Abstract
Bone regeneration is an important objective in clinical practice and has been used for different applications. The aim of this study was to evaluate the effectiveness of nanocomposite tricalcium phosphate (TCP)/collagen scaffolds combined with hydroxyapatite scaffold for bone healing in surgery of femoral defects in rabbits. In this study, 45 mature male New Zealand white rabbits between 6 and 8 months old and weighting between 3 and 3.5 kg were examined. Rabbits were divided into three groups. Surgical procedures were performed after intramuscular injection of Ketamine 10% (ketamine hydrochloride, 50 mg/kg) and Rompun 5% (xylazine, 5 mg/kg). Then an approximately 6 mm diameter–5 mm cylinder bone defect was created in the femur of one of the hind limbs. After inducing the surgical wound, all rabbits were coloured and randomly divided into three experimental groups of 15 animals each. Group 1 received pure medical nanocomposite TCP/collagen granules, group 2 received hydroxyapatite, and third group was a control group which received no treatment. Histopathological evaluation was performed on days 15, 30, and 45 after surgery. On days 15, 30, and 45 after surgery, the quantity and the velocity of stages of bone formation at the healing site in nanocomposite TCP/collagen group were better than HA and control groups and the quantity of newly formed lamellar bone at the healing site in nanocomposite TCP/collagen group were better than onward compared with HA and control groups. In conclusion, it seems that TCP/collagen nanocomposite has a significant role in the reconstruction of bone defects and can be used as scaffold in bone fractures.
Introduction
Tissue engineering aims to reconstruct living tissues for replacement of damaged or lost tissue/organs, hoping to maintain, restore, or enhance part or whole organ function of living organisms [Citation1]. One of the main goals of bone tissue engineering (BTE) is the design of a biodegradable porous material scaffold integrated with biological cells and molecular cues able to guide the process of de novo tissue regeneration [Citation2,Citation3].
Three fundamental components of bone tissue engineering (BTE) are (1) osteoconductive scaffold that promotes the attachment, migration and proliferation of host cells at the implanting site; (2) osteoinductive proteins or growth factors, which stimulate osteoprogenitor cells to differentiate into and synthesize mineralized bone matrix; and (3) osteogenic cells (osteoblast precursors or osteoblasts) that can synthesize bone tissue [Citation4].
Tricalcium phosphate (TCP) is a tertiary calcium phosphate also known as bone ash [Ca3(PO4)]. It serves as a rich source for calcium and phosphorus, which can be easily assimilated and absorbed. Beta-TCP is highly biocompatible and creates a resorbable interlocking network within the defect site to promote healing [Citation5,Citation6].
Collagen is the most abundant protein in the extracellular matrix (ECM) and has been considered to be a group of proteins with a characteristic molecular structure—fibrillar structure, which contributes to the extracellular scaffolding [Citation7]. That is to say, collagen plays an important role in maintaining the biological and structural integrity of ECM and provides physical support to tissues. Collagen possesses extensive sources (such as bone, cartilage, tendon, ligament, blood vessel, nerve, and skin), as it is the main structural protein of most hard and soft tissues [Citation8]. In addition, collagen offers low immunogenicity, a porous structure, permeability, good biocompatibility, and biodegradability and has functions to regulate the morphology, adhesion, migration, and differentiation of cells [Citation9,Citation10].
Nanocomposite biomaterials or bio-nanocomposites offer versatility in designing specific properties due to a better control of interactions between nanoparticles and polymers [Citation11]. Polymer nanocomposite biomaterials possess superior mechanical properties when compared with their macro- and microcomposite counterparts [Citation12]. The aim of this study was to assess new bone regeneration within the rabbit’s femoral defects repaired by the nanocomposite TCP/collagen scaffolds combined with hydroxyapatite scaffold.
Material and methods
Materials
TCP was synthesized by calcining amorphous calcium phosphate. Collagen (Sigma-Aldrich, St. Louis, MO) was used for the collagen matrix, and aqueous solution of glutaraldehyde (Sigma-Aldrich, St. Louis, MO) was used as a cross-linking agent. All other reagents and solvents are of analytical grade and are used as received. Aqueous alkali solution (pH 12) was obtained by dissolving sodium hydroxide into deionized water.
