Abstract
Radiosensitizers that increase cancer cell radio-sensitivity can enhance the effectiveness of irradiation and minimize collateral damage. Nanomaterial has been employed in conjunction with radiotherapy as radiosensitizers, due to its unique physicochemical properties. In this article, we evaluated selenium nanoparticles (Nano-Se) as a new radiosensitizer. Nano-Se was used in conjunction with irradiation on MCF-7 breast cancer cells, and efficacy and mechanisms of this combined treatment approach were evaluated. Nano-Se reinforced the toxic effects of irradiation, leading to a higher mortality rate than either treatment used alone, inducing cell cycle arrest at the G2/M phase and the activation of autophagy, and increasing both endogenous and irradiation-induced reactive oxygen species formation. These results suggest that Nano-Se can be used as an adjuvant drug to improve cancer cell sensitivity to the toxic effects of irradiation and thereby reduce damage to normal tissue nearby.
Introduction
Breast cancer is the most common form of cancer afflicting women worldwide and is also a major cause of cancer-related deaths [Citation1]. Though breast cancer death rate has been decreasing in the past decade owing to widespread employment of surgery as the primary therapeutic method and more effective pre- and post-operative radiotherapy, the mortality is still on the rise [Citation2]. The majority of breast cancer patients are routinely managed with conservative surgery followed by radiotherapy and systemic therapies, including hormonotherapy and chemotherapy [Citation3]. Radiotherapy plays indispensable roles in suppressing metastasis and regeneration of tumour tissue; simultaneously, normal tissue closing to the tumour site is inevitably exposed to irradiation during radiotherapy, especially chest and abdominal tissue moving on account of respiration [Citation4]. Nanomaterial has been more and more widely utilized in cancer research because of its unique physicochemical properties, particularly in radiotherapy, chemotherapy and integrated cancer diagnosis and treatment [Citation5–7].
Recently, selenium, a trace element, has attracted great interest due to its important health effects, especially the effects related to the immune reaction and cancer prevention activity [Citation8,Citation9]. Results of epidemiological, pre-clinical and clinical studies indicated that selenium can reduce risk of a series of cancers, such as liver, mammary, prostate, colon and lung cancer [Citation10,Citation11]. Clinical studies also showed that plasma selenium level in cancer patients was dramatically reduced during radiotherapy [Citation12]. Some molecular selenium compounds, such as sodium selenite, selenomethionine and methylselenocysteine, have more effective anti-cancer activity at high dosage [Citation13–15]. As an anti-tumour agent and essential microelement, the effective dose of selenium is close to the toxic range, which immensely limits its clinical application [Citation16,Citation17]. However, selenium nanoparticles (Nano-Se) attract increasing attention due to its excellent bioavailability, high biological activity and low toxicity [Citation18,Citation19]. The toxicity reported for elemental selenium (Se0) at nano-size is lower than selenite (Se+2 or Se+4) ions; therefore, selenium nanoparticles may be a good candidate to replace other types of selenium in nutritional supplements or pharmaceutical dosage types [Citation20]. Therefore, it is possible that Nano-Se can be utilized as a radiation sensitizer to improve radiotherapy effects and reduce side effects.
Autophagy is a conserved process of protein degradation in which double-membrane vesicles known as autophagosomes engulf intracellular content such as endoplasmic mitochondria, reticulum and ribosomes and fuse with lysosomes for degradation of these organelles [Citation21]. Basal level of autophagy is indispensable for homeostasis maintaining. In the meantime, autophagy provides opportunities for cells to overcome metabolic stress and nutrient deprivation. For example, autophagic level in cancer cells growing in hypoxic environment is frequently elevated as observed [Citation22,Citation23]. Autophagy can be induced by many physical, chemical and biological factors such as starvation, irradiation and chemotherapy. Autophagy and consequent death can be induced by anti-tumour drugs in different types of tumour cells. However, in fact, a variety of materials on nanoscale such as quantum dots [Citation24], gold and iron core-gold shell nanoparticles [Citation25], fullerene C60 [Citation26], grapheme [Citation27], MnO nanocrystals [Citation28], iron oxide [Citation29] and rare earth nanocrystals [Citation30], have also been certified to be inducers of autophagy. Though apoptosis is the primary mechanism of irradiation-induced cell death, autophagy has been determined as a significant mechanism of tumour cell death induced by irradiation. In addition, simultaneous targeting of autophagy and apoptosis during radiotherapy appears to further enhance anti-tumour efficiency [Citation31].
The aim of this study was to assess possible mechanisms through which Nano-Se improve the outcome of irradiation, and to assess the relationship between autophagy induced by Nano-Se and their irradiation enhancing effect, at clinically relevant levels of Megavoltage X-rays. To this end, we researched the anti-cancer effects of the Nano-Se in MCF-7 breast cancer cells. Irradiation treatment combined with Nano-Se induced cell death to a greater degree than irradiation alone, activated autophagy, increased irradiation-induced ROS formation and led to cell cycle arrest at the G2/M phase. These findings could have an important role in biomedical applications of Nano-Se and clinical treatment of breast cancer.
Methods
Materials and methods
MCF-7 breast carcinoma cells were obtained from the School of Biological Science and Medical Engineering of Southeast University (Nanjing, People’s Republic of China). Cells were incubated in Roswell Park Memorial Institute (RPMI) 1640 medium containing 10% fetal bovine serum (FBS), and maintained at 37 °C in a humidified incubator with 95% air/5% CO2. The Cyto-ID Autophagy Detection kit was purchased from Enzo Life Sciences (Plymouth Meeting, PA). Unless otherwise specified, all chemicals were purchased from Sigma-Aldrich (China) or Beyotime Institute of Biotechnology (Nanjing, China), at the highest grade available and used as received.
