Abstract
This work focuses on fabricating poly(2-aminobenzylamine)-modified screen-printed carbon electrode as an electrochemical immunosensor for the label-free detection of human immunoglobulin G. To selectively detect immunoglobulin G, the anti-immunoglobulin G antibody with high affinity to immunoglobulin G was covalently linked with the amine group of poly(2-aminobenzylamine) film-deposited screen-printed carbon electrode. The selectivity for immunoglobulin G was subsequently assured by being challenged with redox-active interferences and adventitious adsorption did not significantly interfere the analyte signal. To obviate the use of costly secondary antibody, the [Fe(CN)6]4-/3- redox probe was instead applied to measure the number of human immunoglobulin G through the immunocomplex formation that is quantitatively related to the level of the differential pulse voltammetric current. The resulting immunosensor exhibited good sensitivity with the detection limit of 0.15 ng mL−1, limit of quantitation of 0.50 ng mL−1 and the linear range from 1.0 to 50 ng mL−1. Given those striking analytical performances and the affordability arising from using cheap screen-printed carbon electrode with label-free detection, the immunosensor serves as a promising model for the next-step development of a diagnostic tool.
Introduction
Humoral immune response executed by immunoglobulins serves as one of the most vital self-defensive mechanisms of the host [Citation1]. Mechanistically, immunoglobulins selectively bind to the recognition sites of antigens to neutralize the toxicity and pathogenicity imposed by invading pathogens [Citation2]. This suggests that the elevated level of immunoglobulins is strongly associated with the number of pathophysiological events occurring in our body [Citation3]. Therefore, much attention has been paid to the construction of analytical device for disease diagnosis at the clinical level using immunoglobulins as the marker [Citation3–5]. In particular, immunoglobulin G, the most abundant immunoglobulin in human serum [Citation6], can be clinically used to evaluate the serologic immunity of measles infection [Citation7,Citation8] and Rift Valley fever [Citation9]. For this reason, there is increasing interest in quantitation of immunoglobulin G concentration using several assays such as enzyme-linked immunosorbent assay (ELISA), radioimmunoassay, surface plasmon resonance, etc. [Citation10–13]. However, these techniques suffer from their high cost and complicated procedures, which require skillful personnel to operate.
Recently, electrochemical immunosensors have shed light on the development of new analytical tools for disease diagnosis due to their advantages over those techniques, including remarkable selectivity towards the analyte resulting from the high affinity of antibody bound to antigen [Citation14], as well as low-cost preparation thanks to various choices of materials for electrode fabrication together with rapid analysis [Citation15]. In principle, the technique essentially transforms the specific molecular binding event between the secondary antibody tagged with enzymes and the analyte (antigen) into electrochemical signal, which in turn yields high specificity and good sensitivity of the sensor [Citation16]. Nevertheless, increasing evidence shows that the secondary antibody can be ignored from immunosensor fabrication (the label-free technique) with good performance of the sensors [Citation17–20]. These label-free immunosensors have proved their use in the detection of different contaminants in food such as viruses, bacteria and chemicals [Citation15,Citation21].
To further enhance the biosensor performance, the electrode is subjected to surface modification by conducting polymers. In particular, polyaniline, one of most important conducting polymers has emerged as an intriguing material because of their specific redox properties, low cost, ease of production and good electrical conductivity along with their excellent biocompatibility [Citation22,Citation23]. In this work, poly(2-aminobenzylamine), a polyaniline derivative, was selected for electrode fabrication in the proposed immunosensor, because 1) poly(2-aminobenzylamine) provides selective detection of biomolecules as reported in the previous article using surface plasmon resonance [Citation24] and 2) poly(2-aminobenzylamine) is easy to manipulate in terms of the deposition and formation rate of the polymer during electropolymerization [Citation24]. In addition, the amino group (-NH2) in the poly(2-aminobenzylamine) structure acts as if it was a chemical scaffold for antibody to immobilize through amide bond on poly(2-aminobenzylamine) thin film. This renders stable analytical platform and high loading of antibody, which immensely boost up the analytical performance that will maximize the number of immunoreaction events. To the best of our knowledge, poly(2-aminobenzylamine) used for biosensing application has never been reported.