Preparation of TCP/collagene nanocomposite
At first, collagen suspension was prepared in aqueous alkali solution (pH 12) at room temperature. Then TCP powder was slowly added into the collagen suspension (2:1) while stirring, after a homogenous suspension was formed, the glutaraldehyde solution was added as a cross-linking agent. The mixture froze in a refrigerator for 5 h at −40 °C. Porous composites were obtained after further lyophilization.
Experimental design
The present study was conducted after obtaining the ethical approval from the Islamic Azad University with number of 5393 (Tehran Science and Research Azad University) Research Committee. In this study, we used 45 mature male New Zealand white rabbits, 6–8 months of age and with an approximate weight of 3–3.5 kg.
All animals were obtained from the same source in order to decrease the genetic variability. The animals were housed separately (one rabbit per cage) and maintained on standard pellet diet and tap water. Animal houses were in standard environmental conditions at the temperature of 18 ± 3 °C, a humidity of 60 ± 5%, and 12 h light/dark cycle. Right Lateral femoral osteotomies were performed surgically. Rabbits were divided into three treatment groups with 15 femur bones in each group.
Surgical procedures
Surgical procedures were done after an intramuscular injection of Ketamine 10% (ketamine hydrochloride, 50 mg/kg) and Rompun 5% (xylazine, 5 mg/kg). The hair was removed from the surgical site and the skin was cleaned with iodinated surgical soap. Aseptic technique was used throughout the surgical procedure. An incision approximately 5 cm long was made along the medial right upper hind limb, and the middiaphyseal surface of the femur was surgically exposed by blunt dissection. The periosteum was stripped from the bone using a periosteal elevator and an approximately a 6 mm diameter–5 mm cylinder bilateral bone defect was created in the femur of one of the hind limbs. This osteotomy site was then irrigated with 0.9% saline, but periosteum around the osteotomy site was preserved and retracted with the overlying muscles. The osteotomy site was then treated according to the treatment protocol for each rabbit.
Treatment
After inducing the surgical wound, all rabbits were coloured with non-toxic colour and randomly divided into three groups by 15 rabbits per group. The segmental defects were implanted with nanocomposite TCP/collagen granules in the first group and hydroxyapatite in the second group. The animals of third group were a control group and received no treatment. The periost and subcutaneous tissues were then closed primarily. Antibiotics (penicillin G procaine 40000 IU/kg IM, bid), dexamethasone (0.6 mg/kg, IM), and analgesics such as tramadol hydrochloride (5 mg/kg, IM, bid) were administered for 3 postoperative days. Experimental animals were kept in separate cages in order to prevent self-injury.
After the procedure, subjects were observed on a daily basis and evidence of infection or other abnormalities were noted. Five experimental rabbits from each group were euthanized with an intravenous infusion of 2 ml per 4.5 kg dose of Euthanyl containing 240 mg pentobarbital per millilitre on days 15, 30, and 45 postoperatively. Then the right femur was extracted and fixed in 10% buffered formalin and stored for histological examination.
Histopathological study and statistical analysis
Slide preparation
For histological examination, the obtained tissues were decalcified with 10% formic acid solution which was replaced daily. The surgical specimens were submitted to decalcification and routine histological processing for slide preparation and then embedded in paraffin blocks. Thereafter, they were sectioned at a thickness of 6 μm in a microtome using the largest diameter of the defect, stained with trichrome and haematoxylin/eosin (samples from 15th day were stained by H&E stain since at this time, evaluation of inflammation phases were considered and on days 30 and 45, the samples were stained by trichrom stain because the grade of osteoclasting was important at this times), and analyzed under a light microscope by pathologist in a double-blind manner.
Recorded factors from specimens were evaluated with a 12-point histological grading scale to determine the quality of the union, appearance and quality of the spongiosa, as well as to evaluate the Bone marrow. The collected data were analyzed statistically with one-way analysis of variance SPSS version 22 (SPSS Inc., Chicago, IL).
