Abstract
Objective: Nanoemulsion of silymarin was developed and optimized.
Materials and methods: Nanoemulsion was made by aqueous titration method. Sefsol 218 (5.8% v/v), Kolliphor RH40 and polyethylene glycol 400 (Smix; 2:1; 28.99% v/v) were used as oil phase, surfactant and co-surfactant while distilled water (65.22% v/v) acted as an aqueous phase. Nanoemulsion was characterized on the basis of particle size, viscosity, electrical conductivity and refractive index. Further, in vitro release, in vivo pharmacokinetic study, stability study and cancer cell line studies were also performed.
Results and discussion: The optimized formulation (NE9) with mean particle size of 21.24 nm showed a minimum viscosity of 9.59 cps, maximum drug release (97.75%) in 24 h. The NE9 formulation also showed higher AUC (p < .01) and Cmax (p < .01) and shorter Tmax (p < .05) compared with conventional and standard suspensions of silymarin. The stability study also showed considerably stable formulations at refrigerator temperature as compared with room temperature (p > .05). The cancer cell line studies also confirmed that silymarin nanoemulsion reduced the cell viability and increased ROS intensity and chromatin condensation (p < .05).
Conclusion: Our results concluded that nanoemulsion may be an efficient carrier for oral delivery of silymarin against human hepatocellular carcinoma without damaging normal cells.
Introduction
Silymarin is obtained from the purified extract of seeds and fruits of Silybum marianum (commonly known as milk thistle plant). It has been used for centuries as an herbal medicine and food supplement for the treatment of liver and gallbladder disorders, including hepatitis, cirrhosis, jaundice, and to protect the liver against poisoning from chemical and environmental toxins, including snake bites, insect stings, mushroom poisoning, and alcohol. Most of the medicinal compounds found in milk thistle are present in high concentrations in the seeds. These compounds include silymarin, which is composed of three isomer flavonolignans: silybin, silydianin, and silychristin [Citation1]. Silybin is considered the major and most active component of silymarin [Citation2,Citation3]. It inhibit the hepatotoxin binding to receptor sites on the hepatocyte membrane; reduction of glutathione oxidation to enhance its level in the liver and intestine; antioxidant activity; and stimulation of ribosomal RNA polymerase and subsequent protein synthesis, leading to enhanced hepatocyte regeneration [Citation4]. However, despite such potential benefits, silymarin is able to produce little effect in vivo both in humans and in animals due to its poor bioavailability. The four major causes of limited silymarin bioavailability are extensive phase II metabolism, low permeability across intestinal epithelial cells, low aqueous solubility, and rapid excretion in bile and urine. These factors necessitate the incorporation of silymarin into a form that can augment its bioavailability [Citation5].
A number of methods including some of the nano-based approaches have been used previously to enhance the oral bioavailability of silymarin and provide them a robust strength against physical, chemical and environmental degradation. Nanostructured biomaterials, featuring a nanoscale morphology and size, exhibit a wide range of advantages over the conventional biomaterials, such as high bioavailability, improved cellular interaction, and specific designed functions [Citation6,Citation7]. It offers a promising solution to many difficulties in drug delivery and tissue engineering [Citation8], a nano-sized drug vehicle has made significant progress in the delivery of conventionally undeliverable molecules, such as compounds with low water solubility and genetic biomolecules [Citation9,Citation10]. The newer formulation design approaches for bioavailability enhancement includes incorporation of the active component into inert lipid vehicles [Citation11], such as oils [Citation12], surfactant dispersions [Citation13–15], self-emulsifying formulations [Citation16,17], emulsions [Citation18–21], micro or nanoemulsions [Citation22–24], and liposomes [Citation25]. Nanoemulsion offers several advantages over these drug delivery systems like higher solubilisation capacity, rapid onset of action (no extra time for dispersion), reduced intersubject variability in terms of gastrointestinal fluid volume and longer shelf life [Citation26], and toxicological safety, a high content of the lipid phase and the possibility of large-scale production by high-pressure homogenization [Citation27].
The aim of the present investigation was to formulate and optimize a stable nanoformulation of silymarin by aqueous titration method. The prepared nanoemulsion was characterized and evaluated on the basis of size, surface morphology, viscosity, conductivity, refractive index, and in vitro drug release study. The pharmacokinetic parameters also showed considerable values than conventional and standard suspensions of silymarin. The optimized silymarin nanoemulsion was also assessing against human liver carcinoma cells.
Materials and methods
Materials used
Silymarin was purchased from Sigma Aldrich (St. Louis, MO), Sefsol 218 and Kolliphor RH40 were obtained as a gift sample from Nikko Chemicals (Tokyo, Japan) and BASF (Mumbai, India), PEG 400 was purchased from SD Fine Chem. (Mumbai, India). Human hepatocellular carcinoma HepG2 and Chang liver cell line was obtained from cell repository-National Centre for Cell Sciences, Pune, India. All other chemicals were of analytical grade and were used as and when required.
Nanoformulation development and its characterization
Solubility studies in various oils, construction of pseudo ternary phase diagram and thermodynamic stability tests were performed so that a stable and robust formulation was developed.
