?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
The aim of this research was to synthesis biocompatible iron disulphide nanocrystals at different reaction temperatures using the colloidal synthesis methodology. Synthesis was conducted at the 220–240 °C range of reaction temperatures at intervals of 5 °C in an inert argon atmosphere. The toxicity of iron disulphide nanocrystals was evaluated in vitro using mouse fibroblast cell line. Two complementary assays were conducted: the first to evaluate cell viability of the fibroblast via an MTT assay and the second to determine the preservation of fibroblast nuclei integrity through DAPI staining, which labels nuclear DNA in fluorescence microscopes. Through TEM and HRTEM, we observed a cubic morphology of pyrite iron disulphide nanocrystals ranging in sizes 25–50 nm (225 °C), 50–70 nm (230 °C) and >70 nm (235 °C). Through X-ray diffraction, we observed a mixture of pyrite and pyrrohotite in the samples synthesized at 225 °C and 240 °C, showing the best photocatalytic activity at 80% and 65%, respectively, for the degradation of methylene blue after 120 minutes. In all experimental groups, iron disulphide nanocrystals were biocompatible, i.e. no statistically significant differences were observed between experimental groups as shown in a one-way ANOVA and Tukey’s test. Based on all of these results, we recommend non-cytotoxic semiconductor iron sulphide nanocrystals for biomedical applications.
Graphical Abstract
Introduction
Metal sulphide nanostructures have garnered much interest in the areas of energy conversion and storage due to their optimal optoelectronic properties [Citation1,Citation2]. Two of these chalcogenides, cadmium (CdS) and lead sulphides (PbS) have been widely studied [Citation1,Citation3,Citation4], but could cause environmental damage. At present, non-toxic metal sulphides, such as copper sulphide, zinc sulphide and iron sulphide, are considered promising semiconductors owing do their optoelectronic properties [Citation2,Citation5,Citation6]. Iron sulphide presents different crystalline phases such as troilite, marcasite, pyrrohotite and pyrite [Citation7,Citation8]. The last crystalline phase is a polymorph iron disulphide (FeS2), which is considered the least expensive and the best earth-abundant and non-toxic semiconductor among 23 semiconductors [Citation9]. In addition, it exhibits a face-centred cubic crystallinity, a high optical absorption coefficient (105 cm−1 at λ <700 nm), excellent mobility (>300 cm2/Vs in single crystal) and a short band gap (Eg≈0.95 eV), all of which are appropriate for optoelectronic and photovoltaic applications [Citation10–12]. Different synthesis and routes have been used to obtain these crystalline phases, including the microwave–hydrothermal method [Citation13–16] and the electrochemical and solvothermal method [Citation17]. Several stabilizing agents have been added in the synthesis in order to keep the pyrite stability at a nanometric size [Citation10,Citation18]. However, the solvent type, the capping ligands and the temperature reaction are also important parameters in the final properties of pyrite for specific applications [Citation8,Citation11,Citation17,Citation19–21]. Pyrite iron disulphide has been mainly used as an absorbing semiconductor in solar cells [Citation12], as well as for stripping mercury [Citation14] and removing industrial pollutants [Citation22]. According to a systematic review recently published by our group [Citation23] concerning nanomaterials made of non-toxic metallic sulphide and their potential biomedical applications, magnetic greigite iron sulphide is a candidate for glucose detection and photothermal activity, among other uses. However, only one report referring to greigite iron sulphide/silver composites (Fe3S4/Ag) presented bacteriostatic effects against Escherichia coli (E. coli) and Staphylococcus aureus (S. aureus), at 86.2% and 90.6%, respectively, and this was attributed to the presence of silver metal [Citation24]. Preliminary results in our group, not yet published, suggest that iron pyrite disulphide nanocrystals could be excellent antimicrobials. Data in the literature support our idea. Other authors [Citation25] have demonstrated that Carbowax sulphur and alcohol sulphur exhibited antimicrobial activity against certain gram-positive microorganisms and fungi. Furthermore, they proposed that antimicrobial and antifungal activity was attributed to the sulphur itself. Thus, we consider that pyrite iron disulphide nanocrystals could be good candidates for biological applications. Based on this consideration, our current research investigates the toxicity of iron disulphides in mammalian cells.
