Abstract
Background: Dysregulated microRNAs (miRNAs) reported to involve into the oncogenesis and progression in various human cancers. However, the roles and mechanism of miR-133 in lung adenocarcinoma remain largely unclear.
Methods: In this study, qPCR assay and western blot were used to detect the expression levels of miR-133, Akt and FLOT2. Luciferase reporter assay was used to identify the target role of miR-133 on FLOT2. The cell invasion and the migration capability were performed using the transwell invasion assay and wound healing assay.
Results: We found that miR-133 expression levels were downregulated in human lung adenocarcinoma specimens and cell lines compared with the adjacent normal tissues and normal human bronchial epithelial cell. miR-133 significantly suppressed metastasis of lung adenocarcinoma cells in vitro. Furthermore, FLOT2 (flotillin-2) identified as a direct target of miR-133, and FLOT2 expression levels were inversely correlated with miR-133 expression levels in human lung adenocarcinoma specimens. And the restoration studies suggested FGF2 as a downstream effector of miR-133 which acted through Akt signalling pathway.
Conclusions: Our study revealed the mechanism that miR-133 suppresses lung adenocarcinoma metastasis by targeting FLOT2 via Akt signalling pathway, implicating a potential prognostic biomarker and therapeutic target for lung adenocarcinoma treatment.
Background
Lung cancer, as one of the most common malignant malignancies, was the leading cause of cancer-related mortality worldwide [Citation1,Citation2]. Lung adenocarcinoma was reported as the most common type of lung cancer, accounting for 30–35% of primary lung tumours [Citation3]. Despite recent advances of diagnosis and treatment strategies in clinical and experimental oncology, the substantial proportion of lung adenocarcinoma patients with localized or locally advanced disease will eventually die, with the 5-year overall survival rate of ∼11%, threatening human health [Citation4]. Thus, it is urgent to elucidate the molecular mechanisms underlying lung adenocarcinoma development and identify novel prognostic markers and molecular therapeutic targets for improving the diagnosis, prevention and treatment of human lung adenocarcinoma.
MicroRNAs (miRNAs), a class of small non-coding RNAs (about 20–23 nucleotides), regulates the expression of its target gene expression by binding to the 3′ untranslated regions (UTRs) at post-transcriptional levels. Accumulating studies showed that dysregulated miRNAs involve in various biological and pathological processes, including cell proliferation, differentiation, apoptosis and tumorigenesis [Citation5–7]. miRNA often function as oncogenes or tumour suppressor, playing crucial roles in various cancer development and progression [Citation8,Citation9]. Recently, Xie' et al. [Citation10] identified seven microRNAs as tumour suppressor and 21 microRNAs as oncogenes in lung adenocarcinoma using high-throughput sequencing. Huang et al. [Citation11] showed that miR-650 expression was significantly upregulated in lung adenocarcinoma tissues compared with corresponding non-tumour tissues and the expression levels of miR-650 was associated with high incidence of lymph node metastasis, advanced clinical stage and poor prognosis of lung adenocarcinoma patients. Moreover, miR-483-5p upregulation is associated with the lung adenocarcinoma progression, and it function as oncogene by promoting the epithelial–mesenchymal transition (EMT) of lung adenocarcinoma [Citation12]. Previously, miR-133, as tumour suppressor, inhibits cell proliferation and metastasis of several cancers, including bladder cancer, prostate cancer and gastric cancer [Citation13–15]. However, the expression and role of miR-133 in lung adenocarcinoma remains unclear.
In the present study, we show that downregulation of miR-133 in lung adenocarcinoma tissue and cell lines, and function as a tumour suppressor by suppressing lung adenocarcinoma metastasis. Further investigations indicated that miR-133 directly targeted the 3′-UTRs of FLOT2, which were inversely correlated with miR-133 expression levels in human lung adenocarcinoma specimens. Moreover, FGF2 as a downstream effector of miR-133 regulated β-catenin signalling pathway in lung adenocarcinoma. Thus, this study identifies novel molecules and its downstream signalling pathway as potential therapeutic targets for the treatment of human lung adenocarcinoma.