Solubility studies for screening of oil phase
An important aspect for the selection of oils for nanoemulsion formulation is the solubility of silymarin in different oils. For this study, three oils, Sefsol 218, Triacetin, and Isopropyl meristate, were selected. An excess amount of drug was added in 2 ml of each oil separately in 5 ml capacity stoppered vials, and mixed using a vortex mixer [Citation28]. These vials were then kept at 25 ± 1.0 °C in an isothermal shaker (IKA® KS 400i, Staufen, Germany) for 72 h to reach equilibrium. The equilibrated vials were removed from shaker and centrifuged at 10,000 rpm for 15 min using centrifuge (REMI, Mumbai, India). The supernatant was taken and filtered through a 0.45-μm membrane filter. The concentration of silymarin was determined in different oils by using HPTLC spectrophotometer at wavelength of 288 nm.
Pseudo ternary phase diagram study
Construction of pseudo ternary phase diagrams was done by in situ emulsification method (aqueous titration method) using three components i.e. oil, Smix (surfactant-co-surfactant mixture) and distilled water [Citation29]. Surfactant and co-surfactant were mixed in different volume ratios in stock of 50 ml to obtain best result. For each phase diagram, oil and a specific Smix ratio was mixed properly in different volume ratios from 1:9 to 9:1 in separate glass vials. Sixteen different combinations of oil and Smix (1:1, 1:2, 1:3, 1:4, 1:5, 1:6, 1:7, 1:8, 1:9, 2:1, 3:1, 4:1, 5:1, 6:1, 7:1, 8:1, 9:1) were slowly titrated with aqueous phase and visually inspected for transparency and flow ability [Citation22]. The physical state of the nanoemulsion was marked on the phase diagrams with three axes representing an aqueous phase, oil phase, and Smix phase. For each phase diagram, nanoemulsion area was plotted and the wider region indicated the better self-nanoemulsifying efficiency. From each phase diagram constructed, different formulations were selected from nanoemulsion region varying the proportion of oil (10–30%v/v) at a minimum concentration of Smix. Selected formulations were subjected to stability and dispersibility tests.
Thermodynamic stability tests
Selected formulations were subjected to thermodynamic stability stress tests as heating cooling cycle, centrifugation, and freeze–thaw cycle:
Heating–cooling cycle: in this study, the prepared formulations were kept at 45 °C and at 0 °C temperature for not less than 48 h for each temperature cycle.
Centrifugation tests: formulations were centrifuged (REMI, Mumbai, India) at 5000 rpm for 30 min and observed for phase separation, creaming, or cracking.
Freeze–thaw cycle: the prepared formulations were exposed at two different temperatures i.e. −20 °C and 20 °C for each temperature cycles not than 24 h. For the better estimation of accelerated stability studies, three such cycles were run for each batch of formulation. The formulations which showed the maximum stability were selected for further study [Citation30].
Dispersibility tests
One millilitre of each formulation was added to 500 ml of 0.1 N HCl and in distilled water in a USP Dissolution apparatus Type II at 37 ± 0.5 °C to assess its efficiency of self-emulsification. A standard stainless steel dissolution paddle rotating at 75 rpm provided gentle agitation. The formulation was visually assessed using the following grading system:
Grade A: Forming (within 1 min) nanoemulsion, having a clear appearance.
Grade B: Rapidly forming, slightly less clear emulsion.
Grade C: Fine milky emulsion formed within 2 min.
Grade D: Dull white emulsion having slightly oily appearance that is slow to emulsify (longer than 2 min).
Grade E: Formulation, exhibiting either poor or minimal emulsification with large oil globules present on the surface. Among the formulations which passed the stability and also dispersibility tests in Grades A and B were selected for preparing drug-loaded batches utilizing minimum concentration of Smix for each percentage of oil.
Preparation of silymarin-loaded nanoemulsions (SLN) by aqueous titration method
The SLN were prepared by dissolving 20 mg of silymarin in oil (10%, 15%, 20%, and 25% v/v). Respective Smix ratio was added to the oil, mixed using vortex mixer, and followed by the addition of aqueous phase to obtain nanoemulsion.
Physicochemical characterization and evaluation of SLN
Visual observation
Visual observation was done to differentiate between SLN and macroemulsion.
Dynamic light scattering (DLS) measurement
The average droplet size and polydispersity index (PDI) of SLN were determined by DLS technique that analyzes the fluctuations in light scattering due to Brownian motion of the particles using a zetasizer ZS 90 (Malvern instruments, Worcestershire, UK). Light scattering was monitored at 25 °C at a 90° angle. Samples were diluted with distilled water and filtered through 0.45 μm membrane filter and then were directly placed into the module [Citation30].
Viscosity determination
Brookfield DV III ultra V6.0 RV cone and plate rheometer (Brookfield Engineering Laboratories Inc. Middleboro, MA) with spindle # CPE40 at 25 ± 0.5 °C was used for the determination of viscosity of the SLN.
Electro conductivity studies
The conductivity (σ) of SLN was determined by using conductometer, CDM 230 (Radiometer, Copenhagen, Denmark). The reading was taken at the frequency of 94 Hz, having a cell constant of 0.11 cm−1. The measurements were performed at 25 ± 1 °C.