Result
Statistical analysis of bone parameters index
Descriptive statistics of union index demonstrated that the highest point of the index union on day 15 were from the nanocomposite TCP-collagen-treated group (3) and the lowest points belonged to the control group and HA-treated groups (1). Plus the highest score on day 30 belonged to the nanocomposite TCP-collagen-treated group (3) and the lowest belonged to the control group (1). And the highest points on day 45 were from nanocomposite TCP-collagen-treated group and HA-treated groups (3.80) and the lowest were from the control group (3) (). Among the days on which the samples were obtained while the average score of union index demonstrated a significant difference in the HA-treated group and the control group (p < .05), such significant difference was not shown in the nanocomposite TCP-collagen-treated group (). Average scores union on days 15, 30, and 45 shows that the highest union score belonged to the nanocomposite TCP-collagen-treated group and the HA-treated group and the control group were in the second and the third place, respectively.
Table 1. Descriptive statistics of union index.
Table 2. Compare average scores of union index.
Descriptive statistics of spongiosa index were demonstrated that the highest scores on day 15 belonged to the nanocomposite TCP-collagen-treated group (0.4) and the control group and HA-treated group were both lower (0.2). On day 30, the highest score was that of the nanocomposite TCP-collagen-treated group (2.2) and the lowest score was shown in the control group (1); moreover, on day 45, nanocomposite TCP-collagen-treated group gained the highest score (3.8) and the control group got the lowest score (2) (). Among the days on which the samples were obtained average scores of spongiosa index demonstrates significant difference in all three groups (p < .05) (). Average scores of spongiosa on days 15, 30, and 45 showed that the highest spongiosa score belonged to the nanocomposite TCP-collagen-treated group. While the HA-treated group and the control group were in the second and the third level, respectively.
Table 3. Descriptive statistics of spangiosa index.
Table 4. Compare average scores of spongiosa index.
The descriptive statistics of bone marrow index indicate that the control group has the highest score by day 15 of the healing process (1.2) and was lower in the nanocomposite TCP-collagen -treated group and HA group (1). On day 30, this score for all three groups was 1.2 and by day 45 of the healing process, the highest score belonged to the nanocomposite TCP-collagen-treated group and the HA-treated group (1), followed by the control group (0.8) (). Among the days on which the samples were obtained average scores of bone marrow index shows no significant difference in groups during this study (p < .05) (). Average scores bone marrow on days 15, 30, and 45 were approximately equal in all three groups.
Table 5. Descriptive statistics of bone marrow index.
Table 6. Compare average scores of bone marrow index.
Histopathological evaluation results
Histopathological evaluation of the healing site was performed on the days 15, 30, and 45 of the healing process. On the day 15, numerous capillary buds were present at the healing site of the control group. The healing site of the HA-treated group demonstrated a fibrous tissue while the healing site from the nanocomposite TCP-collagen-treated group showed a primary bone formation. Samples from the control group on day 30 revealed that the marrow space was filled by a fibrous tissue samples from the HA-treated group demonstrated few primary bone formation and in samples from the nanocomposite TCP-collagen-treated group woven bones were visible. Microscopically, the healing site of the control group on day 45 of healing showed bone deposition within cartilage and primary ossification. The healing site of the HA-treated group on day 45 filling of the defect by primary bone was evident. While the healing site of nanocomposite TCP-collagen-treated group on day 45 of healing showed that the defect is filled with lamellar bones. Advanced stages of remodelling and consolidation and development of haversian system is seen in repaired tissues. The quantity of newly formed lamellar bone in the healing site of nanocomposite TCP-collagen-treated is more than that of the HA-treated group and the control group. The histopathological evaluation of necropsy tissues are illustrated in .
Figure 1. Microscopic section from the healing site of sham group on day 15 of healing shows abundant capillary buds (arrows) in granulation tissue (H&E, ×100).
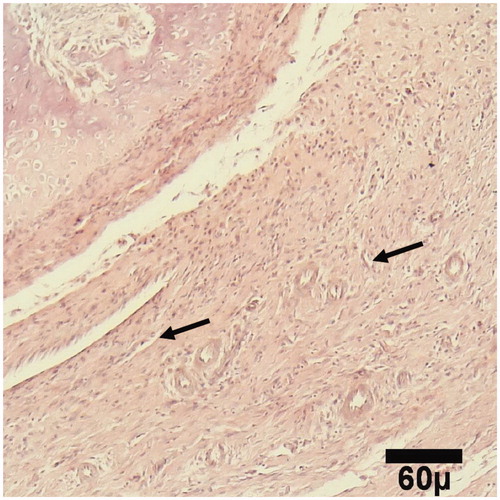
Figure 2. Microscopic section from the healing site of HA-treated group on day 15 of healing shows a fibrous tissue (arrow) (H&E, ×100).
