Abstract
The objective of present study was in vitro and in vivo evaluation of hepatoprotective and antioxidant activity of Quercetin nanoparticles (Q NPs) against toxicity induced by aflatoxin B1. The Q NPs were prepared using precipitation method. Hepatocytes were prepared by the method of collagenase enzyme perfusion via portal vein. The NPs were characterized in terms of size and morphology using dynamic light scattering (DLS) and transmission electron microscopy (TEM), respectively. The level of parameters, such as cell death, ROS formation, lipid peroxidation, mitochondrial membrane potential and cellular glutathione (GSH) content, in the aflatoxin B1-treated and non-treated hepatocytes were determined and the mentioned markers were assessed in the presence of Q NPs. The prepared NPs showed particle size of 52.70 nm with polydispersity index (PDI) of 0.18. In contrast to free Q, the administration of Q NPs more efficiently decreased the rate of ROS formation, lipid peroxidation and improved cell viability, mitochondrial membrane potential and glutathione level and showed a significant hepatoprotective efiect by reducing levels of aspartate aminotransferase, alanine aminotransferase and alkaline phosphatase. It is suggested that the Q NPs is a promising candidate for drug delivery, which enhances the hepatoprotective effect of Q against the cytotoxic effects of aflatoxin B1.
Introduction
Aflatoxins are remarkably potent mycotoxins that can enter the food chain through contaminated cereals as well as foods (i.e. milk, meat and eggs) after consumption of contaminated feed by farm animals [Citation1]. They exhibit a wide range of biological activities, such as carcinogenicity, genotoxicity, nephrotoxicity, hepatotoxicity, reproductive disorders [Citation1]. Aflatoxin B1 (AFB1) is produced by a certain fungus like Aspergillus flavus and Aspergillus parasiticus and is considered to be an unavoidable contaminant of foods [Citation2]. AFB1 has been linked to the aetiology of human liver cancer and also accounts for high rate of hepatocellular carcinoma [Citation2]. Upon metabolism by hepatic cytochrome P450 enzyme system, AFB1 changes into reactive form (AFB1–8, 9-epoxide) which produces both DNA and protein adducts and causes liver damages such as haemorrhage, cell necrosis, intrahepatic bile ducts injury as well as elevated liver enzyme function [Citation2].
Although it has been shown that the formation of reactive oxygen species (ROS) and lipid peroxidation (LPO) are dangerous events leading to the metabolic processing of AFB1 by liver enzymes, the mechanism of AFB1-induced cytotoxicity and carcinogenicity has not been fully elucidated. Several investigations have cited the possible role of antioxidants as an attractive strategy to protect individuals from the risk of hepatotoxicity caused by exposure to the mycotoxins [Citation3]. Quercetin (Q) is one of the most abundant flavonoids in plants and many studies have demonstrated that Q is a promising natural compound in health with antioxidative, anti-inflammatory and anticancer activities [Citation4]. It has been shown that quercetin possess a myriad of hepatoprotective activities against drug/xenobiotics-induced cellular damage such as chlorpyrifos and cadmium [Citation5,Citation6].
Regardless of wide spectrum of pharmacological properties, poor solubility in water, short biological half-life and low oral bioavailability restrict the use of Q as a therapeutic agent [Citation7,Citation8]. Nanotechnology, the procedure to produce designed nanoparticles (NPs <100 nm), holds promise for providing novel solutions to different applications such as drug delivery, electrochemical sensing, diagnostics and treatment of various disease [Citation9–12]. Nanotechnology-based methodologies exhibit beneficial impacts in an increasing absorption of poor water-soluble drugs and also reduce the dose required to achieve therapeutic effects in pharmaceutical investigation [Citation13,Citation14]. Due to smaller size of nanoparticles, which provides a larger surface area, the result is increased efficacy and achievement of drug bioavailability [Citation9,Citation15,Citation16). Since antioxidants are great potentials of disease treatment, researches to improve their performance via novel drug delivery tools are increasing every day [Citation17]. Nanoantioxidants like Q nanoparticles are safe, biocompatible and biodegradable with no toxicity. They also possess intrinsic antioxidant properties in attenuating of free radical-induced oxidative damage. Numerous studies have established that the nanoparticles delivery system could significantly transform the original physicochemical properties of drugs [Citation18].
In the current study, for the first time, we aimed to compare Q and Q nanoparticles (Q-NPs) as hepatoprotective agents against AFB1-induced cytotoxicity and ROS overproduction in isolated rat hepatocytes with accelerated cytotoxicity mechanism screening (ACMS) method. The influence of AFB1 on rat hepatocytes by assessing changes in cell viability, ROS formation, lipid peroxidation, GSH level and mitochondrial function was investigated. The hepatoprotective effects of the Q and Q-NPs were also determined by assessing levels of aspartate aminotransferase (AST), alanine aminotransferase (ALT) and alkaline phosphatase (ALP). Ultimately, the effects of the test substances on the antioxidant enzymes and lipid peroxidation of the liver were also examined by assessing changes in superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GPx) and malondialdehyde (MDA) levels.
Methods and materials
Chemicals
Collagenase (type II) was obtained from Sigma–Aldrich chemical Company (St. Louis, MO). Thiobarbituric acid (TBA), trypan blue, GSH and phenobarbital were prepared from Merck Chemical Company (Darmstadt, Germany). Oxidized glutathione (GSSG) and 4–2-hydroxyethyl-1-piperazineethanesulfonic acid (HEPES) were obtained from Acros (NJ). Other chemicals were obtained from Sigma–Aldrich chemical Company (St. Louis, MO).