Materials and methods
Synthesis of iron disulphide
Iron disulphide was obtained by a colloidal synthetic route as reported by Alam et al. [Citation10], but with minor modifications, as follows: In a 250-ml flask, we mixed 20 ml of dodecylamine (Sigma-Aldrich, St. Louis, MO, 98%) and 500 mg of FeCl2 (Sigma-Aldrich, St. Louis, MO, 98%) at 100 °C for 2 h under continuous argon flow. Simultaneously, in another flask, 20 ml of dodecylamine and 500 mg of sulphur (JT Baker, Center Valley, PA, 98%) were vigorously stirred at 100 °C for 2 h under argon flow. Next, the sulphur–dodecylamine dissolution was added into the solution of FeCl2–dodecylamine. The mixture was kept at a constant temperature (220, 225, 230, 235 or 240 °C) with vigorous stirring for 1 h. Then, drops of chloroform (JT Baker, 99.6%) were added into the dark product, which was washed several times with methanol (JT Baker, 99.6%). At least three syntheses were obtained of each temperature reaction.
Structural, morphological and optical characterization
The structural phases of iron disulphide dark products were determined by X-ray diffraction using Rigaku Miniflex equipment (Cu-Kα radiation, λ≈1.54 Å; 2θ from 10 to 70°; 2° min−1). Morphology and crystalline interplanar distances were observed by transmission electron microscopy (TEM) and by HRTEM using JEOL JEM-1010 and JEOL-JEM-2010 LaB 6, DESA equipment, respectively. The optical absorbance of the colloidal products was measured in a Cary 5000 UV-Vis-NIR spectrophotometer, with superb photometric performance in the 175–3300 nm range using a PbS smart detector.
Photocatalytic activity
Photocatalytic activity was tested using 400 mL of methylene blue (MB) aqueous solution at a concentration of 2 × 1 0−5 mol L−1 and a pH of 6.7. Colloidal iron disulphide obtained at different temperatures were placed in 600 mL of the reactor. A quartz tube was used for the reactor for immersion of the lamp and the solution was stirred for the entire time of the test. The solution in the reactor was irradiated with a commercial germicidal lamp (λ = 252 nm, 11 W). The irradiation times were 0–150 min in incremental steps of 20 min. The residual concentration was quantified by a UV–Visible absorption spectroscopy at 663 nm, previously calibrated with external calibration standards (2, 1.5, 1.0, 0.5 and 0.25 × 10−5 mol L−1). The concentration for photocatalytic assays of the pyrite powder was 1 g L−1 in all cases. The samples were carried out by triplicate and the results shown are the mean value of these three measurements.
Cytotoxicity assay
The cytotoxicity assay was performed using mouse fibroblast NIH-3T3 cell lines (ATCC No. CRL-1658), which were maintained in DMEM medium (Gibco Invitrogen, Carlsbad, CA) at 10% fetal bovine serum (Gibco) and 100 U/mL penicillin–streptomycin at 37 °C in 5% CO2, at 95% humidity (standard culture conditions). The experimental groups were as follows: (a) control without treatment; (b) methanol 1% (vehicle); (c) iron disulphide obtained at 220 °C; (d) iron disulphide obtained at 230 °C; (e) iron disulphide obtained at 235 °C; and (f) iron disulphide obtained at 240 °C. Iron disulphide was added at 250 nM for the treatments.