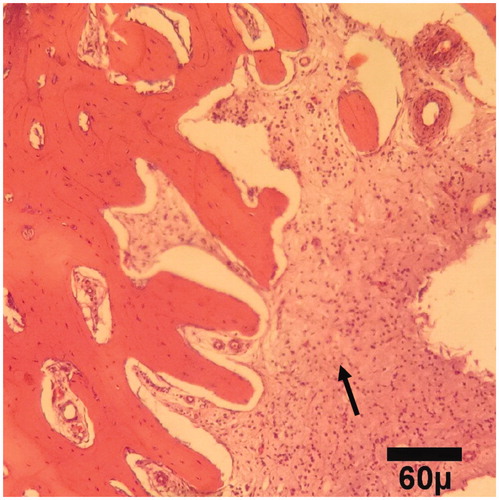
Figure 3. Microscopic section from the healing site of TCP-collagen-treated group on day 15 of healing shows the primary bone (arrow) formation (H&E, ×100).
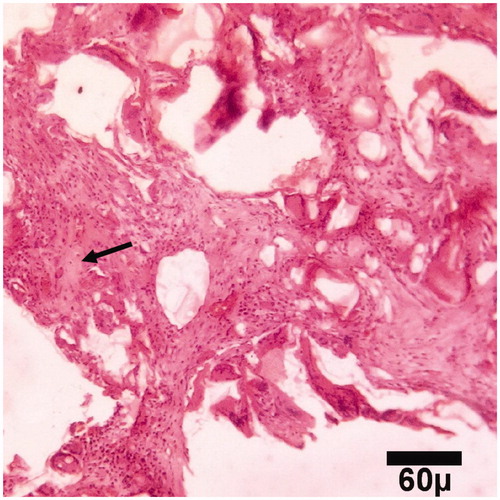
Figure 4. Microscopic section from the healing site of sham group on day 30 of healing shows abundant fibrous tissue (arrows) filled marrow space (trichrom ×100).
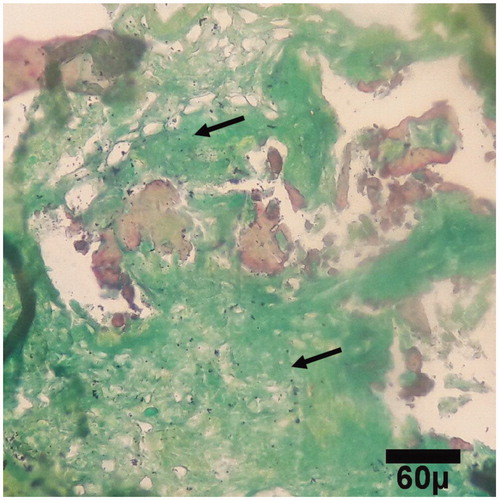
Figure 5. Microscopic section from the healing site of HA-treated group on day 30 of healing. A few primary bones (arrow) are being to produce (trichrom ×100).
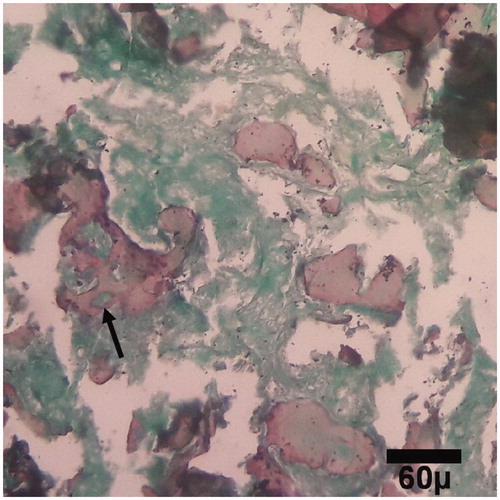
Figure 6. Microscopic section from the healing site of TCP-collagen-treated group on day 30 of healing. Woven bones (arrows) are being to produce (trichrom ×100).