Refractive index and percent transmittance
The refractive index of the SLN was measured by an Abbe refractometer (Bausch and Lomb optical Company, Garden City, NY) by placing one drop of nanoemulsion on the slide at 25 °C. The percent transmittance of the system was measured at 288 nm using HPTLC spectrophotometer [Citation31].
Transmission electron microscopy (TEM)
Surface morphology of SLN was studied by TEM TOPCON 002B (Topcon, Oakland, NJ) [Citation30]. A drop of nanoemulsion was diluted with distilled water (1:100), filtered (0.22 μm), and applied on carbon coated grid with 2% phosphotungestic acid and kept it for 30 s. The dried coated grid was taken on a slide and covered with a cover slip. The slide was observed under the light microscope operating at 200 KV.
In vitro drug release study
In vitro release test was performed in 900 ml of simulated intestinal fluid using dissolution apparatus # 2, at 50 rpm and 37 ± 0.5 °C (Hanson Research SR8 plus, Chatsworth, CA). One millilitre of silymarin nanoemulsion formulation was placed in treated dialysis bag (MWCO 1200 g/mole, Sigma Aldrich, St. Louis, MO). One millilitre of samples was withdrawn at regular time intervals (0, 0.5, 1, 1.5, 2, 2.5, 3, 4, 5, 6, 8, and 24 h) and aliquot amount of simulated intestinal fluid was replaced [Citation32]. The samples were analyzed for the drug content by HPTLC spectrophotometer at 288 nm [Citation31]. The release of the drug from nanoemulsion formulations was compared with the available Limarin® suspension.
Stability studies
The optimized SLN formulations were subjected to accelerated stability studies at 25 ± 2 °C/60 ± 5% RH, 40 ± 2 °C/65 ± 5% RH and 60 ± 2 °C/75 ± 5% RH. The humidity and temperature control stability chambers (Thermolab, Mumbai, India) were used wherein samples were placed in glass bottles and observed at specified time intervals of 0, 30, 60, and 90 d. The droplet size, polydispersity index, refractive index, viscosity, and percent transmittance were determined [Citation32].
In vivo pharmacokinetic study
Animal study protocol
In vivo pharmacokinetic study was carried out after obtaining approval from Integral University, Institutional Animal Ethics Committee, Lucknow, Uttar Pradesh (Registration no. IU/Pharm/Ph.D./CPCSEA/15/06) and CPCSEA guidelines were followed for the whole study. The in vivo study was made to accomplish oral administration of SLN formulations in male Wistar rats (150–180 g). There were six rats in each group. The animals were anesthetized by diethyl ether. The blood samples were withdrawn from the tail vein at 0 (pre-dose), 0.5, 1, 2, 3, 4, 6, 8, 24, and 48 h, centrifuged at 5000 rpm for 20 min in centrifuge tubes restraining 8 mg of EDTA as an anticoagulant. The plasma was separated, stored at −20 °C and analysed by HPTLC method [Citation31]. A treatment schedule is specified in .
Table 1. Treatment schedule of silymarin formulations in male Wistar rats.
Cell line and culture
Human hepatocellular carcinoma HepG2 cell line and Chang liver cells (non-tumor cells) were obtained from cell repository – National Centre for Cell Sciences, Pune, India. Both cell lines were maintained in Eagle’s MEM with 2.0 mM l-glutamine, 1.5 g/l NaHCO3, antibiotic solution (100 U/ml penicillin, and 100 μg/ml streptomycin) and supplemented with 10% (v/v) foetal bovine serum. Cells were grown at 37 °C, 5% CO2 humidified atmosphere.
Cell viability assay
This assay was used to detect the cell viability of silymarin nanoemulsion using following protocol [Citation33]. Approximately 1 × 104 cells/well of HepG2 were seeded in 100 μl complete culture medium in 96-well culture plate and incubated overnight in humidified air. Stock was prepared in phosphate buffer saline (PBS) and diluted into culture medium to the desired concentrations 0.2, 0.5, 1, 2.5, and 5 μg/ml added to the wells. After 24 h of incubation period, 10 μl of MTT (5 mg/ml in PBS) reagent was added and re-incubated at 37 °C until purple formazan crystals developed. Formazan blue crystals were dissolved in 100 μl of DMSO and read at 540 nm using microplate ELISA reader (BIORAD 680, Hercules, CA). The plot of percent cell viability versus SLN concentrations was used to calculate the concentration lethal to 50% of the cells (IC50). The cellular morphological changes were observed under inverted phase contrast microscopy (Nikon ECLIPSE Ti-S, Tokyo, Japan). Similar test of nanoformulation was also performed on Chang liver cells to examine if the treatment had a distinguishable effect between normal and cancer cells.
Intracellular reactive oxygen species (ROS) activity
Intracellular ROS generation was analyzed by using fluorescence microscopic imaging technique as per previous protocol [Citation34]. Cells (1 × 104 per well) were exposed at three effective concentrations i.e. 0.5, 1 and 2.5 μg/ml of silymarin nanoemulsion for 12 h. Subsequently, cells were incubated with DCFH-DA (10 mM) at 37 °C for 30 min and washed with PBS. Intracellular fluorescence intensity of cells was visualized by inverted fluorescent microscope (Nikon ECLIPSE Ti-S, Tokyo, Japan). For quantitative fluorometric analysis, cells (1 × 104 per well) were seeded and treated with SLN in 96-well black bottom culture plate. Fluorescence intensity was measured with a multiwell microplate reader (Synergy H1 Hybrid Multi-Mode Microplate Reader, BioTek, Winooski, VT) at an excitation wavelength of 485 nm and at an emission wavelength of 528 nm. Data were expressed as percentage of fluorescence intensity relative to the control wells.
