?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Herein, ZnO nanoparticles were synthesized using microwave-assisted method in the presence of Vaccinium arctostaphylos L, fruits extract. The structure, size, morphology and optical properties of the samples were characterized by XRD, SEM, TEM, EDX, FT-IR, UV-vis DRS and TGA analysis. A decrease in crystallite size was observed for the biologically synthesized ZnO compared to the chemically synthesized sample. Furthermore, the existence of organic moieties over the biologically synthesized ZnO NPs was approved using characterizing methods. Then, the alloxan-induced diabetic rats were divided into not treated (diabetic control group), and the groups received: insulin, chemically synthesized ZnO, plant extract, biologically synthesized ZnO with a normal healthy control group. After treatment, fasting blood glucose (FBS), high-density lipoprotein (HDL), total triglyceride (TG), total cholesterol (TC) and insulin were measured. Analysis showed a significant decrease in FBS and increase in HDL levels in all groups under treatment. However, the results for cholesterol reduction were only significant for the group treated by biologically synthesized ZnO. Despite the changes in the triglyceride and insulin levels, the results were not significant. For all the studied parameters, bio-mediated ZnO NPs were found the most effective in treating the alloxan-diabetic rats compared to the other studied treatment agents.
Introduction
Amongst group 12 trace elements, zinc is identified as a crucial trace metal for eukaryotes. It is the second most common trace metal in human body after iron, which ranks the fourth among the most consumed metals in the world. This element acts as an actuator for above three hundred enzymes in the body and has an important role in blood sugar regulation and diverse metabolic pathways including glucose metabolism. Herein, zinc improves glucose consumption by stimulating hepatic glycogenesis through its function on the insulin pathways [Citation1,Citation2]. Furthermore, zinc is the central element of insulin structure [Citation3], which has a key role in stabilizing insulin during storage and biosynthesis. Several zinc-transporters, particularly β-cells, are crucial for insulin secretion [Citation4–6]. Moreover, insulin signalling can be enhanced by this element through numerous mechanisms, such as amplified phosphorylation of insulin receptor, improving PI3K activity, and prevention of glycogen synthase kinase-3 [Citation2]. Accordingly, zinc deficiency and homeostasis deviations lead to serious problems in the body and number of diseases such as chronic liver disease, diabetes, mal-absorption syndrome and sickle cell disease [Citation2,Citation7].
Vaccinium arctostaphylos L. is the only member of Vaccinium genus in Iran, which belongs to Ericaceae family. V. arctostaphylos has been traditionally used in Iran, Turkey, and Caucuses to improve the health status of diabetic patients and to treat hypertension [Citation8,Citation9]. Its fruit is rich in bioactive anthocyanins [Citation9]. Anthocyanoside Myrtillin present in this herb has as similar function as insulin. In addition, it has been reported that Vaccinium arctostaphylos L. positively affected the oxidative stress and serum lipid profile in hyperlipidemic patients [Citation10]. The ethanolic extract of the fruits of V. arctostaphylos has antihyperglycemic and antioxidant activities that recovers the lipid profile in the diabetic patients [Citation8]. Consequently, this plant has been studied seriously as a suitable antidiabetic candidate.
The knowledge on and using herbal medicines dates back long history and has occurred in numerous populations. Nevertheless, there are problems which limit the clinical applications of therapeutic plants such as their law solubility and bioavailability, inconstancy and incalculable toxicity and small oral absorption [Citation11]. In consequence, the use of nanoparticles was found as a potential approach to overcome the barriers faced by the application of herbal medicines [Citation12]. In recent times, nanomaterials applications in antidiabetic studies has drawn attention due to their exceptional features such as very little dimensions, aptitude to pass through the cell membrane to transport medications and bio-adaptability [Citation13,Citation14]. The stunning advancement in nanotechnology has opened up innovative applications in various areas such as agricultural, biomedical sciences, catalysis, chemical industry, cosmetics, drug-gene delivery, electronics, energy science, mechanics, optics and space industry [Citation15–21]. The exclusive properties of ZnO nanoparticles (NPs), such as the selective cytotoxicity in cancer cells made them as novel alternatives to cancer therapy [Citation16,Citation22]. Furthermore, ZnO NPs have the antimicrobial [Citation23,Citation24], antioxidant [Citation25,Citation26] and antidiabetic [Citation27,Citation28] activities and have potential for biomedical applications due to better cellular uptake.
There are numerous physico-chemical methods for synthesis of ZnO NPs including chemical vapour deposition [Citation29], thermal decomposition [Citation30], hydrothermal [Citation31,Citation32], sol–gel [Citation33], co-precipitation [Citation34], ultrasound/microwave-assisted combustion methods [Citation35,Citation36], and photochemical and electrochemical reduction techniques [Citation37]. Although these processes give rise to high yields, they suffer not only from the plausible contamination of the prepared sample, but also environmental pollution due to the discharge of toxic by-products. Hence, biological synthesis of ZnO NPs has attracted a great deal of attention due to controlled fabrication with well-defined features using harmless and inexpensive materials [Citation38]. Moreover, biosynthesis methods are green alternatives whereof the outputs are bio-friendly, very soluble in aqueous medium, and free of contaminations, which can actually provide better defined nanoparticles in size and morphology [Citation39]. The bio-agent can provide a desirable reaction medium and act as reducing agent in synthesis process [Citation40,Citation41]. This method offers the application of biological agents including enzymes, microorganisms, and plant extracts for the synthesis of NPs [Citation40,Citation42]. Green synthesis using plant extract, in particular, has attracted a great deal of research interest as they led to facile production of more stable nanoparticles [Citation41,Citation43]. The extracts of potato, tomato, Corymbia citriodora leaf, Ziziphus nummularia leaf, Prunus  yedoensis Matsumura leaf and Abrus precatorius seed, been used as promising choices for facile green synthesis of ZnO NPs in the reported literature [Citation19,Citation20,Citation23,Citation40,Citation41,Citation44].