Preparations of quercetin nanoparticles (Q-NPs)
Q-NPs were prepared according to the precipitation method [Citation19]. Q (10 mg) was dissolved in 3.0 ml of ethanol in a water bath at 40 °C as the organic phase. This was then injected into 27.0 ml of double-distilled water at 14,000 rpm and stirred for 15 s (Heidolph, silence crasher, Germany) to obtain suspensions of Q-NPs. The ethanol was completely removed by rotary vacuum evaporation at 40 °C water bath and the remaining fraction was then lyophilized with a freeze dryer. Moreover, the coarse suspensions were obtained by adding 30.0 mg of Q in 30.0 ml of cold distilled water containing 10% v/v ethanol. The Q content of the two suspensions was adjusted to about 1 mg/ml.
Characterization of the prepared Q-NPs
Dynamic light scattering (DLS) and zeta potential measurements were done using ultra-pure water (Milli-Q Water Purification Systems, Millipore, MA), which was completely particle free medium, by Nanotrac Wave (Microtrac, San Diego, CA) at room temperature. Transmission electron microscopy characterization of the Q-NPs was performed using LEO 906E transmission electron microscope (Zeiss, Germany). Prior to TEM analysis, the samples were stained with 0.5% (w/v) phosphotungstic acid on copper grids.
Animal treatment
Adult male Sprague-Dawley rats (200–250 g) were housed in standard cages with normal chew food, tap water available ad libitum in a laboratory with adjusted ambient temperature (21–23 °C), under a 12-h light/dark cycle obtained from the animal research centre of Tabriz University of Medical Science, Tabriz, Iran. All experiments were conducted according handling protocol of ethical guidelines of the National Institute of Health (NIH publication No. 85–23, revised 1985) and the “Guiding Principles in the Use of Animals in Toxicology”, adopted by the Society of Toxicology in 1989, for the acceptable works on experimental animals. The study protocol was designed and approved by the Ethics Committee for the Use of Animals in Research at Tabriz University of Medical Sciences (No: 91/2–2/5/4 December 2012).
Isolation and incubation of rat hepatocytes
Collagenase perfusion technique was used to prepare rat primary hepatocytes [Citation20]. The isolated rat hepatocytes were incubated in Dulbecco's modified Eagle's medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 1% antibiotic–antimycotic solution (10,000 units penicillin, 10 mg streptomycin, 25 μg amphotericin B), 0.1 U/ml insulin and 0.01 μM dexamethasone for 3 h at 37 °C in a humidified atmosphere of 95% O2 and 5% CO2. Hepatocytes with a density of 4.8 × 104/ml were plated in 96 well plates to perform viability assays. In other biochemical assays, cells (2 × 105/ml) were incubated in 12 well plates. We treated hepatocytes with different concentration of AFB1 for 4 h. Q and Q-NPs were added to the culture medium 1 h after AFB1 treatment. Ultimately, the hepatocytes were assessed for cell viability, ROS formation, the rate of lipid peroxidation (LOP), mitochondrial membrane potential (MMP) change and glutathione level. We also used N-acetyl cysteine as a well-known antioxidant agent to have a positive control group.
Cell viability
Cell viability was determined with 3–(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay which is a colorimetric technique. To do this experiment, 15 μl of a MTT (1 mg/ml) solution was added to the incubation medium and incubated for 4 h at 37 °C. Then, the medium was removed and 200 μl of DMSO was added to dissolve the produced formazan crystals.
The absorbance was measured at 570 nm using an ELISA microplate reader [Citation21] and data were expressed as percentage of control.
Cell lysate preparation
We centrifuged hepatocyte at 5000 g for 5 min upon the treatment period to prepare the cell lysate which was then washed with ice-cold PBS, pH 7.4. At the next step, the cell pellet was resuspended in PBS and sonicated with an ultrasonicator (Parasonic 30 S, Tehran, Iran) for three cycles each time for 30 s and total protein amount was measured with Lowry’s method [Citation22].
Reactive oxygen species (ROS) formation assay
Production of ROS was evaluated by use of 2′,7′- dichlorodihydrofluorescin diacetate (DCFH-DA) as a fluorescent detecting probe [Citation23]. Upon the treatment period, the hepatocytes (2 × 105) were incubated with DCFH-DA solution (100 μM) for 1 h at 37 °C. DFCHDA has the capability to permeabilize membranes and inter the cell where is cleaved by cellular esterases. Inside the cell, DCFH could react with ROS to form highly fluorescent agent, DCF. The dye containing medium was then removed, and the cells were washed and sonicated. The fluorescent DCF generation was measured by a spectrofluorimeter with excitation wavelength at 485 nm and emission wavelength at 535 nm. DCF–standard curve was plotted and results were expressed as pmol DCF/mg protein.
Determination of lipid peroxidation
Oxidative stress is often concomitant with peroxidation of cell membrane and/or subcellular organelles membrane lipids [Citation24]. Hepatocytes LPO was assessed through measuring thiobarbituric acid reactive substances (TBARS) that were generated during the breakdown of lipids. To determine the rate of lipid peroxidation, 0.5 ml of the cell lysate was incubated with 1.0 ml of KCl (0.15 M) and 250 μl of 0.2 mM ferric chloride solution at 37 °C for 30 min after which the reaction was terminated by the addition of 2.0 ml ice-cold mixture of 0.25 N HCl including 0.3% thiobarbituric acid (TBA), 15% trichloroacetic acid (TCA) and 0.05% butylated hydroxyl toluene (BHT) and was heated at 90 °C for 30 min. Then, the mixture was cooled and centrifuged at 7000 g for 5 min and the absorbance of the supernatant was measured spectrophotometrically at 535 nm. The data were expressed as percentage lipid of peroxidation [Citation25].