Cell viability was determined by a 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay. Cells were seeded briefly at 3 × 104 cells/well in culture medium and incubated under standard culture conditions in 24-well sterile plates (Sarstedt, Nümbrecht, Alemania). 24 h later, the medium was changed and a new medium was supplemented with iron disulphide nanocrystals, which were previously solubilized in methanol. The cells were treated in accordance with the recommendations of the aforementioned experimental groups and were immediately incubated for 48 h under standard culture conditions. Subsequently, 20 μL of MTT (5 mg/mL in PBS) was added to each well, and cells were incubated for 4 h under standard culture conditions. The supernatants were removed and 200 μL of dimethyl sulphoxide (DMSO) was added. The absorbance (595 nm) was determined by a microplate reader (Bio-Rad, Hercules, CA). Cell viability was expressed as percent change over control and calculated using the following formula:
In another group of experiments, cell nucleus integrity was analyzed using DAPI staining. The same procedure was carried briefly with all of the experimental groups, but this time DAPI staining was added at 5 µg/mL, followed by incubation for 30 min. and then photographed in an inverted Nikon Eclipse fluorescence microscope.
Statistical analysis
Statistical analysis was carried out to determine toxicity. The data were mean values of at least three different experiments by triplicate, with the values expressed as mean ± SD. The differences between experimental groups were analyzed using a one-way ANOVA and Tukey’s test. The values of p < .05 were considered statistically significant. Thus, no significant difference between experimental groups was observed.
Results and discussion
Determination of crystal structure and morphology
The reaction temperature is a key parameter of the synthesis to adjust the crystalline phase of iron disulfide (). A mixture of pyrrhotite and pyrite were observed by X-ray diffraction of the iron disulfide samples synthesized at 220 and 225 °C. The patterns display peaks at 2θ ≈ 28.52°, 29.48°, 30.02°, 31.5°, 33.12°, 34°, 36.6°, 37.2°, 40.88°, 43.24°, 43.96°, 47.5°, 52.6°, 53.36°, 56.34° and 57.54°. The high intense peaks at 30.02°, 33.12° (2 2 4), 43.96° (2 2 8) and 53.36° (3 2 10) match very well to the orthorhombic pyrrhotite phase standard card (JCPDS#017-0201), while the low intensity peaks at 28.52, 33.12, 37.2, 40.88, 47.5 and 56.34° are associated to the standard card JCPDS#042-1340 of pyrite [6,18,21]. When the temperature of the reaction was increased at 230 °C, the peaks observed at 28.48° (1 1 1), 33.08° (2 0 0), 37.1° (2 1 0), 40.74° (2 1 1), 47.5° (2 2 0) and 56.26° (3 1 1) of the pyrite phase (JCPDS#042-1340) are more intense and defined than those pyrrhotite phase, so that pyrite phase prevails at this temperature. These results were reproducible in at least three syntheses made by separated at the same temperature of 230 °C (). At 235 °C and 240 °C, the peaks associated with pyrite phase are still present but new peaks at 29.56 and 52.6 were not identified with same crystalline phase.
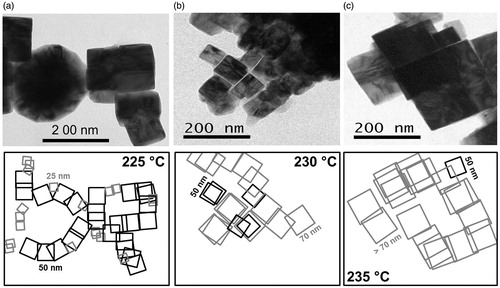
TEM morphology of the iron disulphide samples obtained at different temperatures is shown in . The iron disulphide synthesized at 220 °C presented semi-spherical morphology with a <10 nm (particle size not shown here). From 225 to 240 °C, the iron disulphide samples presented mainly a cubic shape, with the cube sizes increasing as a function of the temperature. For example, at 225 °C, the cube was 25–50 nm; at 230 °C, the cube was 50–70 nm; and at 235 °C, the cube was 70–100 nm (). The cubes appeared arranged in spherical or irregular morphologies (illustrations down of ) similar to that reported by Kim [Citation26], and show promise as electrode material for lithium-ion batteries [Citation27,Citation28] or for mercury removal [Citation14].