Apoptotic effect of SLN using DAPI stain
Florescent nuclear dye DAPI was used to analyze the apoptotic effect of SLN as per previous method [Citation35]. HepG2 cells (1 × 105 cells per well) were seeded in 24-well culture plate overnight and treated with silymarin nanoemulsion for 24 h. Following incubation period, cells were washed and fixed in 4% paraformaldehyde for 15 min followed by permeabilization with permeabilizing buffer (3% paraformaldehyde and 0.5% Triton X-100) for 10 min. After staining with DAPI dye (50 μg/ml), images of condensed nuclei undergoing apoptosis were captured with an inverted fluorescent microscope (Nikon ECLIPSE Ti-S, Tokyo, Japan). Apoptosis was quantitated by morphological changes of nuclei wherein about 500 cells/well representing one sample.
Statistical analysis
The results were expressed as mean values ± SD The analysis of variance (ANOVA) was applied to examine the significance of differences in SLN properties (such as droplet size, polydispersity index, percent transmittance, refractive index, viscosity, conductivity, and drug content). In all cases, p < .05 was considered to be significant.
Result and discussion
Solubility studies
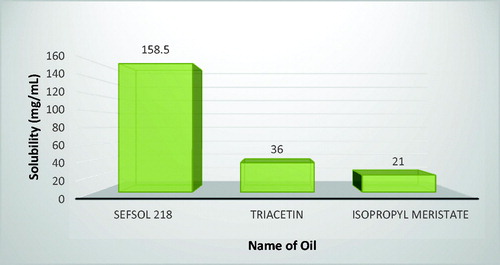
Solubility studies were aimed at identifying a suitable oil phase for the development of silymarin nanoemulsion to achieve optimum drug loading. The higher solubility of the drug in the oil phase is important for the nanoemulsion to maintain the drug in the solubilized form. In the oil phase tested, the solubility of silymarin was found to be the highest in sefsol 218 (158.5 mg/ml) followed by triacetin (36 mg/ml) and iso-propyl meristate (21 mg/ml) (). Thus, sefsol 218 was selected as the oil phase for the development of the formulation.
Pseudo ternary phase diagram study
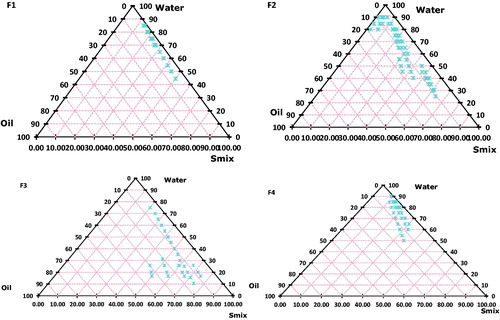
Phase diagram study of SLN containing sefsol 218 as the oil phase and kolliphor RH40 as the surfactant showed the low amount of oil (5.56% v/v) could be solubilized at higher surfactant concentration (F1). It yielded a narrow range for nanoemulsion formation. Generally, surfactant alone cannot lower the oil interfacial tension sufficiently to yield a nanoemulsion; this necessitates the addition of an amphiphilic molecule or co-surfactant to lower the surface tension close to zero. Co-surfactants penetrate into the surfactant monolayer, providing additional fluidity to the interfacial film and thus disrupting the liquid crystalline phases which are formed when the surfactant film is too rigid. This could be attributed to the fact that transient negative interfacial tension and fluid interfacial film is rarely achieved by the use of single surfactant, usually necessitating the addition of a cosurfactant. SLN containing sefsol 218 as the oil phase, kolliphor RH40 as the surfactant, and PEG 400 as the co-surfactant showed the formation of broader nanoemulsion region (F2). Equal Smix ratio (1:1) was used to obtain this region. Nanoemulsions were transparent with slight bluish tint and a maximum amount of oil was solubilized in this region (18.8% v/v). It may be attributed to low interfacial tension between oil and water phases since maximum amount of oil and surfactant were solubilized in this area. Moreover, a combination of a surfactant and co-surfactant in optimal concentration leads to enormous solubilization of oil phase. The presence of PEG 400 as co-surfactant decreases the bending stress of interface and makes the interfacial film sufficiently flexible to take up different curvatures required to form nanoemulsion over a wide range of compositions. Results of F2 phase diagram depict larger nanoemulsion formation in the surfactant-rich region, but this region was comparatively lower than results obtained from F3 (Smix, 2:1) diagram. Therefore, low amount of oil was solubilized in this area; nanoemulsion region was confined to borders along the surfactant region. This may be due to an increase in the concentration of kolliphor RH40 in the system. Phase diagram obtained from F4 indicates nanoemulsion formation in the aqueous-rich region. Smix ratio used for this system was 1:2 and due to increased concentration of PEG 400 in the system interfacial tension seems to have been reduced much lower as compared with other systems.