Glutathione-level determination
The method of Popet et al was utilized to assess the amount of reduced glutathione level in the cell lysate [Citation26]. Briefly, 50 μl of the cell lysate was treated with 150 μL of DTNB reagent which is consisting of 12 mM NADPH, 50 U/ml GSH reductase 0.1 mM DTNB, in 0.1 mM sodium phosphate buffer with 1 mM EDTA, pH 7.5 for 0.5 h. Measurement of absorbance was performed with an ELISA microplate reader at 415 nm by and GSH level was expressed as nmol/mg protein using a calibration curve according to the concentrations of reduced glutathione.
Mitochondrial membrane potential (MMP) assays
Mitochondrial membrane potential (MMP) was measured via the uptake and retention of the cationic fluorescent dye, rhodamine 123 [Citation27]. The dye is accumulated in intact mitochondria via the charge-facilitated diffusion. To do the experiment, at the end of incubation time with AFB1, cells were incubated in culture medium containing 1.6 μM of Rh123 at 37 °C in the dark for 20 min and then were washed and sonicated. The level of rhodamine 123 in the cells was measured spectrophotometrically at 490 nm excitation and 520 nm emission wavelengths and outcomes were expressed as nmol/mg protein [Citation28].
Animal experiments
Rats were randomly divided into four groups of five animals and treated for 30 days. The treatments were as follows: Group 1 (control): vehicle (0.9% saline solution) only. Group 2: AFB 1-treated rats (80 μg/kg body weight orally) [Citation29]. Group 3: AFB 1-treated rats (80 μg/kg body weight orally)+Q (10 mg/kg, PO) [Citation30]. Group 4: FB 1-treated rats (80 μg/kg body weight orally)+Q-NPs (10 mg/kg, PO). Antioxidant administration was performed 3 h before AFB1 administration. Daily observation to detect signs of toxicity was performed during the experimental period. At the end of 30 days, all animals were fasted for 12 h, and then, blood samples were collected from the abdominal vena cava under pentobarbital anaesthesia. The blood was allowed to clot at 25 °C, and serum was prepared by centrifugation (1000 g, for 20 min). Serum ALT, AST and ALP activities were measured with a commercial kit.
The liver tissues were dissected and washed in sodium phosphate buffer (pH 7.2). After washing, samples were taken and homogenized using a Teflon homogenizer (Heidolph Silent Crusher M). Centrifugation of liver homogenates at 1600 g was done for 15 min at 4 °C to obtain a supernatants for future analysis. LPO in the liver homogenate was determined by the formation of malondialdehyde (MDA) as nmol MDA per milligram of protein of liver tissue. Catalase (CAT) activity (K/mg protein) was determined as described by Aebi [Citation31]. Superoxide dismutase (SOD) activity was determined by the method of [Citation32] and total glutathione peroxidase (GPx) was evaluated with method of [Citation33] and the data were presented as unit/mg protein. The protein concentration was measured by the method of Lowry et al [Citation22].
Statistical analysis
The results are shown as the Mean ± SEM for at three independent experiments. Statistical analysis for the control and experimental groups was performed by a one-way analysis of variance (ANOVA) followed by Tukey’s post hoc test to assess significance. Results with values for p < .05 were considered statistically significant.
Results
Quercetin nanoparticles
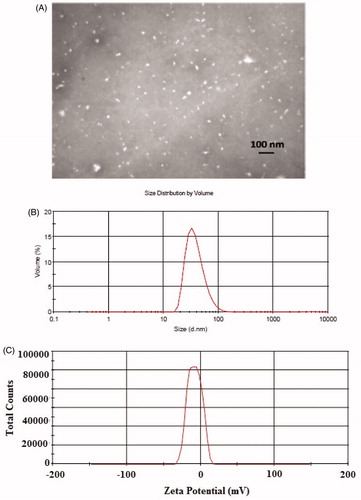
As shown in , TEM shows that Q-NPs are morphologically spherical and uniform in size distributions. Additionally, the Q-NPs displayed a diameter of 52.70 nm with polydispersity index (PDI) of 0.18 (). Zeta potential was found to be −24 mV ().
Cytotoxicity caused by AFB1
Incubation of rat hepatocytes for 4 h at 37 °C with 25 μM AFB1 induced an approximate 50% cell death in a concentration-dependent manner as measured by MTT assay (). This EC50 value was used to investigate potential cytotoxic mechanisms and alterations in biochemical markers.
Figure 2. (A) Effect of the AFB1 on the viability of rat hepatocytes. (B) Effect of quercetin (Q) (100 μM), Q-NPs (quercetin nanoparticles) (100 μM), N-acetyl cysteine (NAC) (200 μM) on the viability of AFB1-treated hepatocytes. Isolated rat hepatocytes were incubated for 4 h with DMEM containing different concentrations of AFB1. Antioxidants were added 1 h after AFB1 (25 μM) treatment. The viability was determined by MTT reduction assay and expressed as percentage of control. Data are expressed as mean ± SEM of three independent experiments. *Different from control group (p < .05). aQ (100 μM) and/or NAC (200 μM) significantly decreased hepatocyte membrane lysis compared to AFB1-treated hepatocytes. **Q-NPs significantly decreased hepatocyte membrane lysis compared to AFB1 and Q-treated hepatocytes.
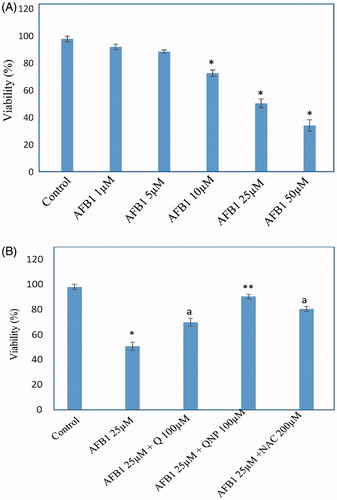
As presented in , 25 μM of AFB1 significantly increased hepatocyte membrane lysis in comparison with control hepatocytes. Q and Q-NPs were added to hepatocytes to determine their effects on AFB1-induced toxicity. The optimum doses for Q was evaluated to be 100 μM which effectively reduced AFB1-induced cell death (). To compare the cytoprotective effect of Q-NPs with Q, we used the same concentration (100 μM). We observed that AFB1-induced toxicity in Q-NPs treated hepatocytes were substantially reduced when compared with the Q treated hepatocytes ().