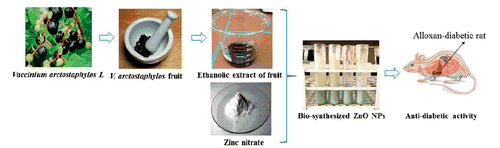
This study aimed at producing ZnO NPs via a green route, using Vaccinium arctostaphylos L. fruit extract as capping agent, and investigate their antidiabetic efficacy on the alloxan- diabetic rats. Subsequently, the effectiveness of the bio-synthesized ZnO NPs were compared with chemically produced sample. For this purpose, the ZnO NPs were prepared using a microwave-assisted method in the presence of ethanolic extract of Vaccinium arctostaphylos L fruit and the characteristics were determined using X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy dispersive analysis of X-rays (EDX), Fourier transform-infrared spectroscopy (FT-IR), thermogravimetric analysis (TGA), and UV-vis diffuse reflectance spectroscopy (UV-vis DRS). Subsequently, the antidiabetic activity of the bio-synthesized NPs were assessed on diabetic rats and compared with the chemically-produced ZnO. The alloxan-induced diabetic male Wistar rats were divided into six groups: (I) normal healthy group; (II) none (not treated) as diabetic control group, and the groups received (III) insulin (10 U/kg), (IV) chemically synthesized ZnO (8 mg/dl), (V) plant extract (150 mg/dl), and (VI) biologically synthesized ZnO (8 mg/dl). The main objective of the present study was to compare the antidiabetic efficacies of the both synthesized ZnO NPs and the extract of Vaccinium arctostaphylos L, fruit exclusively or combined, to explore the most controlling agent.
Materials and methods
Materials
In this study, all the reagents were of analytical grade and used without further purifications. Zinc nitrate (Zn (NO3)2. 4H2O) and sodium hydroxide were purchased from Loba Chemie Company. Alloxan monohydrate was purchased from Sigma Aldrich. Vaccinium arctostaphylos L. dried fruits were bought from a local market in Ardabil province (North West of Iran). The kits for determination of fasting blood sugar (FBS), triglyceride (TG), total cholesterol (TC), and high-density lipoprotein (HDL) were purchased from Pars Azmoun Company (Tehran, Iran). Insulin was determined using commercial ELISA kit (Mercodia, Sweden). Ketamine and xylazine, for the anaesthesia of the studied rats, were purchased from Alfasan, India. Deionized water was used throughout preparation of the ZnO samples.
Animals
Male Wistar rats (near 1 month old) with average weight of 220 ± 20 g were purchased from Tehran University animal house, fed under standard rodent diet and maintained under standard laboratory conditions. All the animal procedures were conducted in accordance with National Institutes of Health guide for the care and use of Laboratory animals (NIH Publications No. 8023, revised 1978) that were approved by Ardabil Animal Care and Use Committee. All procedures of the current experiments were performed at the animal housing department, Faculty of basic Sciences, University of Mohaghegh Ardabili.
Instruments
Philips Xpert X-ray diffractometer with Cu Kα radiation (λ = 0.15406 nm) was used to record the XRD patterns from 20° to 80°. SEM was applied to study the distribution and morphology of the synthesized ZnO particles using LEO1430VP with an accelerating voltage of 15 kV. The EDX on the same SEM instrument was used to analyze the purity and elemental composition of the products. The TEM investigations were performed by a Philips CM30 instrument with an acceleration voltage of 150 kV. A Scinco 4100 apparatus were employed to record the UV–vis DRS data. The FT-IR spectra were obtained using a Perkin-Elmer Spectrum RXI apparatus. The TGA curves of the samples were provided by Linseis STAPT1000 under air atmosphere by heating at 10 °C/min from room temperature to 700 °C. A Metrohm digital pH meter (model 691) was used to measure and adjust the pH of the solutions. The samples were prepared using a domestic microwave oven (2.45 GHz and 1000 W).
Plant extract preparation
The dried fruits of Vaccinium arctostaphylus L. was obtained from a local market (Ardabil, Iran) and authenticated by a botanist. The fruits were washed with water to remove dusts and dried. Then, the dried fruits were ground into uniform powder using a mortar and pestle. A total of 10 mg of the ground powder was weighed out and soaked in 100 ml of 96% ethanol in a conical flask and agitated on an orbital shaker at room temperature for 72 h. Then, the mixture was filtered using Watman No. 1 filter paper. After that, 20 ml of the filtered extract was used in the synthesis and the rest of it was dried on a flat surface to evaporate the solvent and the resultant powder was stored at 4 °C for subsequent experiment [Citation45].