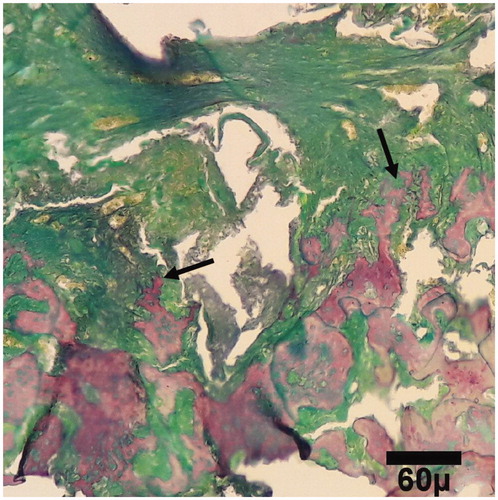
Figure 7. Microscopic section from the healing site of sham group on day 45 of healing. Bone deposition within cartilage (star) in healing site shows primary (arrows) ossification (trichrom ×100).
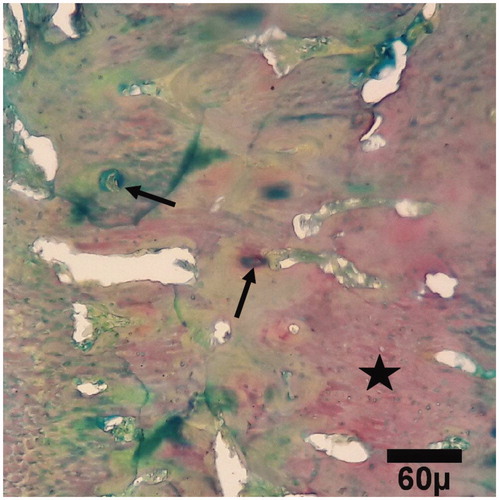
Figure 8. Microscopic section from the healing site of HA-treated group on day 45 of healing. The defect is filled with primary (arrows) bones (trichrom ×100).
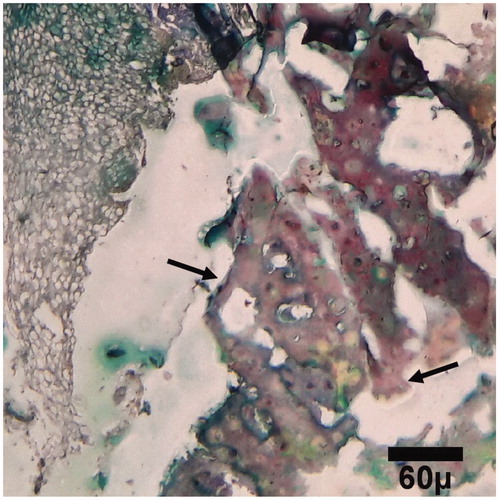
Figure 9. Microscopic section from the healing site of TCP-collagen-treated group on day 45 of healing. The defect is filled with lamellar (arrows) bones (trichrom ×100).
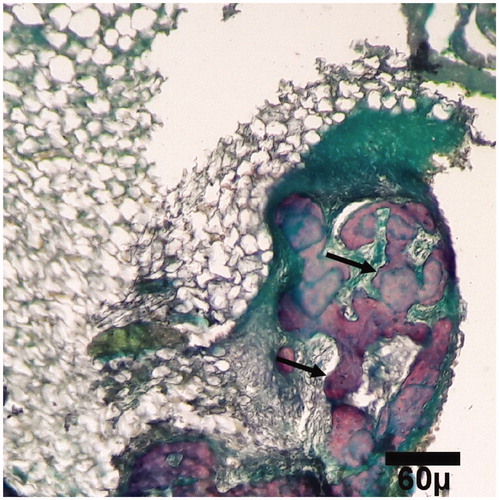
Histopathological evaluation was performed on the days 15, 30, and 45 after surgery. On these days, the quantity and rate of bone formation in the healing site in nanocomposite TCP/collagen group was better than HA and control groups and the quantity of newly formed lamellar bone in the healing site in the nanocomposite TCP/collagen group was more than that of the HA and control groups.
