide nanoparticles: hidden talent. Nat Nanotechnol. 2007;2:535–536.
- Wang N, Zhu L, Wang D, et al. Sono-assisted preparation of highly-efficient peroxidase-like Fe3O4 magnetic nanoparticles for catalytic removal of organic pollutants with H2O2. Ultrasonics Sonochem. 2010;17:526–533.
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021.
- Wei H, Wang E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal Chem. 2008;80:2250–2254.
- Song Y, Qu K, Zhao C, et al. Graphene oxide: intrinsic peroxidase catalytic activity and its application to glucose detection. Adv Mater. 2010;22:2206–2210.
- Huang D-M, Hsiao J-K, Chen Y-C, et al. The promotion of human mesenchymal stem cell proliferation by superparamagnetic iron oxide nanoparticles. Biomaterials. 2009;30:3645–3651.
- Khan MI, Mohammad A, Patil G, et al. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33:1477–1488.
- Fritzsche W, Taton TA. Metal nanoparticles as labels for heterogeneous, chip-based DNA detection. Nanotechnology. 2003;14:R63.
- Luo X, Morrin A, Killard AJ, et al. Application of nanoparticles in electrochemical sensors and biosensors. Electroanalysis. 2006;18:319–326.
- Azzazy HM, Mansour MM. In vitro diagnostic prospects of nanoparticles. Clin Chim Acta. 2009;403:1–8.
- Gao L, Zhuang J, Nie L, et al. the Intrinsic peroxidase-like activity of ferromagnetic nanoparticles. Nature Nanotech. 2007;2:577–583.
- Liang M, Fan K, Pan Y, et al. Fe3O4 magnetic nanoparticle peroxidase mimetic-based colorimetric assay for the rapid detection of organophosphorus pesticide and nerve agent. Anal Chem. 2012;85:308–312.
- Beveridge JS, Stephens JR, Williams ME. The use of magnetic nanoparticles in analytical chemistry. Annu Rev Anal Chem (Palo Alto Calif). 2011;4:251–273.
- Damhorst GL, Watkins NN, Bashir R. Micro- and nanotechnology for HIV/AIDS diagnostics in resource-limited settings. IEEE Trans Biomed Eng. 2013;60:715–726.
- Liu Y, Yuan M, Qiao L, et al. An efficient colorimetric biosensor for glucose based on peroxidase-like protein-Fe3O4 and glucose oxidase nanocomposites. Biosensors Bioelectronics. 2014;52:391–396.
- Mu J, Li J, Zhao X, et al. Cobalt-doped graphitic carbon nitride with enhanced peroxidase-like activity for wastewater treatment. RSC Adv. 2016;6:35568–35576.
- Mu J, Zhang L, Zhao M, et al. Co3O4 nanoparticles as an efficient catalase mimic: properties, mechanism and its electrocatalytic sensing application for hydrogen peroxide. J Mol Catal A Chem. 2013;378:30–37.
- Mu J, Zhang L, Zhao M, et al. Catalase mimic property of Co3O4 nanomaterials with different morphology and its application as a calcium sensor. ACS Appl Mater Interfaces. 2014;6:7090–7098.
- Alizadeh E, Zarghami N, Eslaminejad M, et al. The effect of dimethyl sulfoxide (DMSO) on hepatic differentiation of mesenchymal stem cells. Artif Cells Nanomed Biotechnol. 2016;44:157–164.
- Dong J, Song L, Yin J-J, et al. Co3O4 nanoparticles with multi-enzyme activities and their application in immunohistochemical assay. ACS Appl Mater Interfaces. 2014;6:1959–1970.
- Jia H, Yang D, Han X, et al. Peroxidase-like activity of the Co3O4 nanoparticles used for biodetection and evaluation of antioxidant behavior. Nanoscale. 2016;8:5938–5945.
- Wang T, Su P, Li H, et al. The triple-enzyme mimetic activity of Co3O4 nanotubes and their applications in colorimetric sensing of glutathione. New J Chem 2016;40:10056–10063.
- Liu F, He J, Zeng M, et al. Cu–hemin metal-organic frameworks with peroxidase-like activity as peroxidase mimics for colorimetric sensing of glucose. J Nanopart Res. 2016;18:1–9.