Thus, the phase diagram study () provides valuable information on the role and concentration of surfactants, co-surfactants, and water in formulation of nanoemulsion. It is observed from the diagram that an optimum concentration of Smix ratio (2:1) provided better results.
Thermodynamic stability tests
After optimization of nanoemulsion region, the prepared formulations were subjected to thermodynamic stability tests. Nanoemulsions remain to be stable at stressed conditions. Three tests, heating/cooling cycle, centrifugation, and freeze–thaw cycles, were performed to evaluate the stability of the formulations. Observations made during the tests are given in .
Table 2. Thermodynamic stability tests of prepared nanoformulation.
Preparation of SLN
The optimized formulations that passed all the tests and possessed least concentrations of surfactants were used for incorporating silymarin.
Characterization and evaluation of nanoemulsion
Selected SLN formulations were characterized and evaluated by following parameters.
Visual appearance
SLN was clear and transparent and free from any turbidity. This test was done to differentiate it from macroemulsion which is milky in appearance.
Dynamic light scattering (DLS) measurement
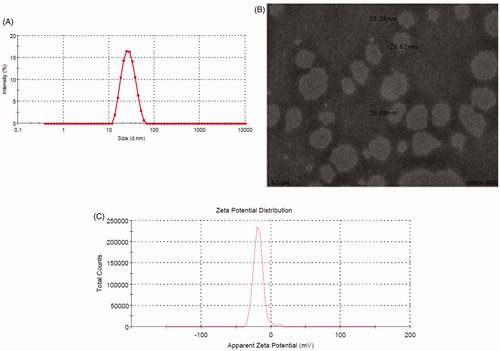
DLS technique was employed to measure the particle size distribution, polydispersity index, and zeta potential (). Formulation NE9 showed the smallest particle size (21.24 ± 0.291 nm) followed by NE3 (29.34 ± 0.634), NE4 (79.02 ± 1.651), and NE14 (31.31 ± 0.298). Polydispersity index of formulations NE3, NE4, NE9, and NE14 were found to be 0.191 ± 0.008, 0.449 ± 0.022, 0.104 ± 0.016, and 0.168 ± 0.022, respectively (). Lower value of polydispersity index indicates that nanoformulations were uniform in their droplet size. The selected combination of oil, Smix and water was able to produce small and stable droplets on nanoscale and the results of particle size and polydispersity index produced from selected composition of formulation was much better than results reported earlier [Citation36–38]. Therefore, the results were indicative of the fact that selection of proper oil, surfactant, and co-surfactant ratios were crucial for obtaining smaller particle size while developing a stable nanoemulsion.
TEM
TEM image of NE9 showed that SLN droplets were less than 50 nm ().
In vitro drug release study
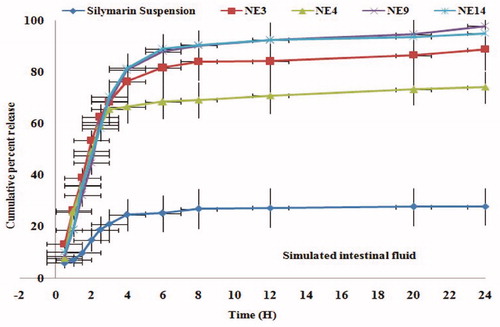
The four nanoformulations (NE3, NE4, NE14, and NE9) were tested and compared with each other and with an available conventional suspension of silymarin i.e. Limarin®. The concentration was calculated by extrapolation of calibration curve and a graph was plotted between time and percent cumulative release (). The nanoformulation NE14 showed 94.99% drug release followed by NE3 (88.69%) and NE4 (74.08%). The NE4 formulation exhibited slowest release due to larger particle size of nanoemulsion. All above nanoformulation exhibited better drug release profile compared to conventional silymarin suspension but only NE9 formulation was selected for further study since it showed maximum release (97.75%), finest globule size (21.24 nm), smallest polydispersity value (0.104), lesser viscosity (9.59 cps), and refractive index (1.316).
Accelerated stability studies
The stability studies revealed that during the storage period of 3 months at 25 ± 2 °C/60 ± 5% RH, 40 ± 2 °C/65 ± 5% RH, and 60 ± 2 °C/75 ± 5% RH, optimized SLN showed non-significant (p > .05) change in mean droplet size, zeta potential, polydispersity index, refractive index, viscosity, electrical conductivity, and transmittance. No phase separation and flocculation were observed, proving its stability. However, data obtained from temperatures 60 °C/65RH for 90 d showed increased droplet size but reduced transparency as compared with optimized SLN formulations at day zero.
Table 3. Droplet size, poydispersity index, zeta potential, viscosity, refractive index, conductivity, and percent transmittance of selected silymarin nanoemusion formulations.