Discussion
Today, many researchers are trying to find materials that can improve the process of bone healing. Bone has a remarkable regenerative ability but a considerable amount of bone loss or development of an adverse microenvironment can hinder this capacity, such as in cases of severe trauma, developmental deformities, revision surgeries, and tumor resection [Citation13,Citation14]. Bone tissue engineering holds the promise of great therapeutic potential [Citation15]. This study aimed to evaluate the positive effects of nanocomposite TCP/collagen scaffold in comparison with hydroxyapatite scaffold in bone healing of the femoral defect in rabbits. In this study, it seems that on days 15, 30, and 45 post-surgery, the quantity and the velocity of bone formation in the healing site of the nanocomposite TCP/collagen group were better than HA and control groups. Additionally, it appears that the quantity of newly formed lamellar bone in the healing site of the nanocomposite TCP/collagen group was more than that of the HA and control groups after 45 d.
Ceramics such as hydroxyapatite (HA), calcium phosphates, and bioactive glasses have been attracted special attention, due to their excellent biocompatibility along with their osteoconductive and osteoinductive properties [Citation16]. Depending on their composition, particle size, and production process, ceramics can have various degrees of bioactivity, which is the ability to chemically bond and be integrated into living bone through the formation of HA [Citation17]. However, these materials are brittle and have low mechanical stability, making them unsuitable for load bearing applications [Citation18].
On one hand, calcium phosphate is among newer generations of bone substitutes, with potential clinical applications in orthopaedics. Calcium phosphate granules have become materials of choice for bone repair, because of their biocompatibility and osteoconductivity [Citation19]. On the other hand calcium phosphate cement (CPC) is an excellent bone-grafting material, with good biocompatibility and a high modelling capacity to fill any bone-defect shape without leaving a gap [Citation20].
On one hand, collagen is the main fibrous structural protein in the bodies of living organisms, and a collagen scaffold is beneficial to cells grown in vivo [Citation21]. On the other hand, collagen-based scaffolds have been proven to possess excellent biocompatibility and sufficient mechanical properties and have gained great achievements in tissue engineering [Citation22].
Design of a well-functioning “constructions” for tissue engineering requires to provide suitable conditions for cell’s attachment, proliferation, and organization, as well as to assure nutrition of the cells and removal of products of their metabolism. Nanocomposite scaffolds containing bioactive nanoparticles may be osteoconductive. Surface properties of nanocomposite scaffolds are a key factor in governing the success of the engineered tissue, since the first interactions between the cells and the substrate are protein adsorption and then cell adhesion Nanocomposite scaffolds containing bioactive nanoparticles may be osteoconductive [Citation23,Citation24].
Nanotechnological approaches have a great potential for medical applications. In particular, the development of electrospinning is very important for health care applications since it is a relatively quick, simple and cost-effective method for producing nanostructured materials desirable for many biomedical applications such as tissue engineering [Citation25,Citation26].
However, most of the studies under the in vitro settings which used TCP and collagen scaffolds in bone healing have showed success.
Inzana et al. [Citation27] fabricated collagen-calcium phosphate scaffolds using low temperature 3 D printing. They maximized the cytocompatibility and mechanical strength of scaffolds by tailoring a certain concentration of phosphoric acid-based blind solution. Then, the scaffolds were implanted into a critically sized murine femoral defect for 9 weeks. Results indicated that the scaffolds were osteoconductive, and the scaffolds were partly broken down with new bone forming [Citation27].
Komaki et al. [Citation28] showed that segmental bone defects were healed with cortical bone 12 weeks after implantation of the complex of β-TCP granules and 5% collagen with rhFGF-2. They hypothesized that the resorption of β-TCP is important for bone formation [Citation28].
Tomonori et al. [Citation29] developed a biodegradable sponge composite for bone tissue engineering by combining βTCP and collagen. In addition, they sought to determine the optimal βTCP granules/collagen ratio by evaluating and bone formation in vivo. Histological evaluation at 4 weeks after implantation revealed that the collagen sponge had degraded and newly formed bone was present on the surface of the B’TCP granules. At 12 weeks, the βTCP granules were completely degraded and remodelling of the lamellar bone was observed [Citation29]. Some researchers have combined nano-sized HA with synthetic biodegradable polymers to produce nanocomposites for bone tissue engineering [Citation30–33].