Preparation and characterization of nano-Se
At the beginning of the experiment, a stock solution of 10 mM sodium selenite was prepared by dissolving 345.9 mg sodium selenite powder in 200 ml Milli-Q water. Forty millilitre of 10 nM sodium selenite was mixed with 160 ml of 10 mM glutathione (GSH) solution containing 200 mg bovine serum albumin (BSA). The mixture was adjusted to 7.1 by the addition of .1 M sodium hydroxide, forming Nano-Se and oxidized glutathione (GSSG) instantly [Citation9]. The resulting red solution was dialysed against double distilled water for 72 h, and the water was changed every 6 h to separate GSSG from the Nano-Se. Nano-Se solution was stored at 4 °C until use.
The synthesized nanoparticles were primarily characterized by UV–vis spectroscopy (Cary UV-2250, Agilent Technologies, Santa, Clara, CA, USA) from 900 to 200 nm followed by high-resolution transmission electron microscope (TEM, JEM-2100, JEOL Ltd., Tokyo, Japan) equipped with an energy-dispersive analyzer. TEM samples were prepared by depositing drops of suspended particle solution onto a carbon-coated copper grid and drying at room temperature [Citation6,Citation7]. The mean size was calculated from a random field of TEM images that showed the general morphology of nanoparticles. Over 150 particles were counted and measured to determine the mean sizes and size distribution [Citation32]. The diameters of Nano-Se were measured by Nano Measurer. The final concentrations of selenium in aqueous solution were determined by Atomic Absorption Spectrophotometer (AAS) using TAS-990 (Beijing Purkinje Central Instrument CO., LTD, Beijing, China).
Irradiation treatment
First, cells were trypsinized, resuspended and dispensed in different culture wells depending on the experiment requirement. After 24 h, various concentrations of Nano-Se were added to the medium. One day later, cells were washed with phosphate buffer saline (PBS), received new culture medium and then radiated by linear accelerator (Varian Medical Systems, Palo Alto, CA) with X-rays (6-MeV, 200 cGy/min). After irradiation, cultures were incubated at 37 °C in 95% air/5% CO2 until harvested at the fixed time points.
Cell vitality assay and cellular uptake of NPs
Cell vitality was detected by CCK-8 cell counting kit (Vazyme biotech Co., Ltd. China) according to the manufacture’s instruction. MCF-7 cells, after pre-treat described above, CCK-8 regent was added to wells and incubated for 4 h, and then the absorbance at 450 nm was measured by a microplate reader. The cells treated with Nano-Se were the experimental controls (Read A), while the medium was the condition controls (Read B). The cells untreated with Nano-Se were set as the negative controls (Read C). The effect of Nano-Se on cells was expressed as the percentage of cell viability calculated as the following formula: Cell vitality (%) = (A − B)/(C − B) × 100% [Citation32].
In this study, internalization of selenium nanoparticles in MCF-7 cells was evaluated by flow cytometry using light scattering method. Cells were washed three times by PBS and cultured in medium containing varying concentrations of selenium nanoparticles. The MCF-7 cells growing in medium lacking nanoparticles serve as negative control. Cells were trypsinized, harvested, washed and finally suspended into 1 ml PBS for flow cytometry for nanoparticle internalization.
Colony formation assay
Radiosensitivity was evaluated with the clonogenic survival assay. Cells were suspended and plated at known numbers. After 1 day for attachment, cells were treated with vehicle or Nano-Se for 24 h before exposure to irradiation at doses of 0, 2, 4, 6 or 8 Gy. After 10 days of incubation, colonies were washed with cold PBS, stained with Giemsa dye and then the surviving fraction was confirmed by counting colonies consisting of >50 cells. The surviving cell rate was normalized to the survival of the control cells. Irradiation survival curves were constructed by normalizing the number of surviving colonies after combined treatment to the number of surviving colonies following vehicle treatments.
Determination of survival and apoptosis rates
To determine the role of apoptosis in the cell death induced by the combined treatment of Nano-Se and irradiation, cells were stained with Annexin V/PI and analysed using flow cytometry. Cells were seeded in 6-well plates. After incubation of 24 h, cells were treated with vehicle control or Nano-Se for 24 h followed by exposure to different doses of irradiation. The next day, cells were trypsinized, resuspended in 10% FBS-containing medium, centrifuged and washed twice in cold PBS, counted and resuspended in annexin-binding buffer; 5 μl Annexin V-fluorescein isothiocyanate (FITC) and 10 μl propidium iodide (PI) were added to a 100-μl cell suspension and the mixture was stored in the dark for 15 min at room temperature. A 400 μl volume of annexin-binding buffer was added to the cells, followed by flow cytometry analysis within 1 h.
Cell cycle analysis
Cycle analysis was implemented by PI/RNase staining (Beyotime Institute of Biotechnology, Nanjing, China) as manual described. In brief, treated cells were collected, fixed in 70% ethanol at 4 °C overnight, washed with cold PBS, labelled with PI/RNase staining buffer, and then sorted using a FACSCalibur instrument by flow cytometry.
Quantitative assay of autophagy
To quantify the intensity of autophagy, cells were labelled and analysed with flow cytometry according to the manufacturer’s protocol. Cells were seeded on coverslips in six-well plates. After 24-h incubation, cells were treated with vehicle or Nano-Se for 24 h at 37 °C followed by exposure to different doses of radiation. The next day, cells were carefully washed with buffer, and detection reagent (prepared by diluting Cyto-ID green autophagy detection reagent) was applied for 30 min in the dark. Cells were then fixed and washed several times, and were analysed with FCM to determine the autophagic potency.