The effects of Q-NPs in AFB1-induced ROS formation and lipid peroxidation
Treatment with AFB1 resulted in a significant (p < .05) increase in dichlorofluorescein (DCF) fluorescence () as compared to normal cells. Incubation of AFB1-treated hepatocytes with antioxidant (Q) showed noteworthy reduction in ROS formation. Interestingly, we also found that in groups treated with Q-NPs, the ROS levels were significantly lower than of non-particulate counterpart (p < .05) ().
Figure 3. (A) Effect of quercetin (Q) (100 μM), Q-NPs (quercetin nanoparticles) (100 μM), N-acetyl cysteine (NAC) (200 μM) on the ROS generation of AFB1-treated hepatocytes. ROS is measured as pmole DCF formed/mg protein. (B) TBARS formation induced by AFB1 and the protective effects of 100 μM Q (quercetin), N-acetyl cysteine (NAC) (200 μM) and Q-NPs (quercetin nanoparticles). Isolated rat hepatocytes were incubated for 4 h with DMEM containing AFB1 (25 μM). Data are expressed as mean ± SEM of three independent experiments. *Different from control group (p < .05). aQ (100 μM) and/or NAC (200 μM) significantly decreased hepatocyte membrane lysis compared to AFB1-treated hepatocytes. **Q-NPs significantly decreased hepatocyte membrane lysis compared to AFB1 and Q-treated hepatocytes.
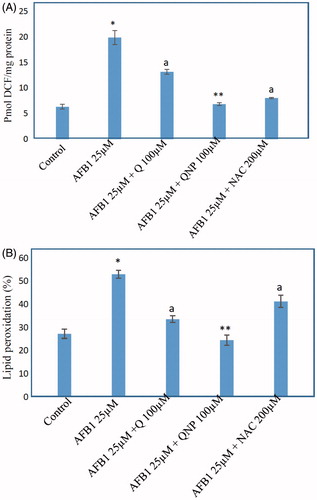
As consequence of ROS generation and oxidative stress peroxidation of membrane lipids increased which was concurrent with the production of thiobarbituric acid reactive substances (TBARS). As presented in , AFB1 toxicity was associated with the generation of a large amount of TBARS. Again treatment with Q significantly (p < .05) protected hepatocytes against TZ-induced LPO (). The comparison of hepatocytes which was treated with Q and Q-NPs demonstrated TBARS formation associated with Q-NPs treatment decreased more effectively ().
The effects of Q-NPs in AFB1-induced GSH depletion
As illustrated in , after incubation with the AFB1 (25 μM), the intracellular GSH level was decreased significantly. Treatment of hepatocytes with Q significantly (p < .05) prevented GSH depletion caused by AFB1 (). Treatment with Q-NPs attenuates the depletion of GSH level meaningfully more than Q-treated primary rat hepatocytes (). All these findings indicate the occurrence of oxidative stress after AFB1 administration in rat hepatocytes. All of the antioxidants did not show any significant change (p > 0.05) in hepatocytes GSH status at concentrations used (data not shown).
Figure 4. Effect of quercetin (Q) (100 μM), Q-NPs (quercetin nanoparticles) (100 μM), N-acetyl cysteine (NAC) (200 μM) on the glutathione depletion induced by AFB1 in primary rat hepatocytes. GSH level is measured as nmole/mg protein. Isolated rat hepatocytes were incubated for 4 h with DMEM containing different AFB1 (25 μM). Data are expressed as mean ± SEM of three independent experiments. *Different from control group (p < .05). aQ(100 μM) and/or NAC (200 μM) significantly decreased hepatocyte membrane lysis compared to AFB1-treated hepatocytes. **Q-NPs significantly decreased hepatocyte membrane lysis compared to AFB1 and Q-treated hepatocytes.
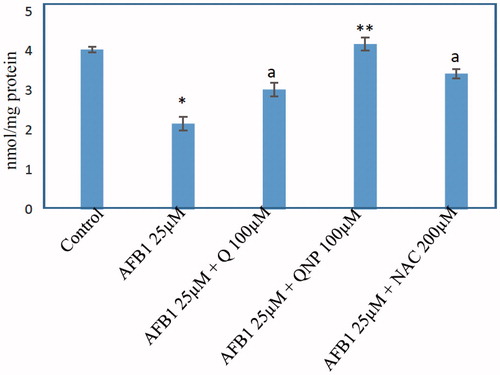
Table 1. Effect of Q and Q-NPs on liver function in AFB1-admministrated rats.
The effects of Q-NPs in AFB1-induced mitochondrial membrane potential collapse
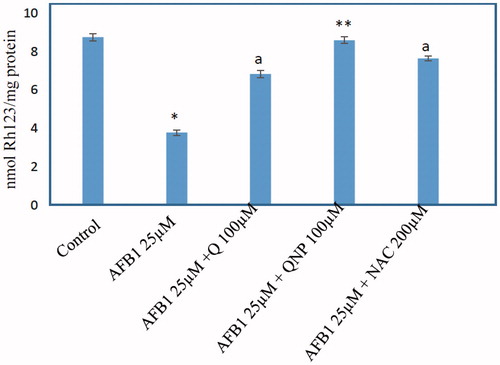
The MMP is the major driving force for ROS generation and ATP production and alteration on this parameter were measured with a potentiometric fluorescent probe rhodamine 123, which can accumulate in the negatively charged mitochondrial matrix. As shown in , AFB1 (25 μM) caused a rapid decline of mitochondrial membrane potential which noticeably was prevented by Q. In addition, it was observed that AFB1-induced MMP collapse significantly decreased in hepatocytes treated with Q-NPs as compared with Q-administered hepatocytes (p < .05) (.