Materials and methods
Tissue samples
A total of 30 primary lung adenocarcinoma tissues were collected from the Respiratory Department, Huangshi Central Hospital between 2012 and 2014. All patients did not receive chemotherapy or radiotherapy prior to surgery. This study was approved by the Research Ethics Committee of Hubei Polytechnic University, and written informed consent was obtained from each patient.
Cell culture and transfection
A normal human bronchial epithelial cell line (HBE) and two human lung adenocarcinoma cell lines (SPC-A1 and PC9) were purchased from the Tumor Cell Bank of Chinese Academy of Medical Science (Shanghai, China). All the cell lines were cultured in RPMI 1640 medium (Invitrogen, CA) supplemented with containing 10% foetal bovine serum (FBS), 100 units/mL of penicillin G sodium, and 100 μg/mL streptomycin sulphate. All the cell lines were cultured in at 37 °C in a humidified atmosphere of 95% air and 5% CO2.
Full-length FLOT2 cDNA was cloned into the pcDNA3.1 vector (Addgene, Cambridge, MA). miR-133 mimic, miR-133 inhibitor and pcDNA/FLOT2 were transfected into HEK293T and PC9 cells using Lipofectamine 2000 (Invitrogen, Carlsbad, CA) according to the instruction from the manufacturer. The cells were harvested for assays 48 h after transfection.
RNA extraction and qRT-PCR assay
We extracted the total RNA of the lung adenocarcinoma tissues or cells using Trizol (Invitrogen, CA). And then 2 μg of total RNA was used to synthesis cDNA according Taqman™ microRNA reverse transcription kit and qPCR was performed using TaqMan™ MicroRNA Assay kit (Applied Biosystems, Waltham, MA). MiRNA expression was normalized to U6.
Western blot assay
We used RIPA lysis buffer to lysates cells for 30 min, and then a BCA assay (Pierce, Rockford, IL) was used for the protein quantification. The SDS-PAGE(10%) was used to separated protein and polyclonal (rabbit) anti-FLOT2 antibody (Abcam, San Francisco, CA), anti-Akt antibody (Santa Cruz Bio-technology, Santa Cruz, CA), anti-Akt antibody (Santa Cruz Bio-technology, Santa Cruz, CA), anti-Bax (Santa Cruz Bio-technology, Santa Cruz, CA) and Bcl2 (Santa Cruz Bio-technology, Santa Cruz, CA) was used in Western blot. Goatanti-rabbit IgG (Pierce, Rockford, IL) secondary antibody conjugated to horseradishper oxidase and ECL detection systems (Super Signal West Femto, Pierce, Rockford, IL) were used for detection.
Luciferase reporter assay
PCR amplification was used to obtain the 3′-UTR of human FLOT2 from HBEs gDNA, and the 3′-UTR was cloned into the pGL3-luciferase reporter plasmid (Promega, Madison, WI). The pGL3-FLOT2-mut vector was built with pGL3-FLOT2 that underwent site-directed mutagenesis of the miR-133 target site, using a Stratagene Quik-Change® Site-Directed Mutagenesis Kit (Stratagene; Agilent Technologies, Santa Clara, CA). Cells were cultured in 24-well plates and transfected with the plasmids and miR-133 mimics using Lipofectamine 2000 for 24 h. The Dual Luciferase Reporter Assay System (Promega Corporation, Fitchburg, WI) was used to detect luciferase activity.
Invasion and migration assay
The cell invasion capability was performed using the transwell invasion assay. Cells were cultivated to 80% confluence on the 12-well plates. Then, we observed the procedures of cellular growth at 24 h. All the experiments were repeated in triplicate. The transwell migration chambers were used to evaluate cell invasion. Then cells invasing cells across the membrane were counted under a light microscope.
For the wound healing assay, cells were seeded in 12-well plates and grown to 90% confluence. Monolayers in the centre of the wells were scraped with pipette tips and washed with PBS. Cell movement into the wound area was monitored and photographed at 0 and 24 h using a light microscope. The migration distance between the leading edge of the migrating cells and the edge of the wound was compared as previous work [Citation16].