In vivo pharmacokinetic study of optimized formulation and its comparison with available commercial product
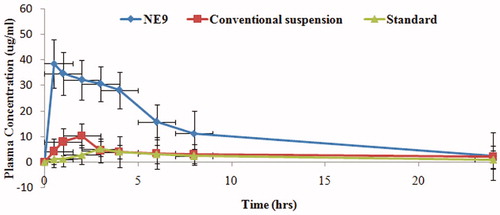
The Cmax of NE9 was found to be 38.62 ± 2.43 μg/ml. That was higher than silymarin-marketed suspension (10.36 ± 1.06) and standard suspension (5.24 ± 4.31). The AUC of nanoemulsion (NE9) was found to be 308.51 ± 4.23 μgh/mL which was 8-fold higher than marketed suspension (37.43 ± 2.89) and 17-fold higher than standard suspension (17.82 ± 7.32) (p < .01) (). The high value of AUC and Cmax in the case of NE9 formulation ensured higher drug absorption and availability at the site of action over a prolonged period of time. The quick onset of the drug action in the body is attributed to the presence of a low Tmax value of NE9 formulation (0.5 ± 0.45 h) as compared with conventional suspension (2.0 ± 0.62 h) and standard suspension (3.0 ± 0.31 h) of silymarin (p < .05). Although nanoemulsion of silymarin showed improved oral absorption [Citation36] but in present study, we were able to make better silymarin nanoemulsion which was more absorbed in less amount of time just by choosing suitable proportions of oil, surfactant, co-surfactant, and water (). The increase in the bioavailability of silymarin using a nanoemulsion might be due to the higher solubilization of drug in oil. Moreover, the presence of a surfactant and cosurfactant in the nanoemulsion system might have caused changes in the membrane permeability, and was able to reach a maximum concentration in minimum possible time [Citation39,Citation40]. Consequently, the present study showed rapid onset and better absorption of orally administered silymarin nanoemulsion than before.
Table 4. Pharmacokinetic parameters obtained after oral administration of silymarin nanoemulsion and its comparison with standard and conventional suspensions in male Wistar rats (n = 6).
Effects of silymarin nanoemulsion on percent cells viability and cellular morphology
In vitro cytotoxicity studies
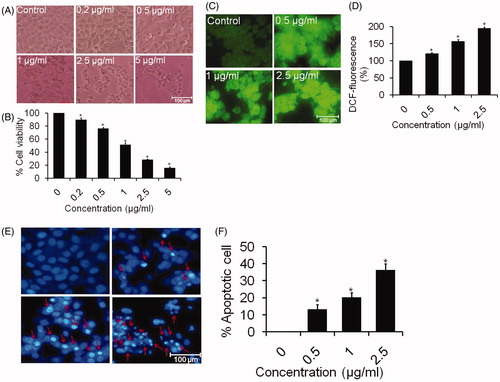
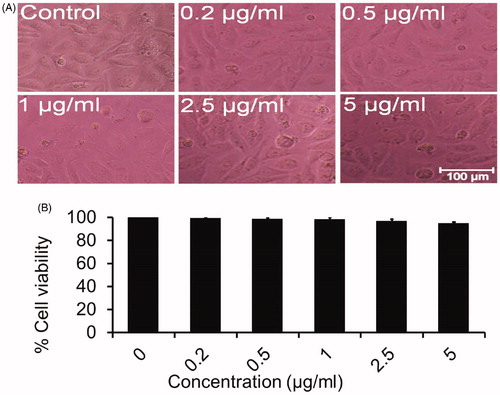
The unexposed cells remained smooth and healthy. However, morphological changes were made in SLN treated cells as revealed by the photomicrograph. The cells exposed to SLN displayed cellular shrinkage and fragmented body as compared with the untreated cells (5 A). This result supports the apoptotic features of the cells [Citation35]. The cytotoxic data indicate that 0.2 μg/ml of SLN reduced the cell viability to approximately 89.83% (p < .05) as compared with control. The cell viability was drastically reduced to 76.25 and 51.41% (p < .05) at 0.5 and 1 μg/ml of SLN, respectively. Further, SLN at a concentrations 2.5 and 5 μg/ml reduced the viability of cells to 28.45 and 15.89% (p < .05), respectively (). The toxicity of oligonucleotide/cationic complexes reduced significantly by the SLN [Citation34]. Although effects of silymarin alone were given on HepG2 cells before [Citation41,Citation42] but the present study showed better effects as SLN reduces the cell viability of cancer cell line in a dose-dependent manner without harming the surrounding Chang liver cells (normal cells) ().
Silymarin nanoemulsion induces intracellular ROS generation
The HepG2 cells treated with nanoemulsion showed a significant increase in ROS intensity in a dose-dependent manner as compared with untreated cells (). The results of quantitative measurement of ROS level showed that 0.5 μg/ml of nanoemulsion induced 121.43% (p < .05) enhancement in ROS production as compared with control. Moreover, ROS production was increased by 156.77 and 195.53% (p < .05) at 1 and 2.5 μg/ml of nanoemulsion when compared with untreated cells (). During apoptosis, ROS are produced by mitochondria which increased the mitochondrial membrane permeability and leads to the apoptotic phenotype [Citation41]. ROS are more reactive than molecular oxygen which may be associated with the activation of signal molecules and destabilization of mitochondrial membrane inducing the release of apoptotic agents resulting in toxicity to cancer cells [Citation42,Citation43]. Our results clearly stated that nanoemulsion provoked the cells death by inducing ROS production.