Figure 1. X-ray patterns of iron disulphide (a) obtained at different temperatures from 220 to 240 °C and (b) reproducibility of pyrite iron disulphide obtained at 230 °C.
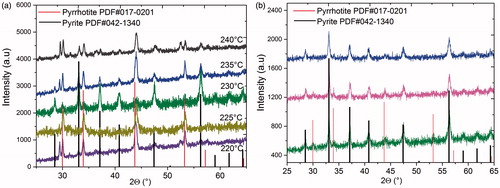
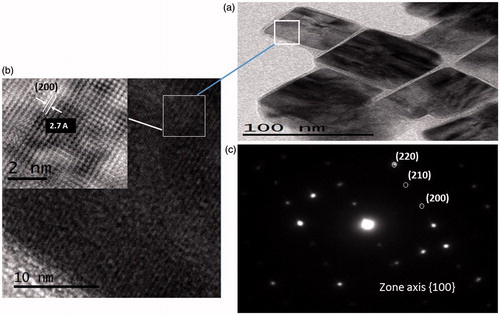
Figure 2. TEM images of iron disulphide obtained at (a) 225 °C, (b) 230 °C and (c) 235 °C. The drawings of each figure illustrate the arrangements of the cubes, seen as spherical or irregular morphologies.
HRTEM images of pyrite nanocrystals obtained at 230 °C are illustrated in . The average size of the cubes is close to 70 nm (), and the interplanar distance was measured from the observed planes in giving a value of 2.7 Å assigned to the (2 0 0) plane of pyrite, which is in accordance with data from JCPDS #042–1340. It confirms the results obtained from XRD patterns. Fast Fourier transformation (FFT) shows (2 0 0), (2 1 0), (2 2 0) planes indexed for the pyrite diffraction patterns () with a crystal size from 14 to 18 nm according to Su-Ching [Citation29].
Optical absorption and photocatalytic response
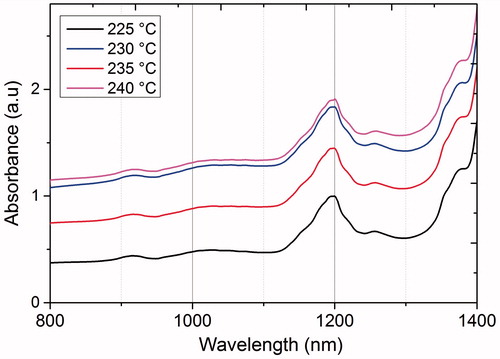
UV-Vis spectra of the iron disulphide samples exhibit a maximum absorption band close to 1200 nm with a band gap of 1.03 eV, independently of the temperature reaction (), in accordance with the band gap reported for pyrite (0.95 (1300 nm) to 1.05 eV (1180 nm)) [Citation11,Citation12].
Figure 4. UV-Vis optical absorbance of iron disulphide synthesized at different temperatures from 220 to 240 °C.
The samples obtained of iron disulphide nanocrystals synthesized at 225, 235 and 240 °C showed photocatalytic activity in water–methylene blue (MB) solution (. Cubic iron disulphide synthesized at 235 °C showed only 10% photocatalytic efficiency, but this is close to the 7.7% and 12% values reported for pristine pyrite for degradation of MB [Citation13,Citation30]. Safranine T, methyl orange Rhodamine B and Pyronine B have also been degraded with pyrite crystallites increasing to only 14.8% after 90 min [Citation30]. In our results, the best degradation rate was found in the samples obtained at 225 and 240 °C with about 80% and 65%, respectively, after 120 min. The samples were kept in the dark for 20 min and the absorption of methylene blue on crystal of iron pyrite was less than 1% in all cases. This indicates that the cubic iron disulphide samples are a good option for the degradation of organic dyes. The efficiency degradation of pyrite could be increased in acidic solution media or in the coexistence of a heavy metal with dissolved oxygen in the organic dye, since the reduction of heavy metal and the degradation of the organic compound would occur simultaneously [Citation31].