Preparation of ZnO samples
The ZnO NPs were prepared biologically by dissolving 20 ml of the prepared extract in 80 ml of water and addition of 6.42 g of zinc nitrate to this solution under stirring at room temperature. In order to adjust pH of the solution at 10, aqueous solution of NaOH (5 M) was slowly dropped into the solution under stirring. Afterward, the suspension was irradiated for 15 min in the microwave oven. The resulted product was centrifuged and the unreacted reagents were removed by washing with water and ethanol and oven-dried at 60 °C for 24 h. For comparison, the ZnO sample was prepared with the same procedure, but in pure water [Citation39].
Experimental design for antidiabetic activity
Induction of diabetes
Prior to the experiments, the overnight fasted Wister rats were diabetic by a single intraperitoneally injection of alloxan monohydrate (170 mg/kg) dissolved in saline. Blood glucose levels were checked one week later. Animals showing hyperglycaemia (∼200–250) were selected and grouped for further studies.
The anti-diabetic studies were carried out by dividing rats into six groups of five rats: (I) normal healthy rats, received physiological saline; (II) diabetic rats, received physiological saline; (III) diabetic rats, received insulin (10 U/kg); (IV) diabetic rats, received chemically synthesized ZnO nanoparticles dissolved in saline (8 mg/dl); (V) diabetic rats, received plant extract (150 mg/dl); (VI) diabetic rats, received biologically synthesized ZnO nanoparticles dissolved in saline (8 mg/dl). Treatment in all the groups was performed by daily intraperitoneal injection, for 16 days. The schematic representation of ZnO NPs bio-synthesis procedure and activity evaluation is provided as Scheme 1.
Biochemical investigations
After 16 days of treatment, the over-night fasted rats were sacrificed under Ketamine (80 mag/Kg)/Xylazine (15 mg/Kg) anesthesia. Blood samples were collected from heart in sterile tubes and centrifuged at 1600 g for 15 min – following 30 min of clotting – to separate the serum. Commercial kits were used to estimate the serum levels of FBS, TG, TC, and HDL. The level of insulin was analyzed by enzyme-linked immunosorbent assay (ELISA) kits obtained from Mercodia AB, Sweden, using Microplate reader, URIT Medical Electronic Co., Ltd, China.
Results and discussion
Characterization of the ZnO samples
The phase structures of the synthesized samples were assessed by XRD patterns. The recorded patterns for the ZnO samples prepared in water and aqueous extract are displayed in . From the figure, all the diffraction peaks of the both samples well-matched to wurtzite hexagonal crystalline phase of ZnO (JCPDS No. 36–3411) that were corresponded to the (100), (002), (101), (102), (110), (103), (200), (112), (201), (004) and (202) planes [Citation47]. However, the intensity and width of the peaks for these samples were evidently different, mainly resulted from the differences in their average crystallite sizes. Hence, the average size of the crystallites (D) was determined using the XRD results by Scherrer’s equation (EquationEquation (1))(1)
(1) .
(1)
(1) where K is the Scherrer’s constant (K = 0.9), λ is the wavelength of X-ray radiation (0.15406 nm), B is the full-width-at-half-maximum of the corresponding plane (in radians) and θ is the characteristic X-ray radiation. The average crystallite sizes for the ZnO samples prepared in water and in the presence of the extract calculated from the (101) plane were 15.5 and 13.9 nm, respectively. Correspondingly, the crystallite sizes estimated from the (110) plane were 16.7 and 15.0 nm for the chemically and biologically prepared samples, respectively. In light of this, it can be concluded that the fruit extract has a considerable effect on the crystallite sizes of the prepared ZnO NPs.
Figure 1. (a) XRD patterns and (b) EDX spectra of the ZnO samples prepared in water and aqueous solution of the extract.
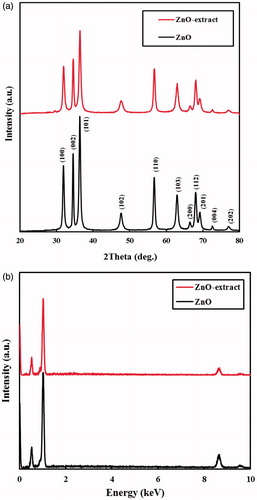
The EDX spectra were used to examine purity of the ZnO samples prepared in water and in the presence of the extract (). Three signals for zinc element and one signal for oxygen element are observed in the EDX spectra of the both ZnO samples. Accordingly, the samples had considerable purity and were composed of Zn and O elements.
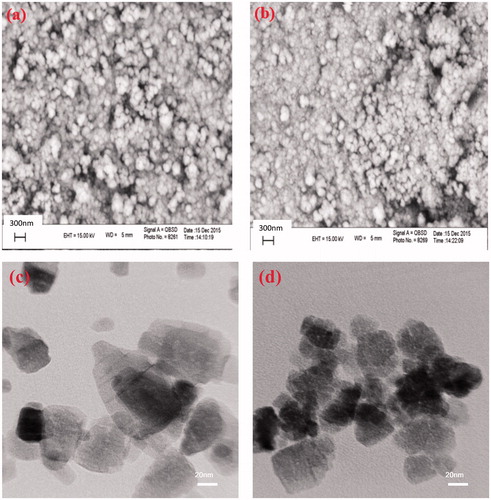
SEM images were used to explore the surface morphology of the ZnO samples (). From these images, it is evident that both of the ZnO samples have almost spherical morphology, but with different sizes and aggregations. As clearly observed in the TEM images, the size of the ZnO NPs prepared in the presence of the extract was considerably decreased (). Similar to the results obtained from XRD patterns, it was concluded that the organic molecules in the extract acted as a capping agent and considerably inhibit the growth of the ZnO NPs during the preparation step [Citation47].