- Hong L, Liu A-L, Li G-W, et al. Chemiluminescent cholesterol sensor based on the peroxidase-like activity of cupric oxide nanoparticles. Biosensors Bioelectron. 2013;43:1–5.
- Hu A-L, Liu Y-H, Deng H-H, et al. Fluorescent hydrogen peroxide sensor based on cupric oxide nanoparticles and its application for glucose and l-lactate detection. Biosens Bioelectron. 2014;61:374–378.
- Nagvenkar AP, Gedanken A. Cu0. 89Zn0. 11O, A New Peroxidase-Mimicking Nanozyme with High Sensitivity for Glucose and Antioxidant Detection. ACS Appl Mater Interfaces. 2016;8:22301–22308.
- Xu Y, Wu X-Q, Shen J-S, et al. Highly selective and sensitive recognition of histidine based on the oxidase-like activity of Cu2+ ions. RSC Adv. 2015;5:92114–92120.
- Liu X, Wang Q, Zhao H, et al. BSA-templated MnO2 nanoparticles as both peroxidase and oxidase mimics. Analyst. 2012;137:4552–4558.
- Gao W, Liu Z, Qi L, et al. Ultrasensitive glutathione detection based on lucigenin cathodic electrochemiluminescence in the presence of MnO2 nanosheets. Anal Chem. 2016;88:7654–7659.
- Natalio F, André R, Hartog AF, et al. Vanadium pentoxide nanoparticles mimic vanadium haloperoxidases and thwart biofilm formation. Nature Nanotech. 2012;7:530–535.
- Rehder D. Vanadate-dependent peroxidases in macroalgae: function, applications, and environmental impact. Oceanography: Open Access; 2014.
- Zhang L, Xia F, Song Z, et al. Synthesis and formation mechanism of VO2 (A) nanoplates with intrinsic peroxidase-like activity. RSC Adv. 2015;5:61371–61379.
- Sun J, Li C, Qi Y, et al. Optimizing colorimetric assay based on V2O5 nanozymes for sensitive detection of H2O2 and glucose. Sensors. 2016;16:584.
- Lin Y, Ren J, Qu X. Nano-gold as artificial enzymes: hidden talents . Adv Mater Weinheim. 2014;26:4200–4217.
- Kabanov AV, Batrakova EV. Polymer nanomaterials for drug delivery across the blood brain barrier. In: Neuroimmune pharmacology. New York: Springer; 2017. p. 847–868.
- Bazin I, Tria SA, Hayat A, et al. New biorecognition molecules in biosensors for the detection of toxins. Biosens Bioelectron. 2017;87:285–298.
- Mühlberg M, Millemaggi A, Coates I, et al. Chemical communications RSC. li/chemcomm editorial board. Chem Commun. 2017;53:271–283.
- Darabdhara G, Sharma B, Das MR, et al. Cu-Ag bimetallic nanoparticles on reduced graphene oxide nanosheets as peroxidase mimic for glucose and ascorbic acid detection. Sensors Actuat B: Chem. 2017;238:842–851.
- Gupta V, Sengupta M, Prakash J, Tripathy BC. Drug Targeting and Delivery. In: Basic and Applied Aspects of Biotechnology. New York: Springer; 2017. p. 279–303.
- Garg B, Bisht T. Carbon nanodots as peroxidase nanozymes for biosensing. molecules. Molecules. 2016;21:1653.
- Cheng H, Lin S, Muhammad F, et al. Rationally modulate the oxidase-like activity of nanoceria for self-regulated bioassays. ACS Sens. 2016;1:1336–1343.
- Ge C, Fang G, Shen X, et al. Facet energy versus enzyme-like activities: the unexpected protection of palladium nanocrystals against oxidative damage. ACS Nano. 2016;10:10436–10445.
- Wang X, Guo W, Hu Y, Wu J, Wei H. Challenges and Perspectives. In: Nanozymes: Next Wave of Artificial Enzymes. New York: Springer; 2016. p. 103–107.
- Nasrabadi HT, Abbasi E, Davaran S, et al. Bimetallic nanoparticles: preparation, properties, and biomedical applications. Artif Cells Nanomed Biotechnol. 2016;44:376–380.
- Akbarzadeh A, Koschny T, Kafesaki M, et al. Graded-index optical dimer formed by optical force. Opt Express. 2016;24:11376–11386.