Figure 5. Photocatalytic activity of iron disulphide obtained at 225, 235 and 240 °C under ultraviolet irradiation (λ = 252 nm, 11 W).
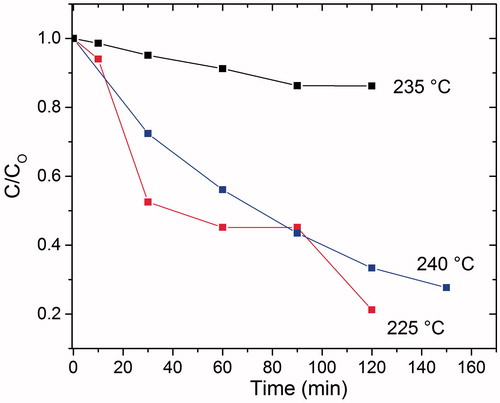
Photocatalysis improved most in the synthesis at a temperature of 225 °C, then for the synthesis at 240 °C and least for the synthesis at 235 °C. This could be the result of the obtained phases and particle sizes (which increased as a function of temperature). The three temperatures showed a range of phases between pyrite (FeS2) and pyrrhotite (Fe1-XS, where X = 0–0.2). The phases only varied in the amount of iron present in the formula. The synthesis at 240 °C showed a mixture of phases between pyrite and pyrrhotite, while synthesis at 235 °C showed a mixture of pyrite and pyrrhotite with a considerable amount of pyrite. Finally, the synthesis at 225 °C showed a greater amount of pyrrhotite. It is well known that the pyrrhotite phase is less stable than pyrite; it reacts quicker to Fe2+ ions than in the presence of oxygen from water. The Fe2+ ion oxidizes to Fe3+, causing greater formations of reactive species in the form of hydroxyl radical. A hydroxyl radical is strongly oxidizing when derived from hydrogen peroxide [Citation22,Citation32–35]. This is known as Fenton's process, which is an advanced oxidation process resulting from the following reaction:
By comparison, Borda et al. [Citation36] showed that the defect structure of the pyrite surface (presence of non-stoichiometric Fe3+ and sulphur (S2-)-deficient defect sites) leads to following the previous step to the oxidation reaction of pyrite:
The combination of two adsorbed hydroxyl radicals (OH*) produces H2O2, which contributes to the photocatalysis activity.
In our results, the best photocatalytic response was obtained in the iron disulphide samples with a mixture of pyrite and pyrrhotite. The photooxidation response of the iron disulphide samples, attributed to either the mixture of crystalline phases or to the defect structure of the pyrite surface, could be related to antimicrobial activity.
Cytotoxicity effect
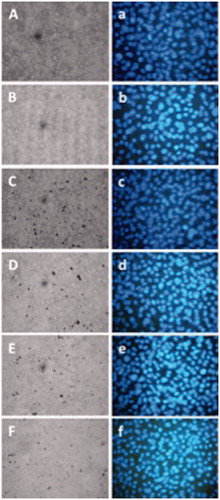
The non-cytotoxic effects of iron disulphide were shown in all experimental groups analyzed. Non-statistically significant differences were observed in the cell viability of experimental groups compared with control cells or control vehicles (. In comparison, cells treated with iron disulphide maintained cell nuclei integrity (. Furthermore, the results showed that the cytoarchitecture and nuclei cells maintain their integrity after a 48-h iron disulphide treatment compared with control cells. This means that the cell monolayer continued to proliferate in the presence of iron disulphide.
Figure 6. Effect of iron disulphide nanocrystals obtained at different reaction temperatures on NIH-3T3 cell viability. NIH-3T3 cells were cultured in the absence of treatment (control) and methanol 1% (vehicle) and 250 nM of iron disulphide nanocrystals was obtained at 220 °C, 230 °C, 235 °C or 240 °C temperature reactions, respectively. After 48 h of treatment, the cells were analyzed by MTT method. No significant differences were observed.