Silymarin nanoemulsion induces nuclear condensation
As observed from photomicrograph (), HepG2 cells treated with increasing concentrations of nanoemulsion increased the chromatin condensation as compared with control cells. However, 1 and 2.5 μg/ml of SLN exhibited maximum condensation as observed under inverted fluorescence microscope. Furthermore, approximately 13.33 and 20.33% of apoptotic cells were observed at 0.5 and 1 μg/ml of SLN, respectively (). Interestingly, 2.5 μg/ml of SLN induced 36.33% apoptotic cells as compared with control. Condensed and fragmented nuclei suggested that SLN caused cell death by an apoptotic process.
Conclusions
Silymarin nanoemulsion was prepared effectively with an optimized composition comprising oil phase (5.8% v/v, sefsol 218), 28.99% v/v of Smix (kolliphor RH40 and PEG 400, 2:1 as a surfactant and co-surfactant, respectively) and 65.22% v/v of distilled water as an aqueous phase. The above formulation was evaluated on the basis of particle size, viscosity, conductivity, and refractive index. That showed maximum drug release in less amount of oil. The NE9 formulation also showed higher Cmax and AUC and low Tmax than conventional suspension and standard suspension of silymarin. Data obtained from the stability studies also demonstrated that the optimized silymarin nanoemulsion remains stable over storage period of 3 months at 25± °C/60 ± 5% RH, 40 ± 2 °C/65 ± 5% RH, and 60 ± 2 °C/75 ± 5% as there was no creaming or phase separation observed in the formulations (p > .05). The optimized formulations also showed reduction in the cell viability and increased ROS intensity and chromatin condensation against human liver carcinoma cells without harming normal cells. The nanosize particles of silymarin followed by higher surface area may permit quicker rate of drug release and improved absorption pursued to enhanced bioactivity in lesser dose of drug.
Acknowledgement
Authors are thankful to Integral University Lucknow for providing the necessary facilities required for successful completion of this research work (IU/R&D/2017-MCN00043).
Disclosure statement
All authors have approved the final manuscript, and no potential conflict of interest was reported by the authors.
References
- Karkanis A, Bilalis D, Efthimiadou A. Cultivation of milk thistle (Silybum marianum L. Gaertn.), a medicinal weed. Ind Crops Prod. 2011;34:825–830.
- Ligeret H, Brault A, Vallerand D, et al. Antioxidant and mitochondrial protective effects of silibinin in cold preservation–warm reperfusion liver injury. J Ethnopharmacol. 2008;115:507–514.
- Kim S, Choi MG, Lee HS, et al. Silibinin suppresses TNF-α-induced MMP-9 expression in gastric cancer cells through inhibition of the MAPK pathway. Molecules. 2009;14:4300–4311.
- Dixit N, Baboota S, Kohli K, et al. Silymarin: a review of pharmacological aspects and bioavailability enhancement approaches. Indian J Pharmacol. 2007;39:172.
- Ahmad U, Faiyazuddin M, Hussain MT, et al. Silymarin: an insight to its formulation and analytical prospects. Acta Physiol Plant. 2015;37:1–7.
- Javed S, Kohli K, Ali M. Reassessing bioavailability of silymarin. Altern Med Rev. 2011;16:239–249.
- Shi J, Votruba AR, Farokhzad OC, et al. Nanotechnology in drug delivery and tissue engineering: from discovery to applications. Nano Lett. 2010;10:3223–3230.
- Wagner V, Dullaart A, Bock AK, et al. The emerging nanomedicine landscape. Nat Biotechnol. 2006;24:1211–1217.
- Zhang P, Ye H, Min T, et al. Water soluble poly (ethylene glycol) prodrug of silybin: design, synthesis, and characterization. J Appl Polym Sci. 2008;107:3230–3235.
- Jain KK. The handbook of nanomedicine. New York: Springer Science and Business Media; 2012.
- Aungst BJ. Novel formulation strategies for improving oral bioavailability of drugs with poor membrane permeation or presystemic metabolism. J Pharm Sci. 1993;82:979–987.
- Burcham DL, Maurin MB, Hausner EA, et al. Improved oral bioavailability of the hypocholesterolemic DMP 565 in dogs following oral dosing in oil and glycol solutions. Biopharmaceutics Biopharm Drug Dispos. 1997;18:737–742.
- Serajuddin A, Sheen PC, Mufson D, et al. Effect of vehicle amphiphilicity on the dissolution and bioavailability of a poorly water‐soluble drug from solid dispersions. J Pharm Sci. 1988;77:414–417.
- Serajuddin A. Solid dispersion of poorly water‐soluble drugs: early promises, subsequent problems, and recent breakthroughs. J Pharm Sci. 1999;88:1058–1066.
- Aungst BJ, Nguyen N, Rogers NJ, et al. Improved oral bioavailability of an HIV protease inhibitor using Gelucire 44/14 and Labrasol vehicles. BT Gattefosse. 1994;87:49–54.
- Toguchi H, Ogawa Y, Iga K, et al. Effects of physiological factors on the bioavailability of ethyl 2-chloro-3-[4-(2-methyl-2-phenylpropyloxy) phenyl] propionate in an emulsion in rats. Chem Pharm Bull. 1990;38:2801–2804.
- Wu W, Wang Y, Que L. Enhanced bioavailability of silymarin by self-microemulsifying drug delivery system. Eur J Pharm Biopharm. 2006;63:288–294.
- Stella V, Haslam J, Yata N, et al. Enhancement of bioavailability of a hydrophobic amine antimalarial by formulation with oleic acid in a soft gelatin capsule. J Pharm Sci. 1978;67:1375–1377.
- Kararli TT, Needham TE, Griffin M, et al. Oral delivery of a renin inhibitor compound using emulsion formulations. Pharm Res. 1992;9:888–893.
- Myers RA, Stella VJ. Systemic bioavailability of penclomedine (NSC-338720) from oil-in-water emulsions administered intraduodenally to rats. Int J Pharm. 1992;78:217–226.
- Palin KJ, Phillips AJ, Ning A. The oral absorption of cefoxitin from oil and emulsion vehicles in rats. Int J Pharm. 1986;33:99–104.
- Lawrence MJ, Rees GD. Microemulsion-based media as novel drug delivery systems. Adv Drug Deliv Rev. 2000;45:89–121.
- Akhtar J, Siddiqui HH, Fareed S, et al. Nanoemulsion: for improved oral delivery of repaglinide. Drug Deliv. 2016;23:2026–2034.
- Jadhav KR, Shaikh IM, Ambade KW, et al. Applications of microemulsion based drug delivery system. Curr Drug Deliv. 2006;3:267–273.
- Schwendener RA, Schott H. Lipophilic 1-β-d-arabinofuranosyl cytosine derivatives in liposomal formulations for oral and parenteral antileukemic therapy in the murine L1210 leukemia model. J Cancer Res Clin Oncol. 1996;122:723–726.
- Shafiq-un-Nabi S, Shakeel F, Talegaonkar S, et al. Formulation development and optimization using nanoemulsion technique: a technical note. AAPS PharmSciTech. 2007;8:E12.7.
- Mehnert W, Mäder K. Solid lipid nanoparticles: production, characterization and applications. Adv Drug Deliv Rev. 2001;47:165–196.
- Shafiq S, Shakeel F, Talegaonkar S, et al. Development and bioavailability assessment of ramipril nanoemulsion formulation. Eur J Pharm Biopharm. 2007;66:227–243.
- Kommuru TR, Gurley B, Khan MA, et al. Self-emulsifying drug delivery systems (SEDDS) of coenzyme Q10: formulation development and bioavailability assessment. Int J Pharm. 2001;212:233–246.
- Akhtar J. Novel oral nanoemulsion based drug delivery system of antidiabetic drugs [dissertation]. Lucknow, India: Integral University; 2015.
- Parveen R, Ahmad S, Baboota S, et al. Stability‐indicating HPTLC method for quantitative estimation of silybin in bulk drug and pharmaceutical dosage form. Biomed Chromatogr. 2010;24:639–647.
- Akhtar J, Hussain Siddiqui H, Fareed S, et al. Nanomulsion as a carrier for efficient delivery of metformin. Curr Drug Deliv. 2014;11:243–252.
- Fraga M, Laux M, Rejane dos Santos G, et al. Evaluation of the toxicity of oligonucleotide/cationic nanoemulsion complexes on Hep G2 cells through MTT assay. Die Pharmazie-Int J Pharm Sci. 2008;63:667–670.
- Siddiqui S, Ahmad E, Gupta M, et al. Cissus quadrangularis Linn exerts dose‐dependent biphasic effects: osteogenic and anti‐proliferative, through modulating ROS, cell cycle and Runx2 gene expression in primary rat osteoblasts. Cell Prolif. 2015;48:443–454.
- Kaleem S, Siddiqui S, Siddiqui HH, et al. Eupalitin induces apoptosis in prostate carcinoma cells through ROS generation and increase of caspase‐3 activity. Cell Biol Int. 2016;40:196–203.
- Parveen R, Baboota S, Ali J, et al. Oil based nanocarrier for improved oral delivery of silymarin: in vitro and in vivo studies. Int J Pharm. 2011;413:245–253.
- Calligaris S, Comuzzo P, Bot F, et al. Nanoemulsions as delivery systems of hydrophobic silybin from silymarin extract: effect of oil type on silybin solubility, in vitro bioaccessibility and stability. LWT – Food Sci Technol. 2015;63:77–84.
- Adhikari M, Arora R. Nano-silymarin provides protection against γ-radiation-induced oxidative stress in cultured human embryonic kidney cells. Mutat Res/Genet Toxicol Environ Mutagen. 2015;792:1–11.
- Woo JS, Kim TS, Park JH, et al. Formulation and biopharmaceutical evaluation of silymarin using SMEDDS. Arch Pharm Res. 2007;30:82–89.
- Kumar N, Rai A, Reddy ND, et al. Silymarin liposomes improves oral bioavailability of silybin besides targeting hepatocytes, and immune cells. Pharmacol Rep. 2014;66:788–798.
- Ramakrishnan G, Lo Muzio L, Elinos, et al. Silymarin inhibited proliferation and induced apoptosis in hepatic cancer cells. Cell Prolif. 2009;42:229–240.
- Fleury C, Mignotte B, Vayssière JL. Mitochondrial reactive oxygen species in cell death signaling. Biochimie. 2002;84:131–141.
- Chibber S, Farhan M, Hassan I, et al. Novel aspect of chemophototherapy in treatment of cancer. Tumour Biol. 2012;33:701–706.