- Cao H, Dan Z, He X, et al. Liposomes coated with isolated macrophage membrane can target lung metastasis of breast cancer. ACS Nano. 2016;10:7738–7748.
- Wang X-Q, Lei Q, Zhu J-Y, et al. Cucurbit [8] until regulated activatable supramolecular photosensitizer for targeted cancer imaging and photodynamic therapy. ACS Appl Mater Interfaces. 2016;8:22892–22899.
- Singh S. Cerium oxide based nanozymes: Redox phenomenon at biointerfaces. Biointerphases. 2016;11:04B202.
- Rahimi-Rad MH, Alizadeh E, Samarei R. Document aquatic leech as a rare cause of respiratory distress and hemoptysis. Pneumologia. 2011;60:85–86.
- Liang H, Lin F, Zhang Z, Liu B, Jiang S, Yuan Q, et al. Multi-copper laccase mimicking nanozymes with nucleotides as ligands. ACS Appl Mater Interfaces. 2017;9:1352–1360.
- Neri S, Garcia Martin S, Pezzato C, Prins LJ. Photoswitchable catalysis by a nanozyme mediated by a light-sensitive cofactor. J Am Chem Soc. 2017;139:1794–1797.
- Liu B, Liu J. Surface modification of nanozymes. Nano Res. 2017:1–24.
- Huang Y, Liu C, Pu F, Liu Z, Ren J, Qu X. GO-Se nanocomposite as an antioxidant nanozyme for cytoprotection. Chem Commun. 2017;53:3082–3085.
- Gao L, Koo H. Do catalytic nanoparticles offer an improved therapeutic strategy to combat dental biofilms? Future Med. 2017;12:275–279.
- Zou HY, Yang T, Lan J, Huang CZ. Scavenging the peroxidase mimetic activity of erythrocyte-like Cu1. 8S nanoparticles for colorimetric detection of glutathione. Anal Methods. 2017;9:841–846.
- Rad MHR, Alizadeh E, Ilkhanizadeh B, et al. Recurrent laryngeal papillomatosis with bronchopulmonaryl spread in a 70-year-old man. Tuberkuloz ve Toraks. 2007;55:299–302.
- Pang L, Zhang C, Qin J, et al. A novel strategy to achieve effective drug delivery: exploit cells as carrier combined with nanoparticles. Drug Deliv. 2017;24:83–91.
- Chen Y, Xianyu Y, Jiang X. Surface modification of gold nanoparticles with small molecules for biochemical analysis. Accounts Chem Res. 2017;50:310–319.
- Lin X, Liu Y, Tao Z, Gao J, Deng J, Yin J, et al. Nanozyme-based bio-barcode assay for high sensitive and logic-controlled specific detection of multiple DNAs. Biosensors Bioelectron. 2017;94:471–477.
- Vallabani NS, Karakoti AS, Singh S. ATP-mediated intrinsic peroxidase-like activity of Fe3O4-based nanozyme: one step detection of blood glucose at physiological pH. Colloids Surfaces B Biointerfaces. 2017;153:52–60.
- Cai K, Wang AZ, Yin L, Cheng J. Bio-nano interface: the impact of biological environment on nanomaterials and their delivery properties. J Control Release. Forthcoming. doi: 10.1016/j.jconrel.2016.11.034
- Abbasi E, Akbarzadeh A, Kouhi M, et al. Graphene: synthesis, bio-applications, and properties. Artif Cells Nanomed Biotechnol. 2016;44:150–156.
- Aiguo S, Jiming H. Research progress of nanozymes and its application in analysis. Chinese J Appl Chem. 2016;33:1245–1252.
- Gao L, Yan X. Nanozymes: an emerging field bridging nanotechnology and biology. Sci China Life Sci. 2016;59:400–402.
- Liang H, Liu B, Yuan Q, et al. Magnetic iron oxide nanoparticle seeded growth of nucleotide coordinated polymers. ACS Appl Mater Interfaces. 2016;8:15615–15622.
- Li H, Huang Y, Yu Y, et al. Self-catalyzed assembly of peptide scaffolded nanozyme as a dynamic biosensing system. ACS Appl Mater Interfaces. 2016;8:2833–2839.