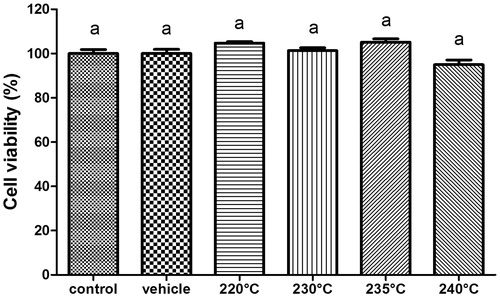
Figure 7. Integrity cell monolayer (capital letter) and cell nuclei (lowercase letter), after 48 h of treatment. Aa; without treatment (control); Bb: methanol 1% as vehicle; Cc: iron disulphide obtained at 220 °C; Dd: iron disulphide obtained at 230 °C; Ee: iron disulphide obtained at 235 °C; or Ff: iron disulphide obtained at 240 °C. All cases at 250 nM doses of treatment. Monolayer was microphotographed under visible light field, and cell nuclei previously stained with DAPI were microphotographed under UV light, 40× magnification.
Biocompatibility is related to the behaviour of nanomaterials in various cells contexts. The term refers to the ability of a material to perform an appropriate response in the material–cell interaction. Therefore, a non-toxicity effect is mandatory in certain biological applications. The toxicity of the iron disulphide nanocrystals was evaluated for the first time and results showed that iron disulphide nanocrystals were biocompatible with NIH-3T3 mouse fibroblast cells at 250 nM, which was determined by measuring the cellular metabolism – specifically, mitochondrial activity – via an MTT assay. Results showed that the cells remained in proliferation 48 h after supplementation of iron disulphide nanocrystals solubilized in methanol, compared with control and vehicle groups. Also, data were reinforced by demonstrating how monolayer cell integrity and nuclei integrity in mammalian cells were maintained. In fact, data suggest that the nanoparticles described here could have future applications in biomedicine.
Other authors showed experimental evidence in which nanoparticulate iron sulphides could be reacting with DNA and modifying their supercoiling. The plasmid DNA incubation at 25 °C with nanoparticulate FeS and a mackinawite structure (FeSm) resulted in nanoparticulate FeSm binds to the DNA molecules. In addition, supercoiled pDNA nicking occurs in solutions with concentrations below the FeSm solubility product. The authors proposed that plasmid DNA appears to be a sensitive proxy for radical reactions [Citation37]. Interestingly, we showed that interaction between iron disulphide nanocrystals and mammalian cells does not affect cell viability. Conversely, Carbowax sulphur and alcohol sulphur exhibited antimicrobial activity via a mechanism mediated by sulphur per se, against certain gram-positive microorganisms, including S. aureus, a pathogenic bacterium [Citation25]. These data could imply a selective cell death mechanism of sulphur against microorganisms. In this context, the possible applications of iron disulphide as antimicrobials, but biocompatible with mammalian cells, are an innovative study.
As concerns the pyrite oxidation mechanism, the formation of hydrogen peroxide (H2O2) was reported after adding pyrite (FeS2) to O2-free water [Citation36]. The authors hypothesized that the defect structure of the pyrite surface (Fe3+ at a sulphur-deficient defect site) generates an adsorbed hydroxyl radical from O2-free water. Later, the same authors showed evidence that suggested the conversion of Fe3+ to Fe2+ at defect sites, the origin of H2O2 from H2O and the existence of hydroxyl radical in the solution. They concluded that this is an initial step of the oxidation reaction of pyrite by other oxidants such as oxygen, i.e. the formation of hydroxyl radical via the interaction of H2O with the defect structure of a pyrite surface, in other words a step in the oxidation mechanism of pyrite [Citation36]. However, hydroxyl radical formation from the interaction of pyrite cells in biological systems such as cell cultures has not been studied.
Conclusions
Biocompatible iron disulphide nanocrystals were obtained successfully via colloidal synthesis as a function of the temperature reaction. A mixture of pyrite and pyrrohotite crystalline phases was present in all samples, but this did not significantly influence the cytotoxicity in the iron disulphide samples. In fact, in all cases, the viability and integrity of monolayer and nuclei cells remained without changes after the treatment of iron disulphide nanocrystals. The biological application of iron disulphide continues to be explored in our laboratory.