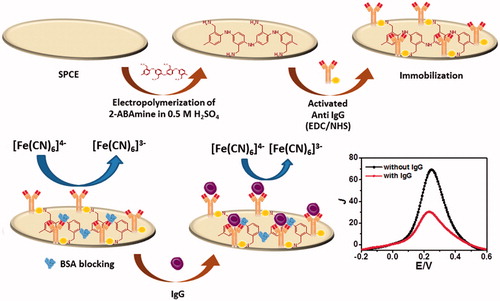
Screen-printed carbon electrodes have been extensively used in the area of biosensors applied for the detection of pesticides and antigens [Citation25,Citation26], since they offer good analytical and electrochemical properties and low background current together with their tiny structure, simple and low-cost fabrication [Citation27–29]. Even though it has been known that screen-printed carbon electrodes contribute to low electroactivity and low sensitivity in the detection, these drawbacks can be easily mitigated and considerably improved by elaborating the sensor with conducting polymers or other electroactive and high conductivity materials. Therefore, the goal of this paper is to develop simple, disposable, inexpensive and label-free electrochemical immunosensor based on poly(2-aminobenzylamine) modified on screen-printed carbon electrode as shown in . In this study, the specific detection of the immunoglobulin G antigen was achieved by the selective molecular recognition of the anti-immunoglobulin G antibody to the immunoglobulin G antigen that is covalently bound on the poly(2-aminobenzylamine) film-based electrode (). The selectivity of the sensor was also corroborated by no significant detection of adventitious binding generated by common interferences, including redox-active biomolecules such as ascorbic acid, glucose, L-cysteine and uric acid. To this end, our developed electrochemical immunosensor showed exceptional sensitivity, selectivity and reproducibility for detection of immunoglobulin G, which could be potentially adopted for non-invasive detection of the immunoglobulin G level in human serum.
Experimental
Chemicals, materials and apparatus
All the reagents used were analytical grade. 2-Aminobenzylamine, immunoglobulin G in human serum, anti-immunoglobulin G antibody and L-cysteine were purchased from Sigma Aldrich (St. Louis, MO). 1-Ethyl-3–(3-dimethylamino-propyl)carbodiimide, N-hydroxysuccinimide, sodium dihydrogen phosphate dehydrate (NaH2PO4·2H2O), ascorbic acid and blocking chemical, bovine serum albumin were obtained from Merck (Darmstadt, Germany). Potassium hexacyanoferrate(II) trihydrate (K4[Fe(CN)6]·3H2O) was ordered from Sigma-Aldrich (St. Louis, MO). Sulfuric acid (H2SO4), potassium ferricyanide (K3[Fe(CN)6]) and sodium hydroxide (NaOH) were purchased from Lab Scan (Gliwice, Poland). Di-sodium hydrogen phosphate dihydrate (Na2HPO4·2H2O) were purchased from Scharlau (Barcelona, Spain) while uric acid was purchased from Hopkin&Williams (Surrey, UK). In addition, phosphate-buffered saline tablets (pH 7.4) were purchased from Sigma-Aldrich (St. Louis, MO). Furthermore, glucose was purchased from Fluka (Switzerland). Deionized water was used throughout this study. Cyclic and differential pulse voltammetric measurements were performed using a conventional three-electrode electrochemical cell driven by μAutolab type II Potentiostat/Galvanostat (Metrohm Siam Ltd., Bangkok, Thailand). The reference electrode was an Ag/AgCl (3 M NaCl) electrode while a platinum wire and screen-printed carbon-based electrodes were used as an auxiliary electrode and working electrodes, respectively.