Detection of ROS
Intracellular ROS levels were confirmed by the membrane-permeable fluorescent probe 2′7′-dichlorofluorescin diacetate (DCFH-DA) using flow cytometry. Cells were seeded in 6-well plates for 24 h, treated with vehicle control or Nano-Se for 24 h and then exposed to different doses of irradiation. The cells were collected 24 h later, washed, incubated with DCFH-DA for 30 min, washed three times with serum-free RPMI 1640 and then sorted using flow cytometry. The intracellular ROS levels are represented as mean fluorescence intensity.
Statistical analysis
Data are presented as mean ± standard deviation of at least three independent experiments and were processed with OriginPro 8.0 (Origin Lab, Northampton, MA, USA). One-way analysis of variance and a Duncan test were used to analyse the difference between the untreated and treated groups, in which a p values < .05 was considered statistically significant.
Results and discussion
Functionalization and characterization of nano-Se
Many kinds of method have been developed for the synthesis of selenium nanoparticles with controllable shapes, including physical, chemical and biological methods [Citation17]. Ultra-small Nano-Se was successfully prepared by a chemical method and characterized by TEM and UV in this study. Besides, GSH, one of the most commonly applied stabilizers and protective agents for Nano-Se synthesis, was used as the protecting agent during the synthesizing to promote nucleation and prevent aggregation [Citation11]. The TEM images and the size distribution patterns showed that the synthesized Nano-Se presents monodisperse spherical shape with diameter about 27.5 ± 4.3 nm (). Nano-Se solution shows a clear red-brown colour and is very stable. It was not sedimented and the average diameter had no significant change for as long as 5 months storage at 4 °C. Nano-Se solution was sterilized by filtration before application.
Figure 1. The representative TEM images (left), size distribution histograms (right), and UV analysis of Nano-Se. (A–D) The morphology of as-synthesized Nano-Se exposure to different irradiation was characterized by TEM and their size distribution histograms were obtained by size analysis of over 150 particles. The mean diameters were 27.5, 25.8, 22.4 and 19.82 nm, respectively. Scale bar were 50 nm. (E) UV–vis spectroscopy of Nano-Se exposure to different irradiation.
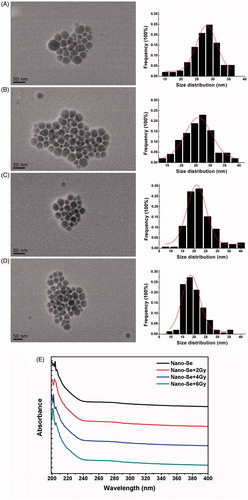
Exposure to 2 Gy irradiation decreased the diameter of Nano-Se in solution to 25.8 ± 5.2 nm (), 4 Gy decreased to 22.4 ± 5.8 nm (), and 6 Gy decreased to 19.82 ± 4.7 nm (). displayed that these nanoparticles were with similar shapes and spherical ends before or after irradiation. These results suggest that small doses of irradiation do not destroy the structures of selenium nanoparticles but may induce a higher concentration of selenium ion. And Clark et al reported that Nano-Se has a 7-fold acute toxicity than sodium selenium in mice (LD50 113 and 15 mg Se/kg body weight, respectively), and high doses of selenite give rise to more concerns about its toxicity [Citation33]. So, the toxicity of intracellular Nano-Se can be increased after irradiation, which may possess excellent potential as an anti-cancer agent in radiotherapy.
The UV-vis spectroscopy has been proved to be a very significant technique for analysis of nanoparticles. As seen in , the results of absorption spectra of Nano-Se under irradiation (0, 2, 4, or 6 Gy) displayed different absorption spectra, 204 nm for Nano-Se, 203 nm for Nano-Se after 2 Gy, 202 nm for Nano-Se after 4 Gy, and 201 nm for Nano-Se after 6 Gy, which were in accordance with the change of average diameters.
The biocompatibility and uptake of nano-Se
To study the biocompatibility of Nano-Se, we executed the cell viability assay. First, MCF-7 cells were treated with different concentrations for 24 h, and cell viabilities were determined by CCK-8 assay 24 h later. As seen in , Nano-Se reduced cell viability in a dose-dependent manner. Cell viability was decreased about 10% in cells incubated with Nano-Se (0.15 μg/ml) compared to untreated controls, as cell viability was decreased about 20% with .3 μg/ml Nano-Se. These results revealed that treating MCF-7 breast cancer cells with Nano-Se led to dose-dependent toxicity. Taken these results into account, .15 and .3 μg/ml Nano-Se was used in the subsequent studies.
Figure 2. The biocompatibility and uptake of Nano-Se. (A) The viability of MCF-7 cells determined 24 h after treatment with Nano-Se at various concentrations. (B) Particles uptake by MCF-7 cells using flow cytometry.
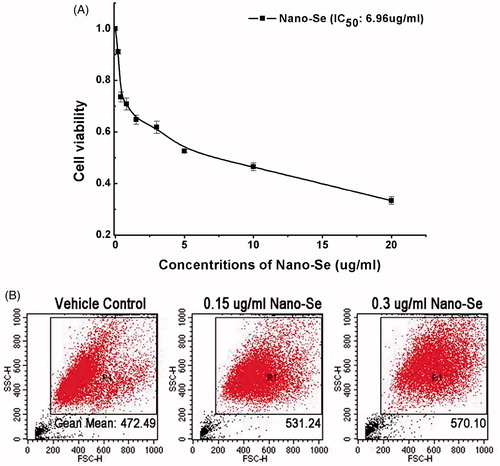
The cellular uptake is a very vital process for the biomedical uses of nanomaterial, as it is critical for the nanomaterial to be able to execute their functions [Citation34]. Quantitative flow-cytometry was used for the quantification of selenium in cell to research the cellular uptake of Nano-Se. The side scatter (SSC) intensity is mainly dependent on internal structure of cells and granularity [Citation35]. An increased SSC suggesting uptake of nanoparticles has been reported in cultured human derived retinal pigment epithelial cells exposed to 0–30 μg/ml TiO2 nanoparticles [Citation35].