Acknowledgements
The authors wish to thank Lourdes Palma Tirado (INB-UNAM) for the TEM characterization and Sergio Martínez González and Dr. María Rodríguez Ceja (LEMA-IF, UNAM) for their excellent technical support.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Chen-Ho L, Ming-Yen L, Lih-Juann C. Metal sulfide nanostructures: synthesis, properties and applications in energy conversion and storage. J Mater Chem. 2012;22:19–30.
- Bhandari KP, Koirala P, Paudel NR, et al. Iron pyrite nanocrystal film serves as a copper-free back contact for polycrystalline CdTe thin film solar cells. Sol Energy Mat Sol C. 2015;140:108–114.
- Martínez-Alonso C, Cortina-Marrero HJ, Coria-Monroy CS, et al. Solution synthesized CdS nanoparticles for hybrid solar cell applications. J Mater Sci: Mater Electron. 2015;26:5539–5545.
- Arenas MC, Mendoza N, Cortina H, et al. Influence of poly3-octylthiophene (P3OT) film thickness and preparation method on photovoltaic performance of hybrid ITO/CdS/P3OT/Au solar cells. Sol Energy Mat Sol C. 2010;94:29–33.
- Quintana-Ramírez PV, Arenas-Arrocena MC, Santos-Cruz J, et al. Growth evolution and phase transition from chalcocite to digenite in nanocrystalline copper sulfide: morphological, optical and electrical properties. Beilstein J Nanotechnol. 2014;5:1542–1552.
- Alam KM, Sarker JC, Lee S, et al. Synthesis, characterization and processing of cubic iron pyrite nanocrystals in a photovoltaic cell. Mater Chem Phys. 2014;148:1022–1028.
- Murphy R, Strongin DR. Surface reactivity of pyrite and related sulfides. Surf Sci Rep. 2009;64:1–45.
- Vedavathi A, Munikrishna R, Ramakrishna R. Effect of precursor concentration on structural and morphological properties of iron pyrite thin films. Procedia Mater Sci. 2015;10:279–284.
- Wadia C, Alivisatos AP, Kammen DM. Materials availability expands the opportunity for large-scale photovoltaics deployment. Environ Sci Technol. 2009;43:2072–2077.
- Alam KM, Manasreh MO, Mook KY. Synthesis, characterization and optoelectronic properties of iron pyrite nanohusks. Mater Lett. 2014;126:181–184.
- Zhai G, Xie R, Wang H, et al. Effect of capping ligands on the optical properties and electronic energies of iron pyrite FeS2 nanocrystals and solid thin films. J Alloy Compd. 2016;674:9–15.
- Yu P, Qu S, Jia C, et al. Modified synthesis of FeS2 quantum dots for hybrid bulk-heterojunction solar cells. Mater Lett. 2015;157:235–238.
- Long F, He J, Zhang M, et al. Microwave-hydrothermal synthesis of Co-doped FeS2 as a visible-light photocatalyst. J Mater Sci. 2015;50:1848–1854.
- Duan Y, Han DS, Batchelor B, et al. Synthesis, characterization, and application of pyrite for removal of mercury. Colloid Surface A. 2016;490:326–335.
- Golsheikh AM, Huang NM, Lim HN, et al. One-pot hydrothermal synthesis and characterization of FeS2 (pyrite)/graphene nanocomposite. Chem Eng J. 2013;218:276–284.
- Jung KE, Batchelor B. Synthesis and characterization of pyrite (FeS2) using microwave irradiation. Mater Res Bull. 2009;44:1553–1558.
- E’jazi N, Aghaziarati M. Determination of optimum condition to produce nanocrystalline pyrite by solvothermal synthesis method. Adv Powder Technol. 2012;23:52–357.