Figure 5. Effect of quercetin (Q) (100 μM), Q-NPs (quercetin nanoparticles) (100 μM), N-acetyl cysteine (NAC) (200 μM) on the mitochondrial membrane potential in AFB1-treated rat hepatocytes. MMP level is measured as nmole Rh 123/mg protein. Isolated rat hepatocytes were incubated for 4 h with DMEM containing AFB1 (25 μM). Data are expressed as mean ± SEM of three independent experiments. *Different from control group (p < .05). aQ (100 μM) and/or NAC (200) significantly decreased hepatocyte membrane lysis compared to AFB1-treated hepatocytes. **Q-NPs significantly decreased hepatocyte membrane lysis compared to AFB1 and Q-treated hepatocytes.
The effects Q-NPs on AFB1-administrated rat liver function
The liver function markers in normal control, AFB1, Q-treated and Q-NPs-treated groups are summarized in . The elevation of the AST, ALT and ALP in AFB1-intoxicated rats was higher than the normal control (p < .05). In AFB1-intoxicated animals treated with Q (10 mg/kg) and Q-NPs (10 mg/kg), the AST, ALT and ALP values were significantly decreased when compared with the AFB1-intoxicated group (p < .05). In addition, there are significant differences between AFB1 groups treated with Q and Q-NPs.
Table 2. Effect of the quercetin and its nanoparticles on liver function markers, antioxidant enzyme activities and lipid peroxidation in rats with aflatoxin B1-induced hepatotoxicity.
The effects of Q-NPs on hepatic antioxidant enzymes in AFB1-administarted rats
As shown in , the activity of the SOD, CAT and GPx levels in AFB1-intoxicated groups were significantly decreased when compared with the normal control (p < .05). Pre-treatment with Q and its nanoparticles significantly raised the antioxidant enzyme levels when compared with the rats intoxicated with AFB1 (p < .05). A comparison was also made between the Q and Q-NPs groups, and the result demonstrated that Q-NPs group had better antioxidant activities than Q group.
Discussion
Among numerous chemical methods for the synthesis of nanoparticles, precipitation method is simple and rapid preparative method and also size-controllable method. The precipitation process offers advantages like simplicity, low cost, high purity of product and not requiring organic solvents [Citation14,Citation34,Citation35]. In this study, we used the nanoprecipitation technique that possesses numerous benefits, which offers reproducible particle size with a narrow distribution (PDI value of 0.18) and is comparatively straightforward and rapid.
DLS method or photon correlation spectroscopy (PCS) presents the intensity-weighted mean diameter of the bulk population (z-average) and the PDI as measures for the width of the size distribution. As shown in , the Q-NPs displayed a diameter of 52.70 nm with PDI of 0.18. According to the ISO standard document 13321:1996 E and ISO 22412:2008; “The Polydispersity Index values smaller than 0.05 are rarely seen other than with highly monodisperse standards. Values greater than 0.7 indicate that the sample has a very broad size distribution”. Furthermore, according to some investigations, a PDI value of 0.1–0.25 indicates a narrow size distribution while a PDI greater than 0.5 refers to a broad distribution [Citation36]. Then, our size distribution result with PDI value of 0.18 specified a narrow size distribution. TEM shows that Q-NPs are morphologically spherical and uniform in size distributions, which was confirmed the results of DLS analysis ().
Zeta potential is a main parameter generally used to predict suspension stability. The higher the zeta potential, the more stable the suspension is. Zeta potential for prepared nanoparticles was found to be −24 mV. As a general rule, a value of zeta potential above ±60 mV yields excellent stability, while zeta potential less than ±5 mV generally results in particle aggregation. The values between this range results good stability or acceptable short-term stability [Citation37,Citation38]. Therefore, the prepared nanoparticles in the present study with zeta potential of −24 mV might be good stability as well as superiorly a high uptake by the liver. Then, in addition to the good physical stability of the nanoparticles, the negative nature of the zeta potential may be a main determinant in the rate and the efficiency of monocyte/macrophage uptake. This process has also been described by other investigators. For example, Gao et al reported the zeta potential value of −21.8 mV for their prepared oridonin nanosuspensions that showed a high uptake by the liver [Citation39]. In another work by Dong et al anticancer agent SNX2112 nanocrystals with a negative zeta potential (−11.6 mV) showed extensive distribution in the liver [Citation40]. Rabinow et al also found that high accumulation of the itraconazole nanosuspension (with a zeta potential of −30.6 mV) happened in the liver [Citation41].
It seems that original physicochemical properties are improved with nanoparticulation, rendering the crystalline structure which may alter the release profile of bare Q. To pinpoint the protective role of Q and its nanoparticles in liver injury, we adopted the isolated rat hepatocyte model of AFB1-induced hepatotoxicity to examine the antioxidant functions and the relevant mechanisms.
ACMS technique as a useful screening system was used to determine the molecular cytotoxic mechanism of AFB1 in a freshly isolated rat hepatocyte suspension over three hours and potential protective effects of antioxidants (Q and its nanocrystals). In order to enhance poor solubility, preparation of Q nanoparticles was performed using precipitation method. An important note using ACMS is that a high drug dose over three hours displays similar hepatotoxic effect in vitro as would do a lower drug dose over a longer period of time (24 to 48 h) in vivo [Citation42]. In other words, this method can be used to predict and characterize hepatotoxicity in vivo.
There are toxicological implications associated with AFB1 intake such as hepatotoxicity and carcinogenicity [Citation43]. Exposure to AFB1 not only overproduces the ROS to exhaust cellular endogenous antioxidants (SOD, CAT and GPx), but also the excess ROS attacks to unsaturated fatty acids of phospholipids bilayer hepatocyte membrane to subsequently initiate LPO [Citation3].