Quantitative flow-cytometry showed an enormous increase in intensity of SSC in MCF-7 cells for active Nano-Se at 24 h in comparison to vehicle control cells shown in , revealing that cellular uptake was higher for high concentration of Nano-Se solution.
Nano-Se enhance irradiation-induced cell death
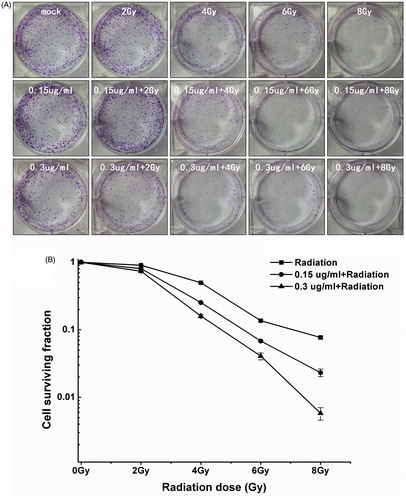
To determine whether Nano-Se increase cellular radio-sensitivity, breast cancer cells stimulated to single or both treating were evaluated with the clonogenic assay. Breast cancer cells were exposed to different concentrations of Nano-Se (0, .15 or .3 μg/ml) for 24 h before irradiation at one of six doses (0, 2, 4, 6 or 8 Gy).
As been seen in , exposure to 4 Gy irradiation decreased the colony formation rate to 49.62 ± .55%, however at .15 or .3 μg/ml Nano-Se under 4 Gy irradiation, colony formation was reduced to 25.27 ± .45% or 15.97 ± .95%, respectively. While exposure to 6 Gy irradiation decreased to 13.58 ± .40%, and the colony formation was reduced to 6.81 ± .17% or 4.06 ± .47% at .15 or .3 μg/ml under 6 Gy irradiation, respectively. The anti-cancer effects of other doses of irradiation on the colony formation were similarly enhanced by combined treatment with Nano-Se, reducing the colony forming efficiency of cancer cells to a larger extent, suggesting that Nano-Se sensitize cells to the effects of irradiation.
Figure 3. Enhancement of irradiation-induced growth inhibition by Nano-Se. (A) Cloning formations of irradiation with or without Nano-Se. (B) The surviving fraction of MCF-7 was decreased by irradiation in a dose-dependent manner, an effect that was potentiated by the addition of Nano-Se.
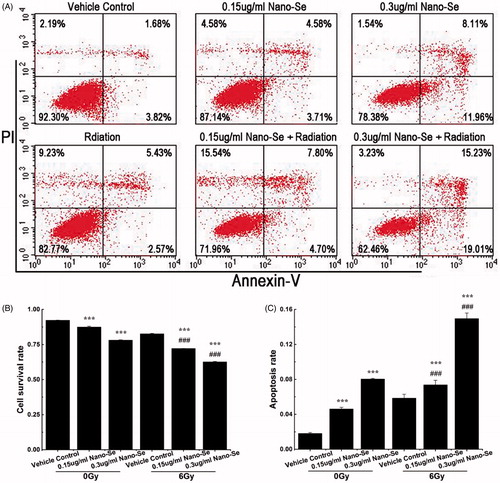
Apoptosis is a process of programmed cell death occurred in multicellular organism, in which morphological and molecular changes, such as nuclear fragmentation, chromatin condensation, cell shrinkage and chromosomal DNA fragmentation can be seen [Citation36]. To determine the rates of cell mortality and apoptosis under combined treatment with Nano-Se and irradiation, cells were stained with Annexin V/PI and analysed by flow cytometry. Annexin V-FITC is used as a non-quantitative probe to label cell surface expression of phosphatidyl serines, early markers for apoptosis. PI can permeate cells with damaged cell membrane and be therefore used to identify apoptotic cells or necrotic cells. In each plot, the lower left corner shows living cells (annexin V-/PI-negative); the lower right corner shows apoptotic cells (annexin V-positive); the top right corner shows dead cells stained with annexin V while membranes permeable to PI (annexin/PI-positive); and the top left corner shows dissociated nuclei (PI positive) [Citation6,Citation7].
Pre-treatment with Nano-Se and radiotherapy inhibited cell proliferation to a greater level than irradiation alone, shown in . Treatment with .15 μg/ml Nano-Se, .3 μg/ml Nano-Se or irradiation at a dose of 6 Gy increased cell death by 4.82 ± .59%, 14.11 ± .21% and 9.63 ± .33%, respectively. However, .15 μg/ml Nano-Se combined with 6 Gy irradiation increased cell death up to 20.16 ± .1% (% apoptosis: 7.37 ± .46%) (p < .001 versus 6 Gy irradiation or Nano-Se alone), while .3 μg/ml Nano-Se combined with irradiation increased cell death up to 29.67 ± .34% (% apoptosis: 14.94 ± .64%) (p < .001 versus 6 Gy irradiation or Nano-Se alone). These results confirm that Nano-Se can exert relatively lower toxicity, potentiate irradiation-induced death of cancer cells, and therefore reduce damages to surrounding tissue in irradiation therapy.
Figure 4. Enhancement of irradiation-induced mortality and apoptosis by Nano-Se. (A) MCF-7 cells were exposed to indicated concentrations of Nano-Se or 6 Gy or both, and stained with Annexin V/PI before flow cytometry analysis. Cell survival rates (B) and quantitative analysis of apoptosis cell percentage (C) obtained after flow cytometry evaluation (*p < .05, **p < .01, ***p < .001, the Nano-Se group (0/6 Gy) compared with vehicle control group (0/6 Gy); #p < .05, ##p < .01, ###p < .001, the combined group (Nano-se and radiation) compared with Nano-Se group).