Ishaug et al. [Citation34] suggested that scaffold materials used for bone formation should meet the following criteria: first, the scaffold material must allow osteoblast attachment, since these anchorage-dependent cells require a supportive matrix to survive. Second, the scaffold must provide an appropriate environment for osteoblast proliferation and function. Third, the scaffold should allow ingrowth of vascular tissue to ensure the survival of transplanted cells. Fourth, the scaffold material should be biodegradable and its degradation products should be easily metabolized and excreted. Finally, the scaffold material should be able to be processed into irregular three-dimensional shapes. The results of the present study indicated that nanocomposite TCP/collagen satisfied all the aforementioned tissue engineering scaffold criteria for bone regeneration [Citation34]. Our results confirm that TCP/collagen nanocomposite has an important role in the reconstruction of bone defects and can be used as scaffold in bone fractures.
Conclusions
The purpose of this study was to review the current state and challenges towards developing bioactive and biodegradable nanocomposite TCP/collagen, while highlighting the promising steps taken to improve the mechanical and biological properties for application in bone regeneration. Due to rapid advances made in the field, it was not possible to include all aspects of the work. However, every effort was made to ensure that seminal works and significant research findings are included, with minimal bias. The need for bone graft materials has led to the synthesis of various materials with different properties. In this study, various attempts have been made to exploit the novel properties of TCP/collagen nanocomposite scaffold for orthopaedic applications. In conclusion, it seems that TCP/collagen nanocomposite has an important role in the reconstruction of bone defects and can be used as scaffold in bone fractures. Nanocomposite TCP/collagen granules exhibited a reproducible bone-healing potential. It seems that TCP/collagen nanocomposite has an important role in the reconstruction of bone defects and can be used as a scaffold in bone fractures.
Acknowledgements
This work was supported by Islamic Azad University, Faculty of Specialized Veterinary Sciences, Tehran, Iran, and Laboratory of Animal Experimentation.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Kim BS, Park IK, Hoshiba T, et al. Design of artificial extracellular matrices for tissue engineering. Prog Polym Sci. 2011;36:238–268.
- Qingchun Z, Ke T, Zhaoyang Y, et al. Preparation of open porous polycaprolactone microspheres and their applications as effective cell carriers in hydrogel system. Mater Sci Eng C. 2012;32:2589–2595.
- Salerno A, Zeppetelli S, Di Maio E, et al. Architecture and properties of bi-modal porous scaffoldsfor bone regeneration prepared via supercritical CO2 foaming and porogen leaching combined process. J Supercrit Fluids*. 2012;67:114–122.
- Beaman FD, Bancroft LW, Peterson JJ, et al. Bone graft materials and synthetic substitutes. Radiol Clin North Am. 2006;44:451–461.
- Ng A, Saim AB, Tan KK, et al. Comparison of bioengineered human bone construct from four sources of osteogenic cells. J Orthop Sci. 2005;10:192–199.
- Tan KK, Shamsui BS, Chua KH, et al. Bone graft substitute hydroxyapatite scaffold seeded with tissue engineered autologous osteoprogenitor cells in spinal fusion: early result in sheep as a model. Med J Malaysia. 2005;60:53–58.
- Gelse K, Pöschl E, Aigner T. Collagens – structure, function, and biosynthesis. Adv Drug Deliv Rev. 2003;55:1531–1546.
- Burgeson RE, Nimni ME. Collagen types. Molecular structure and tissue distribution. Clin Orthop Relat Res. 1992;282:250–272.
- Chevallay B, Herbage D. Collagen-based biomaterials as 3D scaffold for cell cultures: applications for tissue engineering and gene therapy. Med Biol Eng Comput. 2000;38:211–218.
- Wolf K, Alexander S, Schacht V, et al. Collagen-based cell migration models in vitro and in vivo. Semin Cell Dev Biol. 2009;20:931–941.
- Hule RA, Pochan DJ. Polymer nanocomposites for biomedical applications. MRS Bull. 2007;32:354–358.
- Winey KI, Vaia RA. Polymer nanocomposites. MRS Bull. 2007;32:314–322.
- Perka C, Schultz O, Spitzer RS, et al. Segmental bone repair by tissue-engineered periosteal cell transplants with bioresorbable fleece and fibrin scaffolds in rabbits. Biomaterials. 2000;21:1145–1153.