Figure 2. SEM images of the ZnO samples prepared in (a) water and (b) aqueous solution of the extract. TEM images of the ZnO samples prepared in (c) water and (d) aqueous solution of the extract.
FT-IR spectra of the ZnO samples and the extract in the range of 400 to 4000 cm−1 are shown in . For these samples, the wide absorption bands nearby 3400 cm−1 were associated to the O–H stretching vibrations of the water molecules adsorbed onto the samples [Citation48]. In addition, the absorption peak at 570 cm−1 is corresponding to the stretching vibration of Zn–O bond [Citation49]. Regarding the ZnO NPs prepared with the extract, along with the peaks for Zn–O and O–H bonds, other peaks were also observed at 2930, 2855, 1735, 1620 and 1250 cm−1. The absorption peaks at 2930 and 2855 cm−1 are assigned to C-H stretching vibrations of CH3 and CH2 groups, respectively. Furthermore, the vibration peaks for C–O, C = C, and C = O bonds are observed at 1250, 1620, and 1735 cm−1, respectively [Citation50]. Hence, it is clear that during the preparation step, some molecules of the extract tightly covered the ZnO NPs.
Figure 3. (a) FT-IR spectra, (b) TGA curves and (c) UV-vis DRS for the ZnO samples prepared in water and aqueous solution of the extract along with the liquid extract.
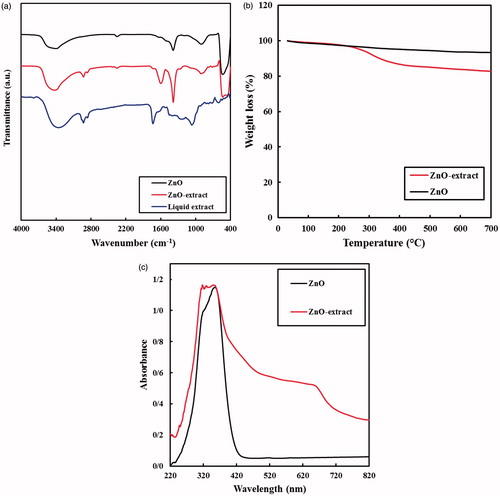
At the next step, the TGA of the ZnO samples were provided with a heating rate of 10 °C min−1 from room temperature to 700 °C under air conditions, in order to estimate the amount of the extract molecules coated the ZnO NPs (). For the ZnO sample prepared in water, a mild continuous decrease of the weigh was observed. This sample lost about 6.4% of its weight up to 700 °C. It is well known that ZnO is stable in this range of temperature, thus, this decrease could be attributed to the evaporation of the intra-particle water of the sample. However, regarding biologically prepared ZnO sample, the observed weight loss was greater, in which almost 17.0% of its weight was decreased by heating up to 700 °C. It is evident from the results that the extract started to decompose into the volatile compounds from 250 °C. This is the typical temperature for decomposition of many organic compounds. The amount of the organic extract over the ZnO sample was estimated to be 10.6% by subtracting the weight losses of the samples.
UV–vis DRS spectra in the range of 220–820 nm were provided to show the electronic absorption characteristics of the prepared samples (). Similar to the literature, the ZnO sample prepared in water only had an absorption peak in the UV region [Citation51]. The absorption edge of this sample was approximately 420 nm, with no absorption in the visible range. However, the ZnO NPs prepared in the presence of the extract owned a sharp absorption in UV range along with a wide absorption in visible wavelengths. The visible-light absorption of the ZnO NPs could be ascribed to the molecules of the extract combined to the ZnO NPs. Thus, the results obtained from the DRS data were concurrent with the TGA and FT-IR analysis, confirming the presence of the extract molecules over the prepared ZnO NPs.
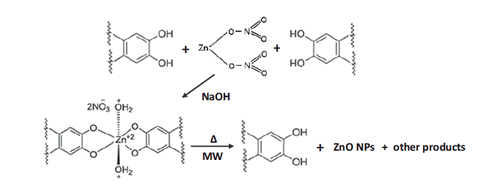
The utilized Vaccinium arctostaphylus L. fruit extract is rich in active constituents, including anthocyanins, flavonoids, polyphenols and vitamins. Phenolic compounds such as gallic, ellagic, p-hydroxybenzoic, m-hydroxybenzoic, chlorogenic, and sinapic acids [Citation50] have particularly significant role in bio-synthesis of ZnO NPs [Citation40]. It is believed that the hydroxyl groups of phenolic compounds form complexes through chelating with Zn2+ ions, forming a p-track conjugation effect. This factor benefits the synthesis process by capping ZnO NPs, thus controlling the shape, minimizing the size and forming more stable NPs after microwave step. The possible mechanism of ZnO NPs bio-synthesis using Vaccinium arctostaphylus L. fruit extract is shown in Scheme 2.
Antidiabetic activities
Fasting blood sugar
After diabetes was diagnosed, the studied groups were subjected to the aforementioned treatments and the blood samplings were carried out every 4 days. gives data regarding the effects of the studied anti-diabetic treatments on the fasting blood sugar (FBS) levels. A major drop in FBS level was detected in all the treated groups from the first sampling (day-4 of the treatment). The exception was group IV wherein the FBS increased in the first sampling and later on, decreased significantly. From the table, the highest decline in FBS was attained by the biologically synthesized ZnO NPs followed by chemically-synthesized ZnO NPs, insulin and extract treatments. The reduction in FBS levels can be attributed to the combination of factors: (i) the insulin-like function of Zn2+ ions released from the ZnO NPs; (ii) effect of zinc on the glucose metabolism through its action on the insulin increment and subsequent hepatic glycogenesis promotion [Citation2,Citation52]; (iii) the synergistic effects of Zn2+ ions on the expression and activity of increased Glucokinase and expression level of IRA, GLUT-2 in diabetic rats [Citation27,Citation53]; (iv) better penetration of bio-synthesized ZnO NPs compared to chem-synthesized ZnO NPs resulted from the smaller size of them, accordingly; (v) higher activities of bio-synthesized ZnO NPs of larger surface area than chem-synthesized ZnO NPs; (vi) presence of numerous bioactive compounds in the V. arctostaphylos fruit extract including polyphenols, flavonoids and anthocyanins [Citation54] with antioxidant and anti-hyperglycemic effects which intensified the expression of GLUT-4 and INS genes [Citation8]. Similar results were reported in the literature when they treated the diabetic rats with biologically prepared ZnO NPs. For instance, Bala et al. [Citation39] reported a significant reduction in FBS levels to 59.58% and 48.27% using Hibiscus subdariffa leaf extract-mediated ZnO NPs, synthesized at 60 °C and 100 °C, respectively.
Table 1. FBS Levels in the studied groups during the experiments.
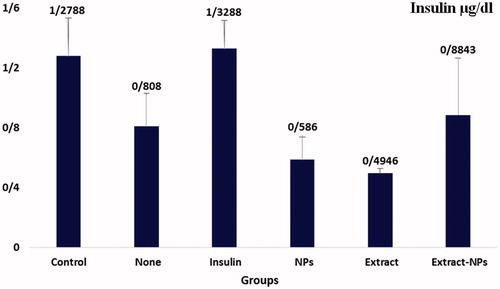
Insulin
Despite the increased insulin levels in the treated groups, the changes were not significant compared to the group (II) as the diabetic control group (. This shows that the decrease in the FBS was predominantly induced by Zn action and the employed plant extract. As mentioned earlier, zinc could act as insulin in the body and increase the glucose utilization by cells. Furthermore, the presence of an anthocyanoside named myrtillin was reported in Vaccinium arctostaphylos L. which has a similar mechanism of action to insulin [Citation55]. As a result, this compound could also have a role in the FBS reduction. Moreover, V. arctostaphylos contains chlorogenic acid and myricetin with antihyperglycemic effects that stimulates the glucose uptake in skeletal muscles and affects the glycogen metabolism respectively [Citation56,Citation57]. In addition, the existence of some bioactive components in the plant extract with α-glucosidase inhibitory effects could be responsible for glucose reduction in plasma [Citation58]. Therefore, bio-synthesized ZnO NPs played a crucial role in controlling the blood sugar level resulted from the interactive effects of zinc and the extract.
Lipid profile
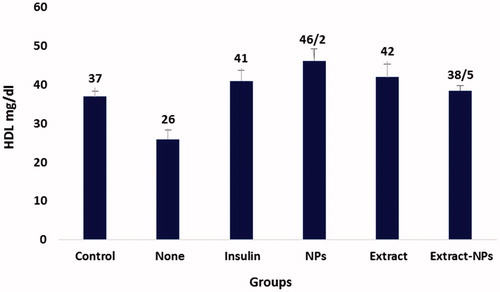
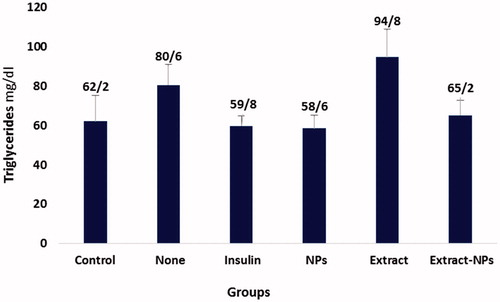
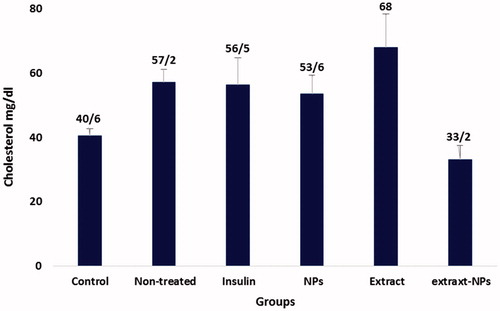
Lipid profile is an important parameter that needs to be controlled in the case of diabetes. The term diabetic dyslipidemia refers to the characteristic pattern in which the levels of serum total cholesterol (TC), triglyceride (TG), and low-density lipoprotein cholesterol (LDL) are elevated and contrariwise, the serum high-density lipoprotein cholesterol (HDL) level is decreased [Citation59,Citation60]. The diabetogenic agent, alloxan, derives hyperlipidemia in healthy rats, as employed in the present study. shows the changes in the HDL level of the studied groups in the sampling days. The HDL level was significantly increased in all groups under the treatment. On the other hand, the decrease in the TG levels was not significant under all treatment conditions and the only significant decrease in the cholesterol level was related to the extract-ZnO NPs ( and ). This could be ascribed to the positive effects of the zinc and extract on the blood glucose catabolism, thus, decreasing the need for lipid utilization and based on the feedback mechanism, the hyperlipidemia decreases in the body. The possible mechanism could be related to the insulin-like action of the both ZnO NPs and plant extract [Citation27,Citation61]. Furthermore, zinc could act similar to α-blocker, as reported for the short-term treatment of the dislipidemic patients [Citation62]. The obtained results evidently show the normalization of cholesterol and HDL levels in the diabetic rats by biologically synthesized ZnO NPs that indicates the efficient treatment of diabetes by this nanomedicine.
Conclusions
Diabetes mellitus is a common metabolic disorder with several complications associated with it. Due to the problems arising from the life-long administration of drugs in chronic disease, emerging alternative treatment strategies with higher bioavailability, lower dosing intervals and side-effects seem to be promising. In this study, the secondary metabolites in Vaccinium arctostaphylos L. fruit extract with antidiabetic potent were used to synthesis ZnO NPs by a process which is known as green synthesis. The presence of the extract molecules over the prepared ZnO NPs was proved using FT-IR, TGA, and UV-vis DRS characterizing techniques. The bio-synthesized ZnO NPs showed great treating efficacies on alloxan-diabetic rats in comparison to the chemically synthesized ZnO due to synergistic effect of the extract. Furthermore, bio-synthesized ZnO NPs were more effective in reducing the FBS level and had good control over the levels of some lipids in the treated diabetic rats. Based on the results obtained in this study, it is suggested that the biologically synthesized ZnO NPs could be considered as nano-antidiabetic drugs. However, more profound studies are required to investigate other aspects of using them.
Acknowledgements
The authors are grateful to the University of Mohaghegh Ardabili which financially supported this work.
Disclosure statement
The authors have no conflicts of interest regarding the publication of this manuscript.
Additional information
Funding
References
- Haase H, Overbeck S, Rink L. Zinc supplementation for the treatment or prevention of disease: current status and future perspectives. Exp Gerontol. 2008;43:394–408.
- Jansen J, Karges W, Rink L. Zinc and diabetes–clinical links and molecular mechanisms. J Nutr Biochem. 2009;20:399–417.
- Sun Q, van Dam RM, Willett WC, et al. Prospective study of zinc intake and risk of type 2 diabetes in women. Diabetes Care. 2009;32:629–634.
- Hasanah AN, Pessagno F, Kartasasmita RE, et al. Tetrabutylammonium methacrylate as a novel receptor for selective extraction of sulphonylurea drugs from biological fluids using molecular imprinting. J Mat Chem B. 2015;3:8577–8583.
- Rungby J. Zinc, zinc transporters and diabetes. Diabetologia. 2010;53:1549–1551.
- Smidt K, Jessen N, Petersen AB, et al. SLC30A3 responds to glucose- and zinc variations in beta-cells and is critical for insulin production and in vivo glucose-metabolism during beta-cell stress. PLoS One. 2009;4:e5684.
- Prasad AS. Biochemistry of Zinc. New York (NY): Springer Science & Business Media; 2013.
- Feshani AM, Kouhsari SM, Mohammadi S. Vaccinium arctostaphylos, a common herbal medicine in Iran: molecular and biochemical study of its antidiabetic effects on alloxan-diabetic Wistar rats. J Ethnopharmacol. 2011;133:67–74.
- Latti AK, Kainulainen PS, Hayirlioglu-Ayaz S, et al. Characterization of anthocyanins in caucasian blueberries (Vaccinium arctostaphylos L.) native to Turkey. J Agri Food Chem. 2009;57:5244–5249.
- Soltani R, Hakimi M, Asgary S, et al. Evaluation of the effects of Vaccinium arctostaphylos L. Fruit extract on serum lipids and hs-CRP levels and oxidative stress in adult patients with hyperlipidemia: a randomized, double-blind, placebo-controlled clinical trial. Evid Based Complement Alternat Med. 2014;2014:217451.
- Bansal SS, Goel M, Aqil F, et al. Advanced drug-delivery systems of curcumin for cancer chemoprevention. Cancer Prevent Res (Philadelphia, Pa). 2011;4:1158–1171.
- Raj KT, Gulam MK, Parajuli-Baral K, et al. Herbal medicine incorporated nanoparticles: advancements in herbal treatment. Asian J Biomed Pharm Sci 2013;3:7–14.
- Karthick V, Kumar VG, Dhas TS, et al. Effect of biologically synthesized gold nanoparticles on alloxan-induced diabetic rats-an in vivo approach. Colloids Surf B Biointerfaces. 2014;122:505–511.
- Sharma R, Gupta U, Garg NK, et al. Surface engineered and ligand anchored nanobioconjugate: an effective therapeutic approach for oral insulin delivery in experimental diabetic rats. Colloids Surf B Biointerfaces. 2015;127:172–181.
- Senapati S, Ahmad A, Khan MI, et al. Extracellular biosynthesis of bimetallic Au–Ag alloy nanoparticles. Small. 2005;1:517–520.
- Kumar VB, Kumar K, Gedanken A, et al. Facile synthesis of self-assembled spherical and mesoporous dandelion capsules of ZnO: efficient carrier for DNA and anti-cancer drugs. J Mater Chem B. 2014;2:3956–3964.
- Zeng K, Li J, Zhang Z, et al. Lipid-coated ZnO nanoparticles as lymphatic-targeted drug carriers: study on cell-specific toxicity in vitro and lymphatic targeting in vivo. J Mat Chem B. 2015;3:5249–5260.
- Erden PE, Zeybek B, Pekyardimc Ş, et al. Amperometric carbon paste enzyme electrodes with Fe3O4 nanoparticles and 1,4-Benzoquinone for glucose determination. Artificial Cells Nanomed Biotechnol. 2013;41:165–171.
- Sutradhar P, Saha M. Green synthesis of zinc oxide nanoparticles using tomato (Lycopersicon esculentum) extract and its photovoltaic application. J Exp Nanosci. 2016;11:314–327.
- Zheng Y, Fu L, Han F, et al. Green biosynthesis and characterization of zinc oxide nanoparticles using Corymbia citriodora leaf extract and their photocatalytic activity. Green Chem Lett Rev. 2015;8:59–63.
- Velmurugan P, Park J-H, Lee S-M, et al. Phytofabrication of bioinspired zinc oxide nanocrystals for biomedical application. Artificial Cells Nanomed Biotechnol. 2016;44:1529–1536.
- Rasmussen JW, Martinez E, Louka P, et al. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Expert Opin Drug Deliv. 2010;7:1063–1077.
- Padalia H, Chanda S. Characterization, antifungal and cytotoxic evaluation of green synthesized zinc oxide nanoparticles using Ziziphus nummularia leaf extract. Artificial Cells Nanomed Biotechnol. 2017. DOI:10.1080/21691401.2017.1282868
- Sharma N, Jandaik S, Kumar S, et al. Synthesis, characterisation and antimicrobial activity of manganese- and iron-doped zinc oxide nanoparticles. J Exp Nanosci. 2016;11:54–71.
- Das D, Nath BC, Phukon P, et al. Synthesis of ZnO nanoparticles and evaluation of antioxidant and cytotoxic activity. Colloids Surfaces B, Biointerfaces. 2013;111:556–560.
- Nagajyothi PC, Sreekanth TVM, Tettey CO, et al. Characterization, antibacterial, antioxidant, and cytotoxic activities of ZnO nanoparticles using Coptidis Rhizoma. Bioorg Med Chem Lett. 2014;24:4298–4303.
- Alkaladi A, Abdelazim AM, Afifi M. Antidiabetic activity of zinc oxide and silver nanoparticles on streptozotocin-induced diabetic rats. Int J Mol Sci. 2014;15:2015–2023.
- El-Mekawy RE, Jassas RS. Recent trends in smart and flexible three-dimensional cross-linked polymers: synthesis of chitosan-ZnO nanocomposite hydrogels for insulin drug delivery. MedChemComm. 2017;8:897–906.
- Stankic S, Suman S, Haque F, et al. Pure and multi metal oxide nanoparticles: synthesis, antibacterial and cytotoxic properties. J Nanobiotechnol. 2016;14:73.
- Yang Y, Chen H, Zhao B, et al. Size control of ZnO nanoparticles via thermal decomposition of zinc acetate coated on organic additives. J Crystal Growth. 2004;263:447–453.
- Cheng B, Samulski ET. Hydrothermal synthesis of one-dimensional ZnO nanostructures with different aspect ratios. Chem Commun. 2004;986–987.
- Stanković A, Dimitrijević S, Uskoković D. Influence of size scale and morphology on antibacterial properties of ZnO powders hydrothemally synthesized using different surface stabilizing agents. Colloids Surfaces B: Biointerfaces. 2013;102:21–28.
- Znaidi L. Sol–gel-deposited ZnO thin films: a review. Mat Sci Eng: B. 2010;174:18–30.
- Mishra SK, Srivastava RK, Prakash S, et al. Photoluminescence and photoconductive characteristics of hydrothermally synthesized ZnO nanoparticles. Opto-Electronics Rev. 2010;18:467–473.
- Nagvenkar AP, Deokar A, Perelshtein I, et al. A one-step sonochemical synthesis of stable ZnO-PVA nanocolloid as a potential biocidal agent. J Mat Chem B. 2016;4:2124–2132.
- Sangshetti JN, Dharmadhikari PP, Chouthe RS, et al. Microwave assisted nano (ZnO–TiO2) catalyzed synthesis of some new 4,5,6,7-tetrahydro-6-((5-substituted-1,3,4-oxadiazol-2-yl)methyl)thieno[2,3-c]pyridine as antimicrobial agents. Bioorg Med Chem Lett. 2013;23:2250–2253.
- Zhang R, Fan L, Fang Y, et al. Electrochemical route to the preparation of highly dispersed composites of ZnO/carbon nanotubes with significantly enhanced electrochemiluminescence from ZnO. J Mater Chem. 2008;18:4964–4970.
- Ramazani SAA, Tamsilian Y, Shaban M. Synthesis of nanomaterials. In: Parameswaranpillai J, Hameed N, Kurian T, et al. Nanocomposite Materials: synthesis, properties and applications. FL: CRC Press; 2016. p. 37–80.
- Bala N, Saha S, Chakraborty M, et al. Green synthesis of zinc oxide nanoparticles using Hibiscus subdariffa leaf extract: effect of temperature on synthesis, anti-bacterial activity and anti-diabetic activity. RSC Adv. 2015;5:4993–5003.
- Velmurugan P, Park J-H, Lee S-M, et al. Eco-friendly approach towards green synthesis of zinc oxide nanocrystals and its potential applications. Artificial Cells Nanomed Biotechnol. 2016;44:1537–1543.
- Buazar F, Bavi M, Kroushawi F, et al. Potato extract as reducing agent and stabiliser in a facile green one-step synthesis of ZnO nanoparticles. J Exp Nanosci. 2016;11:175–184.
- Shanmugasundaram T, Balagurunathan R. Bio-medically active zinc oxide nanoparticles synthesized by using extremophilic actinobacterium, Streptomyces sp. (MA30) and its characterization. Artificial Cells Nanomed Biotechnol. 2017. DOI:10.1080/21691401.2016.1260577
- Ahmed S, Chaudhry SA, Ikram S. A review on biogenic synthesis of ZnO nanoparticles using plant extracts and microbes: A prospect towards green chemistry. J Photochem Photobiol B. 2017;166:272–284.
- Vishwakarma K. Green synthesis of ZnO nanoparticles using abrus precatorius seeds extract and their characterization [dissertation]. 2013. Available from: http://ethesis.nitrkl.ac.in/5012/
- Sabir S, Arshad M, Chaudhari SK. Zinc oxide nanoparticles for revolutionizing agriculture: synthesis and applications. Sci World J. 2014;2014:925494.
- Mishra BG, Rao GR. Promoting effect of ceria on the physicochemical and catalytic properties of CeO2?ZnO composite oxide catalysts. J Mol Catal A: Chem. 2006;243:204–213.
- Javed R, Usman M, Tabassum S, et al. Effect of capping agents: structural, optical and biological properties of ZnO nanoparticles. Appl Surface Sci. 2016;386:319–326.
- Kadam A, Dhabbe R, Gophane A, et al. Template free synthesis of ZnO/Ag2O nanocomposites as a highly efficient visible active photocatalyst for detoxification of methyl orange. J Photochem Photobiol B. 2016;154:24–33.
- Shekofteh-Gohari M, Habibi-Yangjeh A. Fabrication of novel magnetically separable visible-light-driven photocatalysts through photosensitization of Fe3O4/ZnO with CuWO4. J Industrial Eng Chem. 2016;44:174–184.
- Ayaz FA, Hayirlioglu-Ayaz S, Gruz J, et al. Separation, characterization, and quantitation of phenolic acids in a little-known blueberry (Vaccinium arctostaphylos L.) Fruit by HPLC-MS. J Agric Food Chem. 2005;53:8116–8122.
- Lee KM, Lai CW, Ngai KS, et al. Recent developments of zinc oxide based photocatalyst in water treatment technology: a review. Water Res. 2016;88:428–448.
- Rink L, Kirchner H. Zinc-altered immune function and cytokine production. J Nutr. 2000;130:1407S–1411S.
- Garg VK, Gupta R, Goyal RK. Hypozincemia in diabetes mellitus. J Assoc Physicians India. 1994;42:720–721.
- Kianbakht S, Hajiaghaee R. Anti-hyperglycemic effects of Vaccinium arctostaphylos L. fruit and leaf extracts in alloxan-induced diabetic rats. J Med Plants. 2013;1:43–50.
- Murray M. Bilberry (Vaccinium myrtillus). Am J Nat Med. 1997;4:18–22.
- Ong C, Lim JZ, Ng CT, et al. Silver nanoparticles in cancer: therapeutic efficacy and toxicity. CMC. 2013;20:772–781.
- Ong KC, Khoo HE. Effects of myricetin on glycemia and glycogen metabolism in diabetic rats. Life Sci. 2000;67:1695–1705.
- Salehi P, Asghari B, Esmaeili MA, et al. Glucosidase and-amylase inhibitory effect and antioxidant activity of ten plant extracts traditionally used in Iran for diabetes. J Med Plants Res. 2013;7:257–266.
- Buse JB, Tan MH, Prince MJ, et al. The effects of oral anti-hyperglycaemic medications on serum lipid profiles in patients with type 2 diabetes. Diabetes Obes Metab. 2004;6:133–156.
- Chapman MJ, Ginsberg HN, Amarenco P, et al. Triglyceride-rich lipoproteins and high-density lipoprotein cholesterol in patients at high risk of cardiovascular disease: evidence and guidance for management. Uropean Heart J. 2011;32:1345–1361.
- Vardatsikos G, Pandey NR, Srivastava AK. Insulino-mimetic and anti-diabetic effects of zinc. J Inorg Biochem. 2013;120:8–17.
- Grimm JRH, Flack JM, Grandits GA. Long-term effects on plasma lipids of diet and drugs to treat hypertension. Treatment of Mild Hypertension Study (TOMHS) Research Group. JAMA. 1996;275:1549–1556.