Nano-Se enhance irradiation-induced G2/M arrest
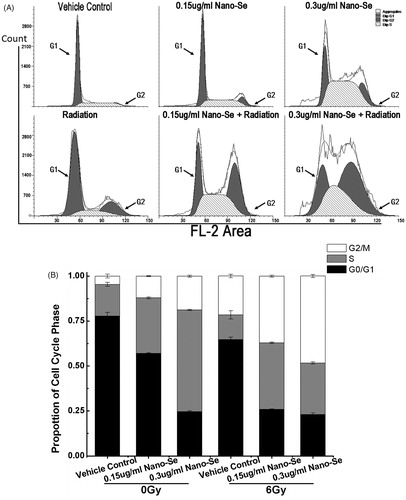
As tumour cells are known to accumulate in the G2 phase of cell cycle after irradiation exposure, we tested the effects of combined treatment on cell cycle regulation using flow cytometry to investigate the detailed mechanism by which Nano-Se enhance cancer cell radio-sensitivity.
Treatment with Nano-Se and irradiation increased the proportion of cells in G2/M phase and decreased in G0/G1 phase as compared to untreated control and irradiation (). For example, 5.65 ± 1.10% of control cells were in G2/M phase, whereas Nano-Se treatment at concentration of .15 μg/ml increased the G2/M fraction to 12.18 ± .21%. The value increased to 37.40 ± .56% (p < .001 versus 6 Gy irradiation or Nano-Se alone) when Nano-Se (.15 μg/ml) were combined with 6 Gy irradiation. Additionally, .3 μg/ml Nano-Se treatment increased the G2/M fraction to 18.80 ± .58%, which further increased to 48.39 ± .82% (p < .001 versus 6 Gy irradiation or Nano-Se alone), when combined with 6 Gy irradiation. These results show that simultaneous exposure of cells to Nano-Se and irradiation causes acceleration through G1/S and arrest in G2/M phase. As cells are most sensitive to irradiation in G2/M and most resistant in G0/G1 phase [Citation37], one potential mechanism by which Nano-Se enhance the effects of irradiation is by regulating cell cycle progression.
Figure 5. Effect of Nano-Se on MCF-7 cell cycle distribution. (A) Cells were pre-treated with the vehicle or Nano-Se for 24 h before exposure to 0 Gy or 6 Gy irradiation, and the fraction of cells in each phase of the cell cycle was analysed by flow cytometry. (B) Quantitative analysis of cell cycle distribution.
Combined nano-Se and irradiation treatment induces autophagy
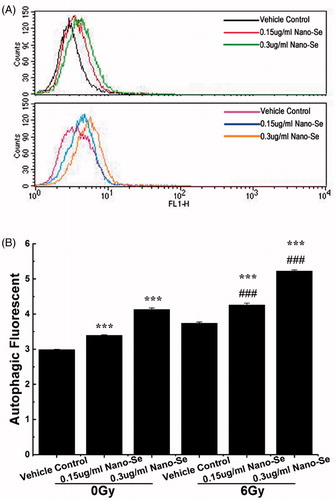
Autophagy can act as programmed cell death (Type II) or play a cytoprotective role in chemotherapeutic treatment. Various kinds of stress signals such as nutrient starvation or treatment with different anti-cancer agents arouse the autophagy process [Citation32]. The final consequence of activation of autophagy program is highly relay on the cellular environment and the strength and duration of the stress-inducing signals [Citation38]. Recent reports suggest that irradiation and certain anti-cancer drugs exert their effects via autophagy and consequently, tumour cell death. Activity of autophagy is typically low under normal conditions, but can be up-regulated by endogenous and external stimulus such as nutrient starvation, energy depletion, irradiation and invasion by foreign matters [Citation38]. Autophagy can be detected by transmission electron microscopy (TEM) or other detection methods that allow the visualization of autophagic vacuole accumulation in the cytoplasm. To confirm whether autophagy was induced by the combined treatment, we used a novel autophagy detection kit that uses the green fluorescent probes Cyto-ID to label autophagic vacuoles, which weakly stains lysosomes and acts as a selective marker of autolysosomes and other early autophagic compartments.
Cells were incubated with Nano-Se of different doses, exposed to 0 or 6 Gy irradiation and then stained with Cyto-ID. Exposure to irradiation or Nano-Se alone did not generate the obvious formation of LC3-positive in flow. However, the combination of Nano-Se and 6 Gy irradiation increased the number of LC3-positive compared to those treated irradiation alone as shown in . These results were determined by flow cytometry and suggest that the augment of autophagic level is a mechanism by which Nano-Se increase sensitivity of radiotherapy on the cells. However, additional studies are needed to study the relationship between the change of autophagic level and the increased rate of cell death.
Figure 6. Detection of autophagy in MCF-7 cells by flow cytometry. (A) Cells were treated with indicated concentrations of Nano-Se without or with subsequent irradiation treatment. A 488 nm laser source and the FL1 channel were used to detect the green florescence of autophagic cells. (B) Quantitative analysis of autophagy (*p < .05, **p < .01, ***p<.001, the Nano-Se group (0/6 Gy) compared with vehicle control group (0/6 Gy); #p < .05, ##p < .01, ###p < .001, the combined group (Nano-se and radiation) compared with Nano-Se group).