- Gugala Z, Gogolewski S. Healing of critical-size segmental bone defects in the sheep tibiae using bioresorbable polylactide membranes. Injury. 2002;33:71–76.
- Cook SD, Salkeld SL, Brinker MR, et al. Use of an osteoinductive biomaterial (rHOP-1) in healing large segmental bone defects. J Orthop Trauma. 1998;12:407–412.
- Schumacher M, Uhl F, Detsch R, et al. Static and dynamic cultivation of bone marrow stromal cells on biphasic calcium phosphate scaffolds derived from an indirect rapid prototyping technique. J Mater Sci Mater Med. 2010;21:3039–3048.
- Yun HS, Park JW, Kim SH, et al. Effect of the pore structure of bioactive glass balls on biocompatibility in vitro and in vivo. Acta Biomater. 2011;7:2651–2660.
- Bhakta S, Pattanayak D, Takadama H, et al. Prediction of osteoconductive activity of modified potassium fluorrichterite glass-ceramics by immersion in simulated body fluid. J Mater Sci Mater Med. 2010;21:2979–2988.
- Komath M, Varma HK, Sivakumar R. On the development of an apatitic calcium phosphate bone cement. Bull Mater Sci. 2000;23:135–140.
- Bohner M, Baroud G. Injectability of calcium phosphate pastes. Biomaterials. 2005;26:1553–1563.
- Zuber M, Zia F, Zia KM, et al. Collagen based polyurethanes—a review of recent advances and perspective. Int J Biol Macromol. 2015;80:366–374.
- Liu S, Wu J, Liu X, et al. Osteochondral regeneration using an oriented nanofiber yarn-collagen type I/hyaluronate hybrid/TCP biphasic scaffold. J Biomed Mater Res. 2015;103:581–592.
- Vitte J, Benoliel AM, Pierres A, et al. Is there a predictable relationship between surface physical-chemical properties and cell behaviour at the interface? eCM. 2004;7:52–63.
- Stodolak-Zych E, Fraczek-Szczypta A, Wiechec A, et al. Nanocomposite polymer scaffolds for bone tissue regeneration. Acta Phys Pol A. 2012;121:518–521.
- Linh NT, Min YK, Song HY, et al. Fabrication of polyvinyl alcohol/gelatin nanofiber composites and evaluation of their material properties. J Biomed Mater Res. 2010;95:184–191.
- Li C, Vepari C, Jin HJ, et al. Electrospun silk-BMP-2 scaffolds for bone tissue engineering. Biomaterials. 2006;27:3115–3124.
- Inzana JA, Olvera D, Fuller SM, et al. 3D printing of composite calcium phosphate and collagen scaffolds for bone regeneration. Biomaterials. 2014;35:4026–4034.
- Komaki H, Tanaka T, Chazono M, et al. Repair of segmental bone defects in rabbit tibiae using a complex of beta-tricalcium phosphate, type I collagen, and fibroblast growth factor-2. Biomaterials. 2006;27:5118–5126.
- Tomonori M, Tatsuo N, Koh-ichi K, et al. Development of P-tricalcium phosphate/collagen sponge composite for bone regeneration. Dent Mater J. 2006;25:1138–1144.
- Boissard CI, Bourban PE, Tami AE, et al. Nanohydroxyapatite/poly(ester urethane) scaffold for bone tissue engineering. Acta Biomater. 2009;5:3316–3327.
- Jack KS, Velayudhan S, Luckman P, et al. The fabrication and characterization of biodegradable HA/PHBV nanoparticle-polymer composite scaffolds. Acta Biomater. 2009;5:2657–2667.
- Jayabalan M, Shalumon KT, Mitha MK, et al. Effect of hydroxyapatite on the biodegradation and biomechanical stability of polyester nanocomposites for orthopaedic applications. Acta Biomater. 2010;6:763–775.
- Asran ASH, Henning S, Michler GH. Polyvinyl alcohol-collagen-hydroxyapatite biocomposite nanofibrous scaffold: mimicking the key features of natural bone at the nanoscale level. Polymer. 2010;51:868–876.
- Ishaug SL, Crane GM, Miller MJ, et al. Bone formation by three dimensional stromal osteoblasts eulture in biodegradable polymer scaffolds. J Biomed Mater Res. 1997;36:17–28.