Q as a potent free radical scavenger is more stronger than well-known traditional antioxidants such as Vitamin C and E [Citation44]. Moreover, Q has shown to exert hepatopreotective effects against various xenobiotic-induced cellular injuries and oxidative hazard [Citation5,Citation6]. However, the solubility of Q is poor due to its chemical structure. Also because of low bioavailability, and only a small percentage of ingested Q is absorbed into circulation.
Our results showed that oxidative stress is firmly involved in the pathogenesis of AFB1-induced cytotoxicity and it was prevented by Q and its nanoparticles. Incubation of isolated rat hepatocytes with Q and Q-NPs significantly protected hepatocytes against cytotoxicity ROS formation and LPO induced by AFB1. In comparison with Q, Q-NPs displayed greater effects in ROS scavenging and achieved higher potency than the free drug form in vitro upon isolated rat hepatocytes treatment.
Since nanoparticle systems have an enhanced aqueous solubility profile and a smaller size, a higher concentration of the bioactive compound could be delivered into the hepatocytes. The aforementioned properties could explain the higher efficacy of Q-NPs than the free drug. The smaller size of Q-NPs in comparison with free Q results in facilitated uptake of the compound to the damaged liver tissue and easier transport across hepatic sinusoid. Also, elevated aqueous solubility gains the nanoparticle the superiority to provide a higher concentration and a further more potent inhibition of the caspase-signalling cascade to prevent hepatocyte cell death.
In the most of mammalian cells, mitochondria are the major cellular source of ROS (reactive oxygen species) and any damage to these powerhouses ultimately leads to depletion of required ATP for cell. Moreover, due to the continual leakage of electrons in the respiratory chain, mitochondria are more susceptible to cellular oxidative hazard [Citation45].
AFB1 induces cellular damages in different points. Induction of oxidative stress, genetic alterations leading to DNA damage and mitochondrial permeability changes have been shown to occur after AFB1 contamination [Citation46]. Besides, it has been reported that AFB1 induces LPO in rat liver lysosomes [Citation47]. Low mitochondrial membrane potential which is a crucial factor of mitopathy and its subsequent events has a tight connection with oxidative stress-associated mitochondrial dysfunction. The results in current paper proposed that AFB1 was a significant inducer of hepatic mitochondrial dysfunction via reduction of MMP and resulted in massive ROS generation to induce oxidative stress.
Furthermore, prevention of MMP collapse with antioxidants (Q and Q-NPs) suggested that MMP collapse was a consequence of ROS production. In comparison with Q-treated hepatocytes, MMP collapse in Q-NPs treated hepatocytes considerably decreased, which can be attributed to sufficient concentration of Q near to mitochondria. In Q-NPs-treated hepatocytes, it is possible that there is an enhanced penetration of Q to the mitochondrial membrane which need further investigations to be proved comprehensively.
GSH as ubiquitous thiol-containing tripeptide is one of the main components of cellular antioxidants reservoirs which contributes to the maintenance of sulfhydryl groups in redox state of many proteins, protecting cell membranes against oxidative damage. GSH is also a vital factor in regeneration of antioxidants such ascorbic acid or α-tocopherol to their active forms [Citation48]. Increased oxidative damage concomitant with GSH depletion has been shown to induce protein oxidation and DNA damage after AFB1 administration in rat liver [Citation49]. We observed a significant GSH depletion that might be a mechanism for the AFB1 toxicity in isolated rat hepatocytes. Q is able to increased hepatocytes glutathione levels and this effect may also participate in protection against AFB1-induced hepatotoxicity. Moreover, for the first time here, we showed that antioxidant potential of Q-NPs was significantly higher than Q in mitigation of GSH depletion in AFB1-exposed isolated rat hepatocytes.
Prevention of AFB1-induced ROS formation, MMP loss and lysosomal membrane leakage by Q and Q-NPs suggests the counter action of aforementioned agents on lysosomal, mitochondrial outer membrane permeability and all subsequent stages of the apoptotic cascade which are in accordance with previous studies. Also, the participation of mitochondria and lysosomes in AFB1-induced cytotoxicity might be a possible proposed mechanism.
Regarding our in vivo AFB1 caused a significant decrease in the activity of antioxidant enzymes. SOD converts superoxide radicals into H2O2, which induces hydroxyl radicals by Fenton and/or Haber–Weiss reactions if the agent is not removed by CAT and/or GSH [Citation50]. The reduction in GPx and SOD activity of AFB-treated rats may indirectly lead to an increase in oxidative damage [Citation3]. Previous experiments on the mechanisms of AFB1-associated hepatotoxicity have revealed that glutathione and SOD play an important role in the detoxification of the reactive and toxic metabolites of this mycotoxin and that the liver necrosis initiates when the glutathione stores are almost depleted [Citation51,Citation52]. In the current study, we showed that the levels of GPX and SOD were diminished in AFB1-treated rats; whereas, this reduction was significantly prohibited by administration with Q and its NPs. On the other hand, MDA is an end product of LPO, and it is considered to be a late marker of oxidative hazard and cytotoxicity [Citation53]. These outcomes indicate that the protective action of Q in the reduction of AFB1s toxicity in part by decreasing oxidative stress through enhancement of antioxidant defence systems. Also, these results strongly suggest that Q-NPs rather than free Q are closely associated with activation of antioxidant defence systems and inhibition of LPO in AFB1-treated animals.
Conclusions
The current results affirmed that, beside the protective role of Q-NPs, it enhanced the antioxidant effect of Q and overcome the problem associated with its poor absorption and bioavailability upon oral administration. Improving effects of Q on mitochondrial electron transport chain to repair the xenobiotic-induced mitochondrial impairment and to decrease ROS formation has been of considerable attention in recent years. In this regard, repression of liver damages caused by AFB1 augmenting ROS production and subsequent toxic events including LPO, mitochondrial membrane potential collapse, GSH depletion membrane injury which is an indicative of an oxidative stress and mitochondrial malfunction was studied by administration of Q-NPs and Q as putative antioxidants were able to inhibit AFB1-induced toxicity while the nanoformulated feature of Q exerted a better effect on neutralization of AFB1-mediated side effects in rat hepatocytes. The results of this experiment are proposed to be used for establishing approaches to protect against adverse effects of AFB1 in hepatocytes and also in other organs. In conclusion, the use of Q-NPs seems to be a very safe as supplementary therapeutics in pathologies associated with xenobiotics-induced hepatotoxicity. Indeed, in addition to the solubility enhancement, nanosized Q particles may be lead to the efficiency of monocyte/macrophage uptake (particularly in the liver and spleen).
Acknowledgements
The authors would like to thank Drug Applied Research Centre of Tabriz University of Medical Sciences, Tabriz, Iran, for providing technical facilities. The authors are also thankful to the University’s “Students’ Research Committee” for providing technical supports to the study.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Khlangwiset P, Shephard GS, Wu F. Aflatoxins and growth impairment: a review. Crit Rev Toxicol. 2011;41:740–755.
- Rawal S, Kim JE, Coulombe R. Aflatoxin B1 in poultry: toxicology, metabolism and prevention. Res Vet Sci. 2010;89:325–331.
- El‐Bahr S. Effect of curcumin on hepatic antioxidant enzymes activities and gene expressions in rats intoxicated with aflatoxin B1. Phytother Res. 2015;29:134–140.
- Boots AW, Haenen GR, Bast A. Health effects of quercetin: from antioxidant to nutraceutical. Eur J Pharmacol. 2008;585:325–337.
- Uzun FG, Kalender Y. Chlorpyrifos induced hepatotoxic and hematologic changes in rats: the role of quercetin and catechin. Food Chem Toxicol. 2013;55:549–556.
- Vicente-Sánchez C, Egido J, Sánchez-González P, et al. Effect of the flavonoid quercetin on cadmium-induced hepatotoxicity. Food Chem Toxicol. 2008;46:2279–2287.
- Cai X, Fang Z, Dou J, et al. Bioavailability of quercetin: problems and promises. Curr Med Chem. 2013;20:2572–2582.
- Murota K, Terao J. Antioxidative flavonoid quercetin: implication of its intestinal absorption and metabolism. Arch Biochem Bioph. 2003;417:12–17.
- Eaton DL, Groopman JD. The toxicology of aflatoxins: human health, veterinary, and agricultural significance. USA: Academic press; 2013.
- Hasanzadeh M, Mokhtari F, Shadjou N, et al. Poly arginine-graphene quantum dots as a biocompatible and non-toxic nanocomposite: layer-by-layer electrochemical preparation, characterization and non-invasive malondialdehyde sensory application in exhaled breath condensate. Mater Sci Eng C. 2017;75:247–258.
- Andishmand H, Tabibiazar M, Mohammadifar MA, et al. Pectin-zinc-chitosan-polyethylene glycol colloidal nano-suspension as a food grade carrier for colon targeted delivery of resveratrol. Int J Biol Macromol. 2017;97:16–22.
- Jafari S, Ahmadian E, Fard JK, et al. Biomacromolecule based nanoscaffolds for cell therapy. J Drug Deliv Sci Technol. 2017;37:61–66.
- Maleki Dizaj S, Lotfipour F, Barzegar-Jalali M, et al. Antimicrobial activity of the metals and metal oxide nanoparticles. Mater Sci Eng C. 2014;44:278–284.
- Maleki Dizaj S, Lotfipour F, Barzegar-Jalali M, et al. Physicochemical characterization and antimicrobial evaluation of gentamicin-loaded CaCO3 nanoparticles prepared via microemulsion method. J Drug Deliv Sci Technol. 2016;35:16–23.
- Maleki Dizaj S, Lotfipour F, Barzegar-Jalali M, et al. Box-Behnken experimental design for preparation and optimization of ciprofloxacin hydrochloride-loaded CaCO3 nanoparticles. J Drug Deliv Sci Technol. 2015;29:125–131.
- Maleki Dizaj S, Barzegar-Jalali M, Zarrintan MH, et al. Calcium carbonate nanoparticles as cancer drug delivery system. Expert Opin Drug Deliv. 2015;12:1649–1660.
- Aldahoun MAA, Jaafar M, Al-Akhras MAH, et al. Enhanced nanocurcumin toxicity against (PC3) tumor and microbial by using magnetic field in vitro. Artif Cells Nanomed Biotechnol. 2017;5:843–853.
- Ghosh A, Mandal AK, Sarkar S, et al. Nanoencapsulation of quercetin enhances its dietary efficacy in combating arsenic-induced oxidative damage in liver and brain of rats. Life Sci. 2009;84:75–80.
- Sun J, Wang F, Sui Y, et al. Effect of particle size on solubility, dissolution rate, and oral bioavailability: evaluation using coenzyme Q(1)(0) as naked nanocrystals. Int J Nanomed. 2012;7:5733–5744.
- Pourahmad J, Amirmostofian M, Kobarfard F, et al. Biological reactive intermediates that mediate dacarbazine cytotoxicity. Cancer Chemother Pharmacol. 2009;65:89–96.
- Neuman MG, Koren G, Tiribelli C. In vitro assessment of the ethanol-induced hepatotoxicity on HepG2 cell line. Biochem Biophys Res Commun. 1993;197:932–941.
- Lowry OH, Rosebrough NJ, Farr AL, et al. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275.
- Lebel CP, Ischiropoulos H, Bondy SC. Evaluation of the probe 2', 7'-dichlorofluorescin as an indicator of reactive oxygen species formation and oxidative stress. Chem Res Toxicol. 1992;5:227–231.
- Eskandari MR, Moghaddam F, Shahraki J, et al. A comparison of cardiomyocyte cytotoxic mechanisms for 5-fluorouracil and its pro-drug capecitabine. Xenobiotica. 2015;45:79–87.
- Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310.
- Popat A, Shear NH, Malkiewicz I, et al. Mechanism of Impila (Callilepis laureola)-induced cytotoxicity in Hep G2 cells. Clin Biochem. 2002;35:57–64.
- Shaki F, Hosseini MJ, Ghazi-Khansari M, et al. Toxicity of depleted uranium on isolated rat kidney mitochondria. Biochim Biophys Acta. 2012;1820:1940–1950.
- Shangari N, O’Brien PJ. The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem Pharmacol. 2004;68:1433–1442.
- Abdel-Wahhab MA, El-Denshary ES, El-Nekeety AA, et al. Efficacy of organo-modified nano montmorillonite to protect against the cumulative health risk of aflatoxin B 1 and ochratoxin A in Rats. SNL. 2015;5:21.
- Janbaz K, Saeed S, Gilani A. Studies on the protective effects of caffeic acid and quercetin on chemical-induced hepatotoxicity in rodents. Phytomedicine. 2004;11:424–430.
- Aebi H. Catalase in vitro. Methods Enzymol. 1984;105:121–126.
- Beauchamp C, Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971;44:276–287.
- Flohé L, Günzler WA. Assays of glutathione peroxidase. Meth Enzymol. 1984;105:114–120.
- Sharma G, Park J, Sharma AR, et al. Methoxy poly (ethylene glycol)-poly (lactide) nanoparticles encapsulating quercetin act as an effective anticancer agent by inducing apoptosis in breast cancer. Pharm Res. 2015;32:723–735.
- Maleki Dizaj S, Lotfipour F, Barzegar-Jalali M, et al. Application of Box–Behnken design to prepare gentamicin-loaded calcium carbonate nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:1475–1481.
- Worldwide MI. Dynamic light scattering, common terms defined. Inform white paper. Malwern Instruments Limited; 2011;2011:1–6.
- Matouskova P, Marova I, Bokrova J, et al. Effect of encapsulation on antimicrobial activity of herbal extracts with lysozyme. Food Technol Biotechnol. 2016;54:304.
- Maleki Dizaj S, Lotfipour F, Barzegar-Jalali M, et al. Ciprofloxacin HCl-loaded calcium carbonate nanoparticles: preparation, solid state characterization, and evaluation of antimicrobial effect against Staphylococcus aureus. Artif Cells Nanomed Biotechnol. 2017;45:535–543.
- Gao L, Liu G, Wang X, et al. Preparation of a chemically stable quercetin formulation using nanosuspension technology. Int J Pharm. 2011;404:231–237.
- Dong D, Wang X, Wang H, et al. Elucidating the in vivo fate of nanocrystals using a physiologically based pharmacokinetic model: a case study with the anticancer agent SNX-2112. Int J Nanomed. 2015;10:2521.
- Rabinow B, Kipp J, Papadopoulos P, et al. Itraconazole IV nanosuspension enhances efficacy through altered pharmacokinetics in the rat. Int J Pharm. 2007;339:251–260.
- O'Brien P, Siraki A. Accelerated cytotoxicity mechanism screening using drug metabolising enzyme modulators. Curr Drug Metab. 2005;6:101–109.
- Omar HEDM. Mycotoxins-induced oxidative stress and disease. Mycotoxin and food safety in developing countries. USA: InTech; 2013. p. 63.
- Geetha T, Malhotra V, Chopra K, et al. Antimutagenic and antioxidant/prooxidant activity of quercetin. Indian J Exp Biol. 2005;43:61–67.
- Rochette L, Zeller M, Cottin Y, et al. Diabetes, oxidative stress and therapeutic strategies. Biochimica Et Biophysica Acta. 2014;1840:2709–2729.
- Shi D, Liao S, Guo S, et al. Protective effects of selenium on aflatoxin B1-induced mitochondrial permeability transition, DNA damage, and histological alterations in duckling liver. Biol Trace Elem Res. 2015;163:162–168.
- Toskulkao C, Glinsukon T. Hepatic mitochondrial function and lysosomal enzyme activity in ethanol-potentiated aflatoxin B 1 hepatotoxicity. Toxicol Lett. 1990;52:179–190.
- Nimse SB, Pal D. Free radicals, natural antioxidants, and their reaction mechanisms. RSC Adv. 2015;5:27986–28006.
- Abdel-Aziem SH, Hassan AM, Abdel-Wahhab MA. Dietary supplementation with whey protein and ginseng extract counteracts oxidative stress and DNA damage in rats fed an aflatoxin-contaminated diet. Mutat Res/Genet Toxicol Environ Mutagen. 2011;723:65–71.
- Johnson F, Giulivi C. Superoxide dismutases and their impact upon human health. Mol Aspects Med. 2005;26:340–352.
- Mary VS, Theumer MG, Arias SL, et al. Reactive oxygen species sources and biomolecular oxidative damage induced by aflatoxin B1 and fumonisin B1 in rat spleen mononuclear cells. Toxicology. 2012;302:299–307.
- Ajiboye TO, Yakubu MT, Oladiji AT. Lophirones B and C extenuate AFB1‐mediated oxidative onslaught on cellular proteins, lipids, and DNA through Nrf‐2 expression. J Biochem Mol Toxicol. 2014;28:558–567.
- Carampin P, Rosan S, Dalzoppo D, et al. Some biochemical properties of melatonin and the characterization of a relevant metabolite arising from its interaction with H2O2. J Pineal Res. 2003;34:134–142.