Fabrication of poly(2-aminobenzylamine) film-modified screen-printed carbon electrode
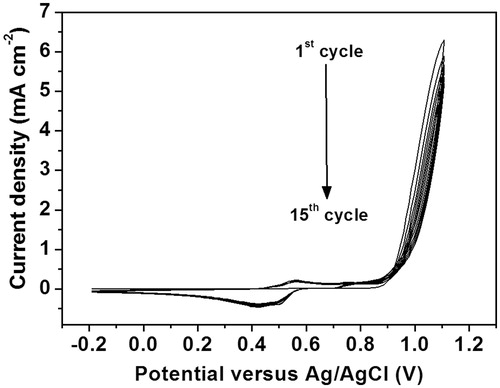
The screen-printed carbon electrodes were in-house fabricated employing an Acheson Electrodag PR-406 carbon ink (Henkel, Rocky hill, CT) and a poly(vinyl chloride) sheet was used as a substrate. Briefly, the ink was screen-printed on poly(vinyl chloride) substrate and then the screen-printed carbon electrodes obtained were incubated at 150 °C in an oven for 1 h. The fabrication process was performed twice to obtain the greater film connection of carbon particles on the substrate, thus possessing the good sheet conductivity. Before use, the screen-printed carbon electrode was used as a working electrode with a diameter about 3.0 mm and was treated by plasma cleaning under the optimum condition. Briefly, the screen printed carbon electrodes were placed in the chamber of the plasma cleaner. The chamber was evacuated to 0.15 torr and then backfilled with air. After that plasma was generated at low radio frequency (RF) and operated at pressure 0.4 torr for 1 min. The electropolymerization of poly(2-aminobenzylamine) film onto screen-printed carbon electrode was undertaken by cyclic voltammetry in 50 mM 2-aminobenzylamine monomer solution containing 0.50 M H2SO4 by an applied potential range from 0.20 to 1.10 V for 15 cycles at a scan rate of 20 mVs-1 as shown in . The electrochemical characterization and optimization of the fabricated poly(2-aminobenzylamine) films are shown in . Surface morphology of the electrodes was investigated using a scanning electron microscope (JEOL, JSM-6335 F, Peabody, MA) (see ).
Figure 2. Cyclic voltammograms for electropolymerization of poly(2-aminobenzylamine) film on the screen-printed carbon electrode in solution containing 50 mM 2-aminobenzylamine and 0.50 M H2SO4.
Construction of immunoglobulin G immunosensor and measurement procedure
Due to lack of the secondary antibody tagged with enzymes, the electrochemical signal of non-redox immunoglobulin G is measured by the voltammetric technique through the mechanism of electron transfer process mediated by the redox coupling agent [Fe(CN)6]4-/3- (). The electrochemical immunosensor was stepwise constructed as follows (see and ). Briefly, anti-immunoglobulin G solution was activated by mixing with a solution containing 0.40 M 1-ethyl-3–(3-dimethylamino-propyl)carbodiimide and 0.10 M N-hydroxysuccinimide for 30 min at 4 °C. The activation of carboxylic groups available on the antibodies was therefore obtained. 20 μL of the activated anti-immunoglobulin G solution was subsequently coated onto the surface of the poly(2-aminobenzylamine) film-modified screen-printed carbon electrode obtained from previous section at the 4 °C to obtain covalent attachment on the electrode surface. After that the electrode was washed with phosphate buffered saline (pH 7.4) several times and dried, nonspecific binding sites were blocked via casting 20 μL of 0.5% bovine serum albumin solution onto the surface at the same temperature for 30 min. After the above electrode was rinsed with phosphate buffered saline again several times and dried, the differential pulse voltammetry was utilized to record the electrochemical signal of the [Fe(CN)6]4-/3- process, which represented a baseline response. The differential pulse voltammetric responses were also measured after incubation of the immunoelectrode surface with 20 μL of different immunoglobulin G concentrations followed by cleaning the surface with phosphate buffered saline several times. The immunocomplex restricted the electron transfer process of the redox probe, causing the reduction of the current signal. The cartoon of the restriction and related current is illustrated in . As shown in , electropolymerization technique was used to prepare the NH2-/amine-terminated polyaniline film for immobilization of antibodies via covalent linkage. To obtain the working calibration data, current differences related to the degrees of immunoreaction at different immunoglobulin G concentrations were calculated due to degree of blockage of the electron transfer at the