Statistical analysis
All data were shown as mean ± sd. Statistical significance was assessed by T-test for two-group comparison. Differences with p values <.05 were considered statistically significant. All data were analyzed with SPSS 17.0 (SPSS Inc., Chicago, IL) to confirm the statistical significance.
Results
MiR-133 was significantly downregulated in lung adenocarcinoma tissues and cell lines
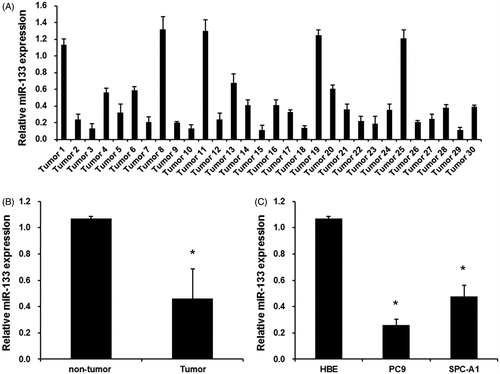
The expression levels of endogenous miR-133 in 30 lung adenocarcinoma tissues and corresponding non-tumour tissues were examined by real-time RT-PCR. As shown in , the miR-133 expression was significantly downregulated in 25 human lung adenocarcinoma tissues and upregulated in five tumour tissues, compared with paired adjacent non-tumour tissues. In total, miR-133 expression levels were much lower in human lung adenocarcinoma tissues than that in corresponding non-tumour tissues (). Next, we analyzed the miR-133 expression in normal human bronchial epithelial cell line (HBE) and two human lung adenocarcinoma cell lines (SPC-A1 and PC9). As shown in , miR-133 expression levels were much lower in SPC-A1 and PC9 cells than that in HBE cells. Thus, these results suggested that miR-133 might be involved in human lung adenocarcinoma development.
Figure 1. The expression levels of miR-133 in lung adenocarcinoma tissues and cell lines. (A) The mean level of miR-133 expression in lung adenocarcinoma tissues (n = 30) was significantly lower than that in corresponding adjacent normal tissues (n = 30). *p < .01 versus normal tissues. (B) qRT-PCR assay revealed the miR-133 expression levels in 30 human lung adenocarcinoma tissues and corresponding adjacent normal tissues. (C) miR-133 was downregulated in SPC-A1 and PC9 cells compared with HBE. *p < .01 versus HBE.
Effects of miR-133 on cell metastasis of lung adenocarcinoma
To further reveal the role of miR-133 in lung adenocarcinoma development, we transfected miR-133 mimics or inhibitors into PC9 cells to overexpress or silence miR-133 expression. As shown in , miR-133 expression was effectively upregulated in cells transfected with miR-133 mimics, and greatly downregulated in cells transfected with miR-133 inhibitors.
Figure 2. miR-133 regulates lung adenocarcinoma cell migration and invasion. (A) miR-133 expression by transfection with miR-133 mimic and miR-133 inhibitor in PC9 cells. *p < .01 versus The control group and the miR-con group. (B) Transwell invasion assay was utilized to analyze the effect of miR-133 on cell invasion of PC9 cells. *p < .05 versus the control group. (C) Wound healing assay was used to analyze the effect of miR-133 on cell migration of PC9 cells. *p < .05 versus the control group.
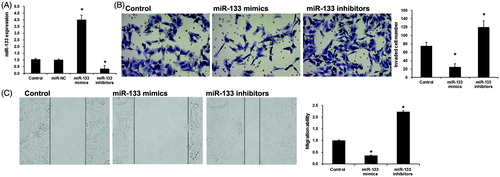
Furthermore, we detected the role of miR-133 on PC9 cells migration and invasion using wound healing assay and transwell invasion assay. Transwell invasion assay showed that miR-133 mimic markedly inhibited lung adenocarcinoma cell invasion (). Consistent with this result, silencing of miR-133 resulted in a significant increase of lung adenocarcinoma cell invasion (). Wound-healing assays indicated that the lung adenocarcinoma migration ability was significantly decreased by miR-133 mimics (), whereas increased by miR-133 inhibitors (). Taken together, the above results indicate that miR-133 performs tumour suppressor role by suppressing cell migration and invasion in lung adenocarcinoma.
FLOT2 is a direct target of miR-133 in lung adenocarcinoma cells
Previous work reported that FLOT2 expression was evidently upregulated in lung cancer NSCLC, and its correlation with tumour progression and patient survival [Citation17]. However, the molecular mechanisms of FLOT2 involved in lung adenocarcinoma remain unclear. In this study, three databases (Pictarget, miRBas and TargetScan) were used to predict that FLOT2 was a direct target of miR-133, suggesting cooperative binding and biologically effective interactions. In this study, we cloned WT or Mut target region sequence of the FLOT2 3′-UTR (), and detected the luciferase activities using Luciferase reporter assays. As shown in , con-transfection of miR-133 mimics suppressed the luciferase activity with wild-type FLOT2 3′ UTR sequence, but failed to inhibit that of mutated FLOT2. These data indicated FLOT2 as a direct target of miR-133. Next, we detect the effects of miR-133 on the expression of FLOT2 in PC9 cells using qRT-PCR and Western blot assay. As shown in , both mRNA and protein expression levels of FLOT2 were significantly downregulated by miR-133 mimics and were upregulated by miR-133 inhibitors in PC9 cells.
Figure 3. miR-133 negatively regulates FLOT2 expression in osteosarcoma cells. (A) Sequence alignment of miR-133 and 3’ UTR of FLOT2 using mirco-RNA.org. Luciferase reporter assay. PC9 cells were transiently cotransfected with Wt/Mut 3′ UTR with miRNAs as indicated. The effects of miR-133 on the expression of FLOT2 at both mRNA (B) and protein (C) level in PC9 cells. *p < .01 versus the control group and the mimics-NC group, **p < .01 versus the control group and inhibitors-NC group. (D) FLOT2 mRNA levels was examined by qRT-PCR in 30 cases of lung adenocarcinoma tissues and adjacent non-tumour tissues. *p < .01 versus the non-tumour group. (E) Correlation of miR-133 levels with FLOT2 mRNA levels was examined by qRT-PCR in 30 cases of osteosarcoma tissues (Pearson's correlation coefficient, r = −0.7315). *p < .01 versus paired non-tumorous tissues.
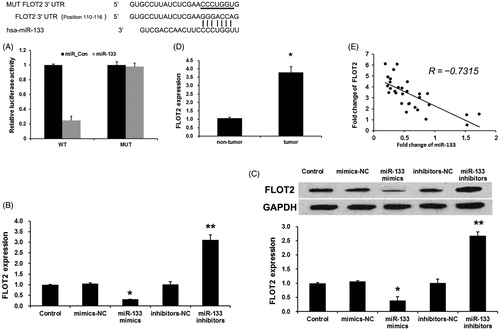
To further confirm the relationship between FLOT2 and miR-133, we detected the expression levels of FLOT2 in lung adenocarcinoma tissues. We found that FLOT2 levels in lung adenocarcinoma tissues were greatly upregulated compared with adjacent non-tumour tissues (). Then, we correlated FLOT2 with miR-133 expression in the same lung adenocarcinoma specimens. As shown in , the mRNA levels of miR-133 and FLOT2 observed a significant inverse correlation by using Spearman’s correlation analysis (R = −0.7315, p < .001). Taken together, these data strongly confirmed that FLOT2 is a direct target of miR-133 in lung adenocarcinoma.
FLOT2 involved the regulation of miR-133 on lung adenocarcinoma metastasis via Akt signalling
To further investigate the roles of FLOT2 on lung adenocarcinoma cells metastasis induced by miR-133, PC9 cells were co-transfected with miR-133 mimics (or control mimics) and pcDNA/FLOT2 vector. FLOT2 expression was rescue by pcDNA/FLOT2 vector using western blotting assay () at 48 h after co-transfection. Meanwhile, overexpressed FLOT2 reversed the inhibitory effect of miR-133 mimics on the migration and invasion ability in PC9 cells (). These results suggest that miR-133 regulated lung adenocarcinoma metastasis by repressing FLOT2 expression.
Figure 4. miR-133 inhibited lung adenocarcinoma metastasis via inactivation of Akt signaling by targeting FLOT2. (A) pcDNA/FLOT2 reversed the repression of miR-133 mimics on FLOT2 expression. (B) Transwell assays revealed the invasion ability of PC9 cell transfected with control, miR-133 or cotransfected with miR-133 and pcDNA-FLOT2. (C) In wound, migration assay revealed the migration ability of PC9 cell transfected with control, miR-133 or cotransfected with miR-133 and pcDNA-FLOT2. (D) Western blotting detection of the expression of pAkt, total Akt, Bcl-2 and Bax proteins in PC9 cell transfected with control, miR-133 or cotransfected with miR-133 and pcDNA-FLOT2. (E) The Bcl-2/Bax ratio assay. *p < .01 versus the control group. **p < .01 versus the miR-133 group.
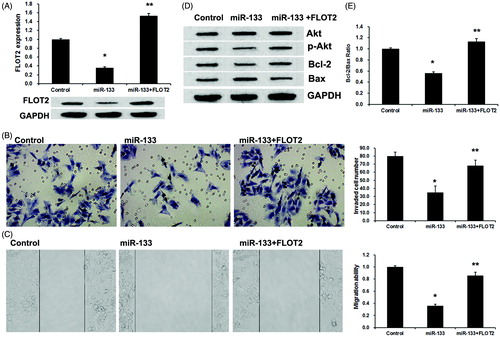
Previously, it has been reported that aberrant FLOT2 impairs the proliferation of breast cancer cells through Akt signalling pathway [Citation18], we then explore whether Akt signalling involved miR-133 and FLOT2 mediated-regulation of lung adenocarcinoma metastasis. phosphorylated Akt (pAkt) protein and total Akt protein were detected by using western blot. As shown in , miR-133 mimics downregulated pAkt expression, which was reversed by pcDNA/FLOT2. However, both miR-133 mimics and pcDNA/FLOT2 did not affect the expression of total Akt protein in PC9 cells. Then, we detect the expression of Bcl-2 and Bax, the downstream targets of Akt signalling. Similarly, miRNA-133 mimics inhibits the Bcl-2 expression and increase the Bax expression (), with a downregulation ratio of Bcl-2/Bax, which was reversed by cotransfected with pcDNA/FLOT2 (). These results indicated that miR-133 inhibited lung adenocarcinoma metastasis via inactivation of Akt signalling by targeting FLOT2.
Discussion
miRNAs always involve in the tumorigenic processes by targeting a variety of tumour suppressors and oncogenes [Citation19]. Thus, it is necessary to explore their roles in tumour development. In the present study, we detected for the first time that miR-133 was significantly downregulated in lung adenocarcinoma tissues and cells, and miR-133 could repressing the invasion and migration in lung adenocarcinoma cells by targeting FLOT2 via inactivation of Akt signalling. These data indicate miR-133 as a novel tumour suppressor in the progression of lung carcinogenesis.
Dysregulated miRNAs is commonly found in cancer and is associated with the pathogenesis of most malignancies, including lung cancer. Recently, the correlations between dysregulated miRNAs and lung carcinogenesis metastasis are increasingly reported. Long et al. [Citation20] showed that microRNA-214 promotes epithelial–mesenchymal transition and metastasis of lung adenocarcinoma by targeting the suppressor-of-fused protein (Sufu). Song et al. [Citation12] reported that miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Hwang et al. [Citation21] showed that overexpression of microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma through downregulation of cyclin D1. However, the roles of miR-133 in lung adenocarcinoma progression remain unknown and need to be further elucidated. In this study, we found that miR-133 expression was evidently downregulated in lung adenocarcinoma tissues and cells. Further functional analysis indicated that miR-133 could inhibit migration or invasion of lung adenocarcinoma cell, indicating miR-133 as a tumour suppressor in lung adenocarcinoma cells. Consistent with our work, it has been reported that miR-133 is also involved in other tumours and may regulate cell metastasis in these cancer cells, including bladder cancer, prostate cancer and gastric cancer [Citation13–15]. Here, we detected the role of miR-133 on lung adenocarcinoma migration and invasion for the first time, indicating miR-133 as a tumour suppressor in various human lung adenocarcinoma.
Flotillin-2 (FLOT2) belongs to the flotillin family, which was a protein family on micro-domain lipid rafts, which play a key role in signal transduction pathways, organization of the cytoskeleton, as well as synaptic transmission and involves lots of biological processes, such as cell proliferation, apoptosis, adhesion, and motility and so on [Citation22]. FLOT2 has been found to be associated with various cancer progression and poor survival outcomes, including breast cancer, melanoma, renal cell carcinoma and cervical carcinoma [Citation23–26]. Recently, FLOT2 expression was associated with lung cancer. For example, Arkhipova et al. [Citation27] revealed downregulated FLOT2 expression in the majority of NSCLC tissues. Berger et al. [Citation28] showed that FLOT2 deficiency leads to reduced lung metastases in a mouse breast cancer model. Subsequent study shows that the expression of FLOT2 in human non-small cell lung cancer and its correlation with tumour progression and patient survival [Citation17]. However, the mechanism of FLOT2 involves in lung adenocarcinoma metastasis needs to be further elucidated. In this study, we showed that FLOT2 was a direct of miR-133 and miR-133 could regulate FLOT2 expression. A negative correlation between miR-133 and FLOT2 expression was observed in clinical lung adenocarcinoma tissue samples. The Akt pathway is known to play a critical role in human cancer initiation and progression, and is also associated with cancer metastasis [Citation29]. In this study, we found that miR-133 mimics could downregulate the expression of phosphorylated Akt protein and eventually induced the decreased of Bcl-2/Bax ratio, which was reversed by FLOT2. These results support the notion that miR-133-repressed FLOT2 involves the regulation of lung adenocarcinoma metastasis via Akt signalling pathway.
Conclusions
In conclusion, the present study shows for the first time that miR-133 is downregulated in lung adenocarcinoma and miR-133 efficiently inhibit the metastasis progression in lung adenocarcinoma cells by targeting FLOT2 via Akt signalling pathway. These results indicated that miR-133 is a promising biomarker and a therapeutic target for lung adenocarcinoma in future.
| Abbreviations | ||
| miRNAs | = | microRNAs |
| EMT | = | epithelial–mesenchymal transition |
| FLOT2 | = | flotillin-2 |
| HBE | = | human bronchial epithelial cell line |
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Mokdad AH, Marks JS, Stroup DF, et al. Actual causes of death in the United States, 2000. JAMA. 2004;291:1238–1245.
- Jemal A, Siegel R, Ward E, et al. Cancer statistics, 2009. CA: Cancer J Clin. 2009;59:225–249.
- Wan L, Zhu L, Xu J, et al. MicroRNA-409-3p functions as a tumor suppressor in human lung adenocarcinoma by targeting c-Met. Cell Physiol Biochem. 2014;34:1273–1290.
- Gettinger S, Lynch T. A decade of advances in treatment for advanced non-small cell lung cancer. Clin Chest Med. 2011;32:839–851.
- Li Z, Lei H, Luo M, et al. DNA methylation downregulated mir-10b acts as a tumor suppressor in gastric cancer. Gastric Cancer: Off J Int Gastric Cancer Assoc Jpn Gastric Cancer Assoc. 2015;18:43–54.
- Xiao X, Tang C, Xiao S, et al. Enhancement of proliferation and invasion by MicroRNA-590-5p via targeting PBRM1 in clear cell renal carcinoma cells. Oncol Res. 2013;20:537–544.
- Yang X, Ni W, Lei K. miR-200b suppresses cell growth, migration and invasion by targeting Notch1 in nasopharyngeal carcinoma. Cell Physiol Biochem. 2013;32:1288–1298.
- Liu Z, Mai C, Yang H, et al. Candidate tumour suppressor CCDC19 regulates miR-184 direct targeting of C-Myc thereby suppressing cell growth in non-small cell lung cancers. J Cell Mol Med. 2014;18:1667–1679.
- Zhang HH, Pang M, Dong W, et al. miR-511 induces the apoptosis of radioresistant lung adenocarcinoma cells by triggering BAX. Oncol Rep. 2014;31:1473–1479.
- Xie L, Yang Z, Li G, et al. Genome-wide identification of bone metastasis-related microRNAs in lung adenocarcinoma by high-throughput sequencing. PLoS One. 2013;8:e61212.
- Huang JY, Cui SY, Chen YT, et al. MicroRNA-650 was a prognostic factor in human lung adenocarcinoma and confers the docetaxel chemoresistance of lung adenocarcinoma cells via regulating Bcl-2/Bax expression. PLoS One. 2013;8:e72615.
- Song Q, Xu Y, Yang C, et al. miR-483-5p promotes invasion and metastasis of lung adenocarcinoma by targeting RhoGDI1 and ALCAM. Cancer Res. 2014;74:3031–3042.
- Tao J, Wu D, Xu B, et al. microRNA-133 inhibits cell proliferation, migration and invasion in prostate cancer cells by targeting the epidermal growth factor receptor. Oncol Rep. 2012;27:1967–1975.
- Zhou Y, Wu D, Tao J, et al. MicroRNA-133 inhibits cell proliferation, migration and invasion by targeting epidermal growth factor receptor and its downstream effector proteins in bladder cancer. Scand J Urol. 2013;47:423–432.
- Cheng Z, Liu F, Wang G, et al. miR-133 is a key negative regulator of CDC42-PAK pathway in gastric cancer. Cell Signal. 2014;26:2667–2673.
- Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333.
- Wang YL, Yao WJ, Guo L, et al. Expression of flotillin-2 in human non-small cell lung cancer and its correlation with tumor progression and patient survival. Int J Clin Exp Pathol. 2015;8:601–607.
- Xie G, Li J, Chen J, et al. Knockdown of flotillin-2 impairs the proliferation of breast cancer cells through modulation of Akt/FOXO signaling. Oncol Rep. 2015;33:2285–2290.
- Xu JQ, Zhang WB, Wan R, et al. MicroRNA-32 inhibits osteosarcoma cell proliferation and invasion by targeting Sox9. Tumour Biol: J Int Soc Oncodev Biol Med. 2014;35:9847–9853.
- Long H, Wang Z, Chen J, et al. microRNA-214 promotes epithelial-mesenchymal transition and metastasis in lung adenocarcinoma by targeting the suppressor-of-fused protein (Sufu). Oncotarget. Nov 17 2015;6:38705–38718.
- Hwang SJ, Lee HW, Kim HR, et al. Overexpression of microRNA-95-3p suppresses brain metastasis of lung adenocarcinoma through downregulation of cyclin D1. Oncotarget. 2015;6:20434–20448.
- Babuke T, Tikkanen R. Dissecting the molecular function of reggie/flotillin proteins. Eur J Cell Biol. 2007;86:525–532.
- Wang X, Yang Q, Guo L, et al. Flotillin-2 is associated with breast cancer progression and poor survival outcomes. J Transl Med. 2013;11:190.
- Yan Y, Yang FQ, Zhang HM, et al. Up-regulation of flotillin-2 is associated with renal cell carcinoma progression. Tumour Biol: J Int Soc Oncodev Biol Med. 2014;35:10479–10486.
- Liu R, Xie H, Luo C, et al. Identification of FLOT2 as a novel target for microRNA-34a in melanoma. J Cancer Res Clin Oncol. 2015;141:993–1006.
- Liu Y, Lin L, Huang Z, et al. High expression of flotillin-2 is associated with poor clinical survival in cervical carcinoma. Int J Clin Exp Pathol. 2015;8:622–628.
- Arkhipova KA, Sheyderman AN, Laktionov KK, et al. Simultaneous expression of flotillin-1, flotillin-2, stomatin and caveolin-1 in non-small cell lung cancer and soft tissue sarcomas. BMC Cancer. 2014;14:100.
- Berger T, Ueda T, Arpaia E, et al. Flotillin-2 deficiency leads to reduced lung metastases in a mouse breast cancer model. Oncogene. 2013;32:4989–4994.
- Wu X, Sun L, Wang X, et al. Breast cancer invasion and metastasis by mPRalpha through the PI3K/Akt signaling pathway. Pathol Oncol Res. 2016;22:471–476.