Determining roles of autophagy in combined treatments
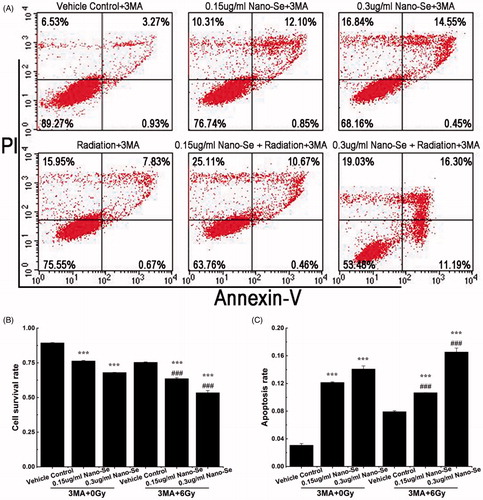
In this study, our main purpose was to find out the significance of autophagy to the irradiation sensitizing effect of Nano-Se. To investigate the relationship between anti-cancer effect and autophagy, we pre-treated MCF-7 cells with the autophagy inhibitor, 3-methyladenine (3-MA) and then processed cells with vehicle control, Nano-Se or Nano-Se plus irradiation. 3-MA is an inhibitor of phosphatidylinositol 3-kinases (PI3K), which plays important roles in many biological processes including controlling the activation of mTOR [Citation31]. The cross talk between induction of autophagy and apoptosis in tumour is complicated. On one hand, apoptotic signalling leads to protective autophagy; on the other hand, excessive autophagy may swallow the cellular organelles beyond a certain threshold, causing irreversible disorder of vital functions in cells, as the volume occupied by autophagic vacuoles may be roughly equivalent to the free cytosol and organelles during autophagy [Citation39,Citation40].
As shown in , .15 μg/ml Nano-Se followed by 3-MA (5 μM) increased cell death to 23.66 ± .34% (% apoptosis: 12.14 ± .09%), while .3 μg/ml Nano-Se followed by 3-MA increased cell death to 32.04 ± .17% (% apoptosis: 14.08 ± .45%). Moreover, Nano-Se (.15 μg/ml) combined with irradiation followed by 3-MA increased cell death up to 36.42 ± .70% (% apoptosis: 10.66 ± .01%), while Nano-Se (.3 μg/ml) combined with irradiation followed by 3-MA increased cell death up to 46.61 ± 1.67% (% apoptosis: 16.54 ± .58%). The results showed that the mortality rate resulting from combined treatment with Nano-Se plus irradiation was significantly improved by 3-MA. In this study, autophagy induced by Nano-Se or/and irradiation in MCF-7 cells was determined to be protective, which was a prosurvival process of the cancer cells facing Nano-Se associated irradiation, while the apoptosis rate was dramatically improved after 3-MA added into the culture medium. After treatment with inhibitors, the increase in rate of apoptosis caused by combined treatment of Nano-Se and irradiation indicated that there was an association between autophagy and apoptosis.
Figure 7. The effect of autophagy in the co-treatment. (A) Flow cytometry of MCF-7 cells treated with completed medium, irradiation (6 Gy), Nano-Se, combination of the nanoparticles and irradiation respectively with 3-MA (5 μM). Cell survival rates (B) and quantitative analysis of apoptosis cell percentage (C) obtained after flow cytometry evaluation (*p < .05, **p < .01, ***p < .001, the Nano-Se group (0/6 Gy) compared with vehicle control group (0/6 Gy); #p < .05, ##p < .01, ###p < .001, the combined group (Nano-se and radiation) compared with Nano-Se group).
Nano-Se augment the generation of ROS
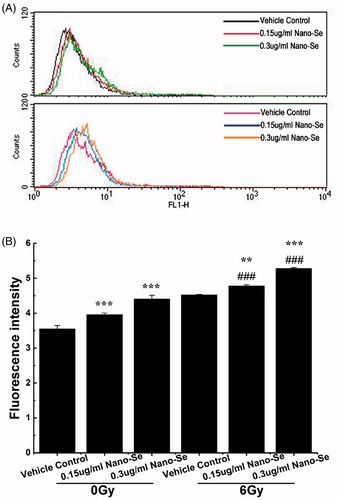
Oxidative stress, which induces major disorders in cellular function like the induction of autophagy, cell cycle arrest and apoptosis, is frequently the cause of irradiation-induced toxicity [Citation41]. Some metal nanomaterials and irradiation have been shown to cause ROS-mediated cell deaths [Citation42]. Given that ROS can also induce autophagy [Citation43], we studied the changes in ROS levels resulting from combined treatment of Nano-Se and irradiation with a fluorometric kit that detects intracellular oxidation of DCFH-DA using flow cytometry. DCFH-DA is hydrolysed by esterases to DCFH, remaining trapped within the cell and oxidized to the fluorescent molecule dichlorofluorescein (DCF) by the induction of cellular oxidants; the detection of DCF provides an indication of intracellular ROS level.
Measurement of ROS using DCFH-DA, in , showed that treatment of MCF-7 cells with .15 μg/ml or .3 μg/ml Nano-Se induced increase of ROS, and increase of ROS by .15 μg/ml Nano-Se alone did not induce obvious cell death. Interestingly, significantly increased ROS was detected in cancer cells following exposure to Nano-Se and irradiation, in comparison with irradiation alone, which were in accordance with results of mortality. These results suggest that increase of ROS is a mechanism by which Nano-Se sensitize cancer cells to the effects of irradiation.
Figure 8. Detection of ROS in MCF-7 cells by flow cytometry. (A) Cells were treated with indicated concentrations of Nano-Se without (top) or with (bottom) subsequent irradiation treatment. A fluorometric assay based on the oxidation of DCFH-DA by intracellular oxidants was used in conjunction with flow cytometry to detect ROS. (B) Quantitative analysis of ROS (*p < .05, **p < .01, ***p < .001, the Nano-Se group (0/6 Gy) compared with vehicle control group (0/6 Gy); #p < .05, ##p < .01, ###p < .001, the combined group (Nano-se and radiation) compared with Nano-Se group).
Conclusions
Radiation therapy is one of the most widely used processes for the treatment of cancer. Though it is really efficient at reducing and eliminating cancer cells, normal tissues proximity to the irradiation site are inevitably exposed to the harmful effects of irradiation. During the process, free radicals are formed via ionizing reactions that are then capable of destroying normal cells. To date, several kinds of radiosensitizers have been developed and used in cancer treatment, while the most common in current clinical application are still chemotherapeutics [Citation30]. An ideal schedule would be to identify a radiosensitizer that shows weak cytotoxicity without irradiation and effectively enhances the process of irradiation-induced cell death in tumour cells. Selenium has membrane stabilizing property, as an essential micronutrient for both animals and humans. Selenium is toxic above certain level, however nano-selenium has been also well certified to be less poisonous than inorganic selenium as well as some organoselenium compounds [Citation20].
In our study, Nano-Se was used in conjunction with irradiation, against MCF-7 breast cancer cells and the efficacy and mechanisms of action of these combined treatment approaches were evaluated. Radiation do not destroy the structures of selenium nanoparticles but induce a higher concentration of selenium ion, which can increase the toxicity of intracellular Nano-Se. MCF-7 breast cancer cells were treated with Nano-Se in conjunction with irradiation; the combined treatment induced cell death to a greater extent than irradiation alone, and also led to autophagy, cell cycle arrest at the G2/M phase and increase of ROS. Indeed, it was suggested that in early or later phases of tumour progression, the level of autophagy was augmented in metabolically stressful regions of tumour mass. Thus, we suspect that, in the present study, inhibition of autophagy may not only impair the adaptive responses of tumour cells to radiation, but aggravate the metabolic stress in tumour cells, and eventually lead to cell death and inhibition of cell proliferation. These results suggest that adjunctive use of Nano-Se can increase the effectiveness of radiotherapy in breast cancer treatment.
| Abbreviations | ||
| 3-MA | = | 3-methyladenine |
| DCFH-DA | = | 2′7′-dichlorofluorescin diacetate |
| DMSO | = | dimethyl sulphoxide |
| FBS | = | fetal bovine serum |
| FITC | = | fluorescein isothiocyanate |
| GSH | = | glutathione |
| GSSG | = | glutathione |
| Nano-Se | = | selenium nanoparticle |
| PI | = | fluorescein isothiocyanate |
| PI3K | = | phosphatidylinositol 3-kinases |
| ROS | = | reactive oxygen species |
| SSC | = | side scatter |
| SQSTM1 | = | sequestosome |
| RPMI 1640 medium | = | Roswell Park Memorial Institute 1640 medium |
| TEM | = | transmission electron microscopy |
Disclosure statement
The authors declare that they have no conflict of interest, and thank the administration of the Jiangsu Laboratory for Biomaterials and Devices, Southeast University and College of Materials Science and Technology, Nanjing University of Aeronautics and Astronautics for providing technical support for this research.
Additional information
Funding
References
- Ly D, Forman D, Ferlay J, et al. An international comparison of male and female breast cancer incidence rates. Int J Cancer. 2013;132:1918–1926.
- Montazerabadi AZ, Sazgarnia A, Bahreyni-Toosi MH, et al. The effects of combined treatment with ionizing irradiation and indocyanine green-mediated photodynamic therapy on breast cancer cells. J Photochem Photobiol B. 2012;109:42–49.
- Sami B, Anais A, Faten M, et al. High-LET irradiation combined with oxaliplatin induce autophagy in U-87 glioblastoma cells. Cancer Lett. 2008;264:63–70.
- Xie Q, Zhou YL, Lan GQ, et al. Sensitization of cancer cells to irradiation by selenadiazole derivatives by regulation of ROS-mediated DNA damage and ERK and AKT pathways. Biochem Biophys Res Commun. 2014;449:88–93.
- Lin J, Huang ZH, Wu H, et al. Inhibition of autophagy enhances the anticancer activity of silver nanoparticles. Autophagy. 2014;10:2006–2020.
- Chen F, Zhang XH, Hu XD, et al. Synthesis and characteristics of nanorods of gadolinium hydroxide and gadolinium oxide. J Alloy Compd. 2015;664:311–316.
- Chen F, Zhang XH, Hu XD, et al. Enhancement of radiotherapy by ceria nanoparticles modified with neogambogic acid in breast cancer cells. Int J Nanomedicine. 2015;10:4957–4969.
- Yazdi MH, Mahdavi M, Kheradmand E, et al. The preventive oral supplementation of a selenium nanoparticle-enriched probiotic increases the immune response and lifespan of 4T1 breast cancer bearing mice. Arzneimittelforschung. 2012;62:525–531.
- Gao FP, Yuan Q, Gao L, et al. Cytotoxicity and therapeutic effect of irinotecan combined with selenium nanoparticles. Biomaterials. 2014;35:8854–8866.
- Helzlsouer KJ, Huang HY, Alberg AJ, et al. Association between alpha-tocopherol, gamma-tocopherol, selenium, and subsequent prostate cancer. J Natl Cancer Inst. 2000;92:2018–2023.
- Kong L, Yuan Q, Zhu HR, et al. The suppression of prostate LNCaP cancer cells growth by Selenium nanoparticles through Akt/Mdm2/AR controlled apoptosis. Biomaterials. 2011;32:6515–6522.
- Oliver M, Lutz S, Jens B, et al. Selenium in oncology: from chemistry to clinics. Molecules. 2009;14:3975–3988.
- Zeng HW, Cheng WH, Johnson LK. Methylselenol, a selenium metabolite, modulates p53 pathway and inhibits the growth of colon cancer xenografts in Balb/c mice. J Nutr Biochem. 2013;24:776–780.
- Bi XL, Pohl N, Dong HL, et al. Selenium and sulindac are synergistic to inhibit intestinal tumorigenesis in Apc/p21 mice. J Hematol Oncol. 2013;6:8.