immunoelectrode. Between the incubation with each immunoglobulin G solution, the immunosensing electrode was regenerated using a 5 mM NaOH solution for 2 min. To monitor the electroactivity change of electrode surface due to surface coverage, cyclic voltammograms between each step of the immunosensor construction were determined (data not shown), which are related to differential pulse voltammetric responses as seen in . demonstrates the electrochemical responses relating to each step of fabrication process of the immunosensor. Firstly, the peak current of the redox process at the screen-printed carbon electrode is improved 85.7% by deposition of the electrochemically synthesized (2-aminobenzylamine) film. Then, the peak current is reduced sequentially due to degree of surface coverage with non-conducting molecules. A decrease in the peak current from 280 to 80 μA cm−2 was observed due to covalent immobilization of 60 μg mL−1 activated anti-immunoglobulin G onto polymer film-based screen-printed carbon electrode and a further current decrease of ca. 55 μA cm−2 is from immunoreaction of immunoglobulin G (50 ng mL−1) and anti-immunoglobulin G at the electrode surface. The parameters such as concentration of antibody, incubation periods of antibody attachment and antigen binding, pH, temperature, were optimized to obtain the best electrochemical response. Without use, the immunoelectrode was stored at 4 °C in refrigerator.
Results and discussion
Electropolymerization of 2-aminobenzylamine
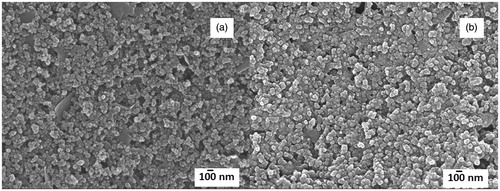
Electropolymerization technique was used to manipulate the polymer thickness and mitigate the side reactions of polymerization. Cyclic voltammetry was performed in this work to polymerize 2-aminobenzylamine to generate poly(2-aminobenzylamine). As illustrated in , the polymerization was successfully performed through consecutive 15 cycles of cyclic voltammetry with the applied potential from 0.20 to 1.10 V at a scan rate of 20 mV s−1. To elaborate, the first scan reveals the first oxidation peak of 2-aminobenzylamine at 0.80 V, corresponding to the 2-aminobenzylamine oxidation potential and the formation of poly(2-aminobenzylamine) film on the screen-printed carbon electrode [Citation30]. As to the rest of the cyclic voltammetric scans (2nd to 15th cycle), the dedoping peak at 0.45 V and the doping peak at 0.55 V derived from the cathodic and anodic scans are observed respectively, reflecting the electron transfer process mediated by the polymerized aniline chain in poly(2-aminobenzylamine) [Citation30,Citation31]. In addition, the more polymerization cycle is performed, the higher intensities of the doping and dedoping peaks are obtained, suggesting the higher amount of the conductive poly(2-aminobenzylamine) deposited on screen-printed carbon electrode. To optimize the thickness of the polymer film, cyclic voltammetry was again employed to the 2-aminobenzylamine monomer solution with five to twenty cyclic voltammetric cycles (). shows the electrochemical responses of redox process of solution-phase [Fe(CN)6]4-/3- at poly(2-aminobenzylamine) films electropolymerized on screen-printed carbon electrode with different numbers of cyclic voltammetric cycles, resulting in different thicknesses. The higher number of electropolymerization cycle would be expected to give higher thickness of the film. Cyclic voltammograms and differential pulse voltammograms of the [Fe(CN)6]4-/3- redox system at the films electrosynthesized with different polymerization cycles are illustrated in ), respectively. Cyclic voltammograms were recorded in range from -0.40 to 1.0 V at a scan rate of 50 mV s−1 while differential pulse voltammograms were recorded in range from −0.20 to 0.60 V using a step potential of 5 mV, modulation amplitude of 50 mV and modulation time of 20 ms at a scan rate of 10 mV s−1. It was found that the poly(2-aminobenzylamine) films can improve the electrochemical response at the modified screen-printed carbon electrode when the current is increased with the deposited polymer. With increasing the polymerization cycle, current responses from both techniques are increased and reach a maximