?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Green synthesis of AgNPs has gained many research interests as a low cost and eco-friendly approach. This work reported on the use of bacterial genomic DNA as the alternative biopolymer for a green production of AgNPs under a neutral pH. Although both ssDNA and dsDNA could function as stabilizing agents during the synthesis process, the ssDNA, generated by pre-heating the dsDNA at 100 °C, was more efficient to produce AgNPs with higher yield as determined by the intensity of the surface plasmon resonance (SPR) peak of silver at 475 nm. The obtained AgNPs were spherical with the average diameter of 15.0 ± 7.6 nm, which their identity was confirmed by X-ray diffraction, high-resolution transmission electron microscopy, and selected area electron diffraction analyses. The synthesized AgNPs exhibited the potent antibacterial activity against both Escherichia coli and Staphylococcus aureus with the minimum bactericidal concentrations at 250 and 500 μg/ml, respectively, suggesting their potential antibacterial activity against both Gram-negative and Gram-positive bacteria.
Introduction
Silver nanoparticles (AgNPs) have received many research interests owing to their optical, electronic and antibacterial properties resulting from their quantum size effect and large surface energy, which their various applications are proposed such as catalysis, microbial agents, anticancer agents, and colorimetric sensors [Citation1]. AgNPs are commonly produced by chemical and physical approaches such as evaporation-condensation, irradiation, colloidal dispersion, chemical reduction, microemulsion preparation, photochemical reduction, and sol-gel [Citation2]. Chemical approaches offer a simple large-scale production of AgNPs but usually generate hazardous wastes potentially unsafe to the environment. Physical approaches require no toxic chemicals and yield monodispersed particles but usually require a high energy in the processes [Citation3]. Recently, biological methods have received increasing interests as the alternative approach for a production AgNPs due to the eco-friendly and cost-effective advantages. For biological methods, AgNPs are produced either in vivo or in vitro, which biomolecules function as reducing and stabilizing agents to mediate the formation of the particles. Some bacteria, yeasts, and fungi can produce AgNPs from the uptake silver ions, which their cellular antioxidants and various reductase enzymes play the important roles in these transformation processes [Citation4–6]. Nevertheless, the syntheses by these organisms are still not practical for a mass industrial production due to the difficulty to maintain a septic condition for their growth and to control a production of nanoparticles [Citation7]. In recent years, many works focus on the in vitro syntheses of AgNPs mediated by biomolecules extracted from organisms, especially plant extracts, because of their simple, fast, and high productive advantages [Citation6]. In principle, silver ions are reduced to zero-valent silvers and eventually AgNPs are formed, which these processes are mediated by some phytochemicals (such as phenolics, tannins, saponins and terpenoids) and water-soluble biopolymers such as proteins and polysaccharides [Citation8]. Examples of polysaccharides and proteins reported to mediate a formation of AgNPs are starch [Citation9], cellulose [Citation10], silk fibroin [Citation11], apple snail egg proteins [Citation12], and cytochrome c [Citation13]. In addition, a nucleic acid is the other natural biopolymer that potentially facilitates a green production of AgNPs with the less study. Nithyaja and colleagues reported the synthesis of highly stable AgNPs of in an aqueous solution using the herring sperm double-stranded (ds) DNA as a stabilizing agent and sodium borohydride (NaBH4) as a reducing agent [Citation14]. Later, Takeshima and colleagues reported the production of AgNPs using the single-stranded (ss) DNA of salmon milt under a basic condition (10% ammonium) and sodium borohydride (NaBH4) as a reducing agent [Citation15]. Although the greater production of AgNPs was obtained via the use of ssDNA under an alkaline condition, this pH may not be suitable for biological applications of AgNPs. Thus, this work is interesting in a production of AgNPs using ssDNA under a neutral condition, which the dissociation of dsDNA to ssDNA is controlled by heat to destroy the hydrogen bonding between nitrogenous bases of each DNA strand. Alternative to eukaryote DNA, the bacterial genomic DNA is used in this work due to its simple preparation at low cost. In addition, effects of reaction temperature, DNA content, reaction time on the production of AgNPs as well as the characterization and antibacterial activity of the produced AgNPs are investigated.
Experiment
Genomic DNA extraction
A single colony of Escherichia coli strain DH5α was cultured in 5 ml Luria–Bertani (LB) broth as a starter at 37 °C for 6 h. The cultured cells were transferred to a fresh medium (1000 ml) and further incubated for 18 h before the bacterial cells were harvested by centrifugation at 8000 × g for 2 min. The cell pellet was washed twice with 40 ml STE buffer (100 mM NaCl, 10 mM Tris/HCl, 1 mM EDTA, pH 8.0), before resuspending in 20 ml TE buffer (10 mM Tris/HCl, 1 mM EDTA, pH 8.0). Tris-saturated phenol, pH 8.0 (10 ml) was then added, followed by a vortex-mixing step of 60 s to lyse cells. After centrifuging at 13,000 × g for 5 min at 4 °C, the aqueous phase was removed to mix with chloroform (1:0.5 volume ratio) and the mixture was centrifuged at 13,000 × g at 4 °C for 5 min. The aqueous phase was removed to mix with 200 μl RNase (stock 10 mg/ml) and the mixture was incubated at 37 °C for 20 min to digest RNA. Chloroform was added and mixed before centrifuging for 5 min. The aqueous phase was removed to a new tube and DNA in the aqueous phase was precipitated by adding 2.5 volumes of ethanol and 0.1 volume of 3 M sodium acetate. After incubating at –20 °C for 1 h, DNA was precipitated by centrifuging at 13,000 × g at 4 °C for 15 min. The purity and yield of the DNA were determined from the absorbance values at 260 and 280 nm. The pattern of genomic DNA was analyzed by 0.8% agarose gel electrophoresis and stained with 0.5 μg/ml ethidium bromide.
Green synthesis of AgNPs using bacterial genomic DNA
To synthesize AgNPs, the reaction contained 45 mM silver nitrate, 180 mM glucose, and 5.0 μg genomic DNA. The reactions were set at 25, 40, 60, and 80 °C for 24 h and the presence of AgNPs was observed forming a colour change from transparent to yellow-brown. In addition, a formation of AgNPs was monitored by the absorbance scanning from 300 – 900 nm using a spectrophotometer (Analytik Jena Specord® 250 Plus, Germany) at a resolution of 1 nm. To study the effect of denaturing temperatures, similar reactions were prepared except the genomic DNA solution was heated in boiling water at 100 °C for 5 min to obtain ssDNA before it was immediately added into each pre-heated reaction at 25, 40, 60, and 80 °C for 24 h. To study the effects of DNA amounts, the reactions containing silver nitrate, glucose, and the heated DNA (0.25, 1.25, 2.5, 5.0, and 10.0 μg) were incubated at 60 °C for 24 h. In addition, the formation of AgNPs at 60 °C was monitored in a time course of 72 h.
Characterization of AgNPs
The morphology and size of the produced AgNPs were determined by transmission electron microscopy (TEM) images using a Tecnai G2 20 S-Twin TEM (FEI, USA) with operating at accelerating voltage 120 kV. The suspension of AgNPs was dropped onto a carbon-coated copper grid (200 Mesh, Electron Microscope Science, Hatfield, PA) and dried at room temperature. The crystalline nature of the synthesized AgNPs was determined by selected area electron diffraction (SAED) pattern and high-resolution transmission electron microscope (HR-TEM) operating at 200 kV with LaB6 filament and equipped with a Gatan Orius 200 CCD Camera (Gatan, Pleasanton, CA). In addition, the X-ray diffraction (XRD) analysis was performed to investigate the crystallographic nature of the synthesized AgNPs. The suspension of AgNPs (1 ml) was dropped onto a glass slide and dried at room temperature for 1 h. The XRD pattern was recorded using a Bruker D8 Advance (D8 Advance, Bruker, UK) with the operating voltage of 40 kV and the current of 40 mA. The samples were subjected to the Cu kα radiation with a copper monochromator in the 2θ range of 30–80 radians.
Antibacterial tests
The antibacterial activity of the produced AgNPs was determined by the minimal inhibitory concentration (MIC) and minimal bactericidal concentration (MBC) against the Gram-positive (S. aureus, ATCC 25923) and Gram-negative (E. coli, ATCC 25922) bacteria [Citation16]. The suspension of AgNPs (1000 μg/ml) was serially twofold diluted by Muller–Hinton (MH) broth to obtain the concentration in a range of 1 – 1000 μg/ml. The suspension of AgNPs (100 μl) at each concentration was incubated with the tested bacteria (100 μl) at a concentration of 1 × 106 colony-forming units/ml (CFU/ml) in MH broth at 37 °C with shaking at 80 rpm. The relative numbers of bacteria were determined by measuring the optical density at 600 nm. The MIC value was determined at the minimal concentration of AgNPs that was completely inhibited the bacterial growth. To determine MBC value, the bacterial cultures (100 μl) containing the suspension of AgNPs at MIC and two above concentrations were spread on MH agar plates. After incubating at 37 °C for 24 h, the MBC value was determined at the minimal concentration of AgNPs that killed 100% of the bacterial population.
In addition, the bacterial activity of the produced AgNPs was determined by a disc diffusion assay [Citation17]. A single colony of each bacterium was initially cultured in MH broth at 37 °C and shaken at 200 rpm for 4 h. The bacterial culture was prepared at 0.5 McFarland standard, equally 1 × 108 colony-forming units/ml (CFU/ml). The bacterial culture (0.2 ml) was mixed with MH agar (20 ml) at 45 °C before pouring into a petri dish plate. The filter papers (Whatman No. 1) were punched into circular discs (6 mm in diameter) and sterilized by autoclaving at 121 °C. The suspension of AgNPs (1, 5, and 10 mg) was added onto each circular disc and dried in a laminar flow cabinet. The filter papers were placed on the prepared bacterial plate and incubated at 37 °C for 24 h. The antibacterial activity was determined by the bacterial growth inhibition zone, the diameter of the clear zone around the filter paper disc. The genomic DNA (200 ng/disc) and ampicillin (25 μg/disc) were used as the negative and the positive controls, respectively. The results were expressed as the mean of three independent experiments.
Statistical analysis
The data were expressed as the means of at least three replicates ± standard deviation (SD). For statistical analysis, a one-way ANOVA was used to compare the means of different data set with SPSS 18.0 for windows software (SPSS, Chicago, IL). The differences with a value of p < .05 were considered statistically significant.
Results and discussion
Green synthesis of AgNPs using bacterial genomic DNA
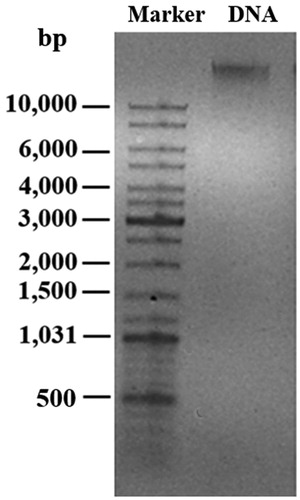
In this work, a bacterial genomic DNA was used for a green synthesis of AgNPs. To obtain the purified genomic DNA, a fast phenol-chloroform extraction method was employed, which yielded 44.5 ± 5.6 μg genomic DNA from 1 l of bacterial culture. The ratio of absorbance at 260 and 280 nm (A260/A280) was at 1.82, suggesting low or no protein contamination in the nucleic acid extracts [Citation18]. Generally, the A260/A280 ratio at 1.8 is considered as a pure DNA [Citation19]. shows a single band of extracted genomic DNA of larger than 10 kb on a 0.8% agarose gel.
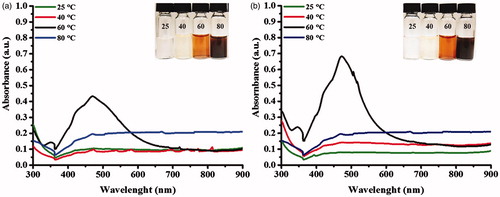
shows the UV-Vis spectra of the produced AgNPs using dsDNA under different incubating temperatures (25, 40, 60 and 80 °C). The formation of stabilized AgNPs was obviously detected at the incubating condition at 60 °C, as seen from the surface plasmon resonance (SPR) peak of silver at 475 nm and the reaction turned yellow-brown. For the reactions incubating at 25 and 40 °C, no obvious SPR peak was observed. However, the light yellow colour of the reactions suggested a formation of small amounts of AgNPs. For the condition at 80 °C, it was likely that AgNPs were formed as seen from the dark-brown solution. But due to their aggregation, an SPR peak was not detected. In addition, the ssDNA obtaining by pre-heating at 100 °C was used for a synthesis of AgNPs at 25, 40, 60 and 80 °C for 24 h. shows that the use of denatured DNA in the reaction at 60 °C yielded the higher production of AgNPs as determined by the highest intensity of the SPR peak. At 25 and 40 °C, the faint yellow colour of the reactions was observed but no obvious SPR peak was detected. At 80 °C, the reactions turned brown but the SPR peak was not detected due to the aggregated stage of AgNPs. Taken together, these results suggested that both dsDNA and ssDNA could facilitate a formation of AgNPs under a neutral pH, but ssDNA promoted more production of AgNPs. By heating at 100 °C, dsDNA is dissociated due to the destroy of hydrogen bonds, allowing Ag+ ions to interact with negatively charged groups of nitrogenous bases of DNA strands. These Ag+ ions are reduced to Ag0 by aldehyde groups of glucose and eventually AgNPs are formed, which the wrapped NDA chain maintains their stability [Citation20,Citation21].
Figure 2. UV-Vis spectra of the produced AgNPs in the reactions at 25, 40, 60 and 80 °C for 24 h using (a) unheated DNA and (b) heated DNA at 100 °C for 5 min.
shows the UV-Vis spectra of AgNPs in the reactions using different amounts of preheated DNA (0.25, 1.25, 2.5, 5.0 and 10.0 μg), which the obvious SPR peaks were detected at 415 – 499 nm in the reactions using 1.25 – 10.0 μg genomic DNA. Although the condition using 10.0 μg genomic DNA gave the highest yield of AgNPs, the SPR peak was slightly shifted to 499 nm, suggesting the larger size distribution of AgNPs. In addition, the reaction was performed without glucose and no formation of AgNPs was detected, suggesting a requirement of the reducing agent in this reaction. Glucose is a weak reducing agent that can reduce silver ions to metallic silver and oxidize itself into a gluconic acid [Citation22], which the reaction to form AgNPs can occur in the EquationEquation (1)(1)
(1) [Citation23].
(1)
(1)
Figure 3. UV-Vis spectra of the produced AgNPs under the conditions using (a) different amounts of heated DNA and (b) different reaction times.
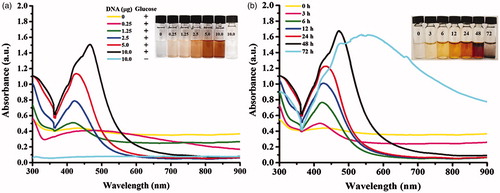
shows the formation of AgNPs observing in a time course of 72 h. In the reactions at 3 – 24 h, the SPR peak was detected at 414 nm and shifted to the longer wavelengths at 471 and 579 nm in the reactions at 48 and 72 h, respectively. These shifted wavelengths indicated a forming of increasing particle sizes [Citation24], well corresponding to an observation of dark precipitated particles in the reaction tubes. With increasing reaction time, the particles are allowed to attach each other via van der Waals forces or other chemical bonds, resulting in their aggregation and precipitation [Citation25].
Characterization of the produced AgNPs
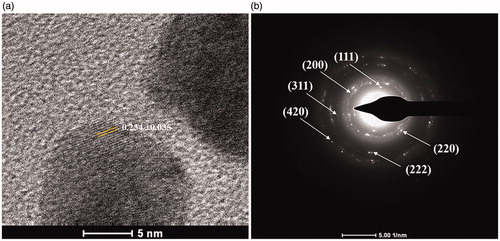
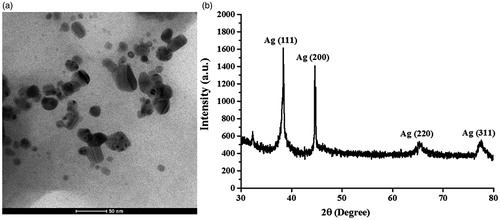
shows the representative TEM image of the produced AgNPs. The size distribution histogram corresponding to the TEM images represented spherical AgNPs with the diameters ranging from 5 and 45 nm with the average diameter of 15.0 ± 7.6 nm. shows the XRD pattern of the synthesized AgNPs. The 2θ peaks were observed at 38.14°, 44.37°, 64.45°, and 77.42°, corresponding to (111), (200), (220), and (311) reflection planes of the face-centred cubic (fcc) lattices of silver (JCPDS No. 04 – 0783), respectively, suggesting the identity of the synthesized particles as AgNPs [Citation26].
Figure 4. The representative images of (a) TEM and (b) XRD of the produced AgNPs in a reaction at 48 h.
The identity of the produced AgNPs was also confirmed by HR-TEM and SAED-TEM analyses. From the HR-TEM image in , the lattice spacing corresponding to the nearest spot was 0.234 ± 0.035 nm, corresponding to the (111) plane of the fcc lattices of silver [Citation27]. In , the SAED patterns demonstrated the concentric diffraction rings at 2.37, 2.04, 1.44, 1.24, 1.17 and 0.91 Å, corresponding to the hlk planar of (111), (200), (220), (311), (222), and (420), respectively, suggesting a characteristic polycrystalline pattern of AgNPs [Citation21].
Antibacterial activity of the synthesized AgNPs
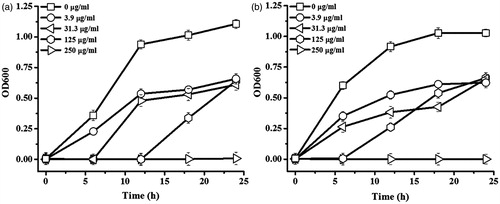
shows the growth curves of E. coli and S. aureus in response to various concentrations of AgNPs in a time course of 24 h. With the increasing concentrations of AgNPs, the growths of both bacteria were significantly reduced. The minimal concentration of AgNPs to inhibit E. coli growth (or MIC value) was at 250 μg/ml as seen in , which equalled the minimal concentration to completely kill the bacteria (or MBC value). Although the MIC value against S. aureus was at 250 μg/ml as seen in , the MBC value was higher at 500 μg/μl. The antibacterial activity of AgNPs was proposed to involve the adhesion of AgNPs on the negatively charged membrane of bacteria, leading to a disruption of membrane permeability and leakage of intracellular structure [Citation28]. In addition, small AgNPs can penetrate inside the bacterial cell and induce a formation of reactive oxygen species (ROS), which are toxic to cells. The released silver ions from AgNPs can interact with thiol groups of proteins and enzymes, causing the disruption of their functions. Silver ions can also interact with phosphate groups of DNA structure and interfere a regular DNA replication process [Citation29].
Figure 6. Growth curves of (a) E. coli and (b) S. aureus in response to AgNPs in a time course of 24 h.
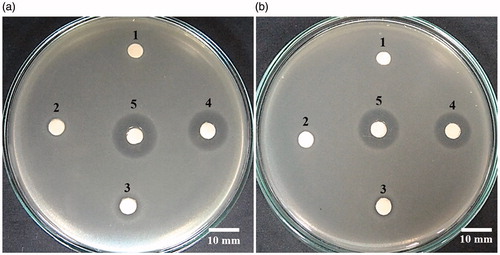
The antibacterial activity of AgNPs was also evaluated by a disc diffusion assay, which the suspension of AgNPs was absorbed in the sterile filter paper discs and placed on the bacterial plates. The antibacterial activity was determined by the growth inhibition zone, or clear zone, around the paper disc. shows the growth inhibition zones of AgNPs (1, 5 and 10 mg) against E. coli were 7.03 ± 0.58, 9.10 ± 0.29, and 16.07 ± 0.24 mm, respectively, while those against S. aureus were 6.90 ± 0.77, 8.33 ± 0.44, and 13.90 ± 0.50 mm, respectively. The inhibition zones of ampicillin (the positive control) against E. coli and S. aureus were 17.34 ± 0.46 and 16.38 ± 0.58 mm, respectively, while the negative control (DNA solution) showed no bacterial inhibition zone. These results demonstrated the antibacterial activity of AgNPs in a dose response. The antibacterial activity of AgNPs was likely attributed to the released silver ions from AgNPs and the interaction of AgNPs to the bacterial cell surface, causing cell damage and death [Citation30]. The greater antibacterial activity of AgNPs against E. coli than S. aureus was likely due to the thinner peptidoglycan layer of the Gram-negative bacterial cell wall [Citation31].
Conclusions
This work reported the use of genomic DNA of E. coli as the alternative biopolymer for a facile green synthesis of AgNPs. Via a heat denaturation, ssDNA was obtained under a neutral pH and could facilitate a formation of stabilized AgNPs more efficient than dsDNA. The obtained AgNPs were small spherical with the average diameter of 15.0 nm, which their identity was confirmed by HR-TEM, SAED-TEM, and XRD analyses. The synthesized AgNPs exhibited the potent antibacterial activity against both E. coli and S. aureus, suggesting their potential application as the effective antibacterial agent in both Gram-negative and Gram-positive bacteria.
Acknowledgements
This work was financially supported by Suranaree University of Technology [SUT1 – 104 – 58 – 24 – 11] and the student academic study was supported by the SUT high potential student scholarship.
Disclosure statement
No potential conflict of interest was reported by the author(s).
Additional information
Funding
References
- Saion E, Gharibshahi E, Naghavi K. Size-controlled and optical properties of monodispersed silver nanoparticles synthesized by the radiolytic reduction method. Int J Mol Sci. 2013;14:7880–7896.
- Abou El-Nour KMM, Eftaiha AF, Alwarthan AA, et al. Synthesis and applications of silver nanoparticles. Arabian J Chem. 2010;3:135–140.
- Iravani S, Korbekandi H, Mirmohammadi SV, et al. Synthesis of silver nanoparticles: chemical, physical and biological methods. Res Pharm Sci. 2014;9:385–406.
- Korbekandi H, Mohseni S, Mardani Jouneghani R, et al. Biosynthesis of silver nanoparticles using Saccharomyces cerevisiae. Artif Cells Nanomed Biotechnol. 2016;44:235–239.
- Elgorban AM, Al-Rahmah AN, Sayed SR, et al. Antimicrobial activity and green synthesis of silver nanoparticles using Trichoderma viride. Biotechnol Biotechnol Equip. 2016;30:299–304.
- Rafique M, Sadaf I, Rafique MS, et al. A review on green synthesis of silver nanoparticles and their applications. Artif Cells Nanomed Biotechnol 2016; [Epub ahead of print]. doi: 10.1080/21691401.2016.1241792
- Mohammadi S, Pourseyedi S, Amini A. Green synthesis of silver nanoparticles with a long lasting stability using colloidal solution of cowpea seeds (Vigna sp. L). JECE 2016;4:2023–2032.
- Ahmed S, Ahmad M, Swami BL, et al. A review on plants extract mediated synthesis of silver nanoparticles for antimicrobial applications: a green expertise. J Adv Res. 2016;7:17–28.
- Vasileva P, Donkova B, Karadjova I, et al. Synthesis of starch-stabilized silver nanoparticles and their application as a surface plasmon resonance-based sensor of hydrogen peroxide. Colloids Surf A. 2011;382:203–210.
- Wu J, Zhao N, Zhang X, et al. Cellulose/silver nanoparticles composite microspheres: eco-friendly synthesis and catalytic application. Cellulose. 2012;19:1239–1249.
- Fei X, Jia M, Du X, et al. Green synthesis of silk fibroin-silver nanoparticle composites with effective antibacterial and biofilm-disrupting properties. Biomacromolecules. 2013;14:4483–4488.
- Janthima R, Khamhaengpol A, Siri S. Egg extract of apple snail for eco-friendly synthesis of silver nanoparticles and their antibacterial activity. Artif Cells Nanomed Biotechnol. 2017; [Epub ahead of print]. doi: 10.1080/21691401.2017.1313264
- Macdonald IDG, Smith WE. Orientation of cytochrome c adsorbed on a citrate-reduced silver colloid surface. Langmuir. 1996;12:706–713.
- Nithyaja B, Misha H, Nampoori V. Synthesis of silver nanoparticles in DNA template and its influence on nonlinear optical properties. Nanosci Nanotechnol. 2012;2:99–103.
- Takeshima T, Sun L, Wang Y, et al. Salmon milt DNA as a template for the mass production of Ag nanoparticles. Polym J. 2014;46:36–41.
- Clinical Laboratory Standards Institute (CLSI). Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically; approved standard—ninth edition, M07-A9. PA: Clinical Laboratory Standards Institute Wayne; 2012.
- Pandey S. Preliminary phytochemical screening and in vitro antibacterial activity of Bauhinia variegata Linn. against human pathogens. Asian Pac J Trop Dis. 2015;5:123–129.
- Ozdemir-Kaynak E, Yesil-Celiktas O. Microwave-assisted digestion combined with silica-based spin column for DNA isolation from human bones. Anal Biochem. 2015;486:44–50.
- Arruabarrena A, Benítez-Galeano MJ, Giambiasi M, et al. Application of a simple and affordable protocol for isolating plant total nucleic acids for RNA and DNA virus detection. J Virol Methods. 2016;237:14–17.
- Darroudi M, Ahmad MB, Abdullah AH, et al. Green synthesis and characterization of gelatin-based and sugar-reduced silver nanoparticles. Int J Nanomedicine. 2011;6:569–574.
- Takeshima T, Tada Y, Sakaguchi N, et al. DNA/Ag nanoparticles as antibacterial agents against gram-negative bacteria. Nanomaterials (Basel). 2015;5:284–297.
- Susilowati E, Triyono T, Santosa SJ, et al. Synthesis of silver-chitosan nanocomposites colloidal by glucose as reducing agent. Indones J Chem. 2015;15:29–35.
- Yan J, SOH K, Cheng J. Antimicrobial yarn having nanosilver particles and methods for manufacturing the same. European Patent EP1490543B1. 2006.
- Farah MA, Ali MA, Chen SM, et al. Silver nanoparticles synthesized from Adenium obesum leaf extract induced DNA damage, apoptosis and autophagy via generation of reactive oxygen species. Colloids Surf B Biointerfaces. 2016;141:158–169.
- Li D, Kaner RB. Shape and aggregation control of nanoparticles: not shaken, not stirred. J Am Chem Soc. 2006;128:968–975.
- Deepak P, Sowmiya R, Ramkumar R, et al. Structural characterization and evaluation of mosquito-larvicidal property of silver nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner) J. Agardh 1848. Artif Cells Nanomed Biotechnol. 2016. [Epub ahead of print]. doi: 10.1080/21691401.2016.1198365
- Majeed Khan MA, Kumar S, Ahamed M, et al. Structural and thermal studies of silver nanoparticles and electrical transport study of their thin films. Nanoscale Res Lett. 2011;6:1–8.
- Durán N, Durán M, de Jesus MB, et al. Silver nanoparticles: a new view on mechanistic aspects on antimicrobial activity. Nanomed Nanotechnol Biol Med. 2016;12:789–799.
- Benjamin LO, Francesco S. Antibacterial activity of silver nanoparticles: a surface science insight. Nano Today. 2015;10:339–354.
- Sheehy K, Casey A, Murphy A, et al. Antimicrobial properties of nano-silver: a cautionary approach to ionic interference. J Colloid Interface Sci. 2015;443:56–64.
- Wang L, Liu CC, Wang YY, et al. Antibacterial activities of the novel silver nanoparticles biosynthesized using Cordyceps militaris extract. Curr Appl Phys. 2016;16:969–973.