- Mangham SC, Alam KM, Benamara M, et al. Synthesis of iron pyrite nanocrystals utilizing trioctylphosphine oxide (TOPO) for photovoltaic devices. Mater Lett. 2013;97:144–147.
- Mao B, Dong Q, Exstrom CL, et al. Surface thermal stability of iron pyrite nanocrystals: role of capping ligands. Thin Solid Films. 2014;562:361–366.
- Lucas JM, Tuan CC, Lounis SD, et al. Ligand-controlled colloidal synthesis and electronic structure characterization of cubic iron pyrite (FeS2) nanocrystals. Chem Mater. 2013;25:1615–1620.
- Binxia Y, Weiling L, Shan-Tung T. Synthesis of air stable and pure phase pyrite FeS2 nanoparticles in water. Mater Lett. 2015;142:160–162.
- Kaur G, Singh B, Singh P, et al. Preferentially grown nanostructured iron disulfide (FeS2) for removal of industrial pollutants. RSC Adv. 2016;6:99120–99128.
- Argueta-Figueroa L, Martínez-Alvarez O, Santos-Cruz J, et al. Nanomaterials made of non-toxic metallic sulfides: a systematic review of their potential biomedical applications. Mat Sci Eng C. 2017;76:1305–1315.
- He Q, Huang C, Liu J. Preparation, characterization and antibacterial activity of magnetic greigite and Fe3S4/Ag nanoparticles. Nanosci Nanotechnol Lett. 2014;6:10–17.
- Weld JT, Gunther A. The antibacterial properties of sulfur. J Exp Med. 1947;85:531–542.
- Kim HT, Nguyen TPN, Kim CD, et al. Formation mechanisms of pyrite (FeS2) nano-crystals synthesized by colloidal route in sulfur abundant environment. Mater Chem Phys. 2014;148:1095–1098.
- Yoder TS, Tussing M, Cloud JE, et al. Resilient carbon encapsulation of iron pyrite (FeS2) cathodes in lithium ion batteries. J Power Sources. 2015;274:685–692.
- Qiu W, Xia J, Zhong H, et al. L-cysteine-assisted synthesis of cubic pyrite/nitrogen-doped graphene composite as anode material for lithium-ion batteries. Electrochim Acta. 2014;137:197–205.
- Su-Ching H, Chih-Ming H, Szu-Ying C, et al. Facile synthesis and characterization of high temperature phase FeS2 pyrite nanocrystals. Mater Lett. 2012;75:152–154.
- Liu S, Li M, Li S, et al. Synthesis and adsorption/photocatalysis performance of pyrite FeS2. Appl Surf Sci. 2013;268:213–217.
- Zeng-Hui D, Xiang-Rong X, Fu-Ming L, et al. Photocatalytic degradation of malachite green by pyrite and its synergism with Cr(VI) reduction: performance and reaction mechanism. Sep Purif Technol. 2015;154:168–175.
- Balcioglu IA, Arslan I, Sacan MT. Homogenous and heterogenous advanced oxidation of two commercial reactive dyes. Environ Technol. 2001;22:813–822.
- Zhong X, Royer S, Zhang H, et al. Mesoporous silica iron-doped as stable and efficient heterogeneous catalyst for the degradation of C.I. Acid Orange 7 using sono–photo-Fenton process. Sep Purif Technol. 2011;80:163–171.
- Bernal-Martinez LA, Barrera-Diaz C, Solis-Morelos C, et al. Synergy of electrochemical and ozonation processes in industrial wastewater treatment. Chem Eng J. 2010;165:71–77.
- Soo-Myung K, Alfons V. Degradation of organic pollutant by the photo-Fenton-process. Chem Eng Technol. 1998;21:187–191.
- Borda MJ, Elsetinow AR, Strongin DR, et al. A mechanism for the production of hydroxyl radical at surface defect sites on pyrite. Geochim Cosmochim Acta. 2003;67:935–939.
- Rickard D, Hatton B, Murphy DM, et al. FeS-induced radical formation and its effect on plasmid DNA. Aquat Geochem. 2011;17:545–566.