- Li ZS, Carrier L, Belame A, et al. Combination of methylselenocysteine with tamoxifen inhibits MCF-7 breast cancer xenografts in nude mice through elevated apoptosis and reduced angiogenesis. Breast Cancer Res Treat. 2009;118:33–43.
- Narajji C, Karvekar MD, Das AK. Biological importance of organoselenium compounds. Indian J Pharm Sci. 2007;69:344–351.
- Arin B, Abhishek B, Prosenjit G, et al. Protective effect of Selenium nanoparticle against cyclophosphamide induced hepatotoxicity and genotoxicity in Swiss albino mice. J Biomater Appl. 2014;29:303–317.
- Zhang J, Wang X, Xu T. Elemental selenium at nano size (Nano-Se) as a potential chemopreventive agent with reduced risk of selenium toxicity: comparison with se-methylselenocysteine in mice. Toxicol Sci. 2008;101:22–31.
- Li YH, Li XL, Wong YS, et al. The reveal of cisplatin-induced nephrotoxicity by selenium nanoparticles functionalized with 11-m ercapto-1-undecanol by inhibition of ROS-mediated apoptosis. Biomaterials. 2011;32:9068–9076.
- Wang HL, Zhang JS, Hai-Qing YU. Effect of elemental selenium at nano size (Nano-Se) with lower toxicity on the anticancer effect of cisplatin. Acta Nutr Sin. 2007;29:445–444.
- Joshua DR, Eileen W. Autophagy and metabolism. Science. 2010;330:1344–1348.
- Levine B. Cell biology: autophagy and cancer. Nature. 2007;446:745–747.
- Kondo Y, Kanzawa T, Sawaya R, et al. The role of autophagy in cancer development and response to therapy. Nat Rev Cancer. 2005;5:726–734.
- Seleverstov O, Zabirnyk O, Zscharnack M, et al. Quantum dots for human mesenchymal stem cells labeling. A size-dependent autophagy activation. Nano Lett. 2006;6:2826–2832.
- Wu YN, Yang LX, Shi XY, et al. The selective growth inhibition of oral cancer by iron core-gold shell nanoparticles through mitochondria-mediated autophagy. Biomaterials. 2011;32:4565–4573.
- Zhang Q, Yang W, Man N, et al. Autophagy mediated chemosensitization in cancer cells by fullerene C60 nanocrystal. Autophagy. 2009;5:1107–1117.
- Markovic ZM, Ristic BZ, Arsikin KM, et al. Graphene quantum dots as autophagy inducing photodynamic agents. Biomaterials. 2012;33:7084–7092.
- Lu Y, Zhang L, Li J, et al. MnO nanocrystals: a platform for integration of MRI and genuine autophagy induction for chemotherapy. Adv Funct Mater. 2013;23:1534–1546.
- Khan MI, Mohammad A, Patil G, et al. Induction of ROS, mitochondrial damage and autophagy in lung epithelial cancer cells by iron oxide nanoparticles. Biomaterials. 2012;33:1477–1488.
- Man N, Yu SH. Rare earth oxide nanocrystals as a new class of autophagy inducers. Autophagy. 2010;6:310–311.
- Yuk JM, Shin DM, Song KS, et al. Bacillus calmette-guerin cell wall cytoskeleton enhances colon cancer radiosensitivity through autophagy. Autophagy. 2010;6:46–60.
- Wu H, Lin J, Liu PD, et al. Is the autophagy a friend or foe in the silver nanoparticles associated radiotherapy for glioma? Biomaterials. 2015;62:47–57.
- Clark LC, Combs CF Jr, Turnbull BW, et al. Effects of selenium supplementation for cancer prevention in patients with carcinoma of the skin. A randomized controlled trial. Nutritional Prevention of Cancer Study Group. JAMA. 1997;277:1520.
- Zhao F, Zhao Y, Liu Y, Chang X, et al. Cellular uptake, intracellular trafficking, and cytotoxicity of nanomaterials. Small. 2011;7:1322–1337.
- Khatoon I, Vajpayee P, Singh G, et al. Determination of internalization of chromium oxide nano-particles in Escherichia coli by flow cytometry . J Biomed Nanotechnol. 2011;7:168–169.
- Kim HS, Hwang KK, Seo JW, et al. Apoptosis and regulation of B ax and B cl -X proteins during human neonatal vascular remodeling. Arterioscler Thromb Vasc Biol. 2000;20:957–963.
- Roa W, Zhang XJ, Guo LH, et al. Gold nanoparticle sensitize radiotherapy of prostate cancer cells by regulation of the cell cycle. Nanotechnology. 2009;20:375101.
- Lao YZ, Wan G, Liu ZY, et al. The natural compound oblongifolin C inhibits autophagic flux and enhances antitumor efficacy of nutrient deprivation. Autophagy. 2014;10:736–749.
- Rosenfeldt MT, Ryan KM. The role of autophagy in tumour development and cancer therapy. Expert Rev Mol Med. 2009;11:e36.
- Mathew R, Karantza-Wadsworth V, White E. Role of autophagy in cancer. Nat Rev Cancer. 2007;7:961–967.
- Gao Z, Sarsour EH, Kalen AL, et al. Late ROS accumulation and radiosensitivity in SOD1-overexpressing human glioma cells. Nanomed Nanotechnol Biol Med. 2013;9:558–569.
- Shi JM, Bai LL, Zhang DM, et al. Saxifragilolin D induces the interplay between apoptosis and autophagy in breast cancer cells through ROS-dependent endoplasmic reticulum stress. Biochem Pharmacol. 2013;85:913–926.
- Azad MB, Chen YQ, Gibson SB. Regulation of autophagy by reactive oxygen species (ROS): implications for cancer progression and treatment. Antioxid Redox Signal. 2009;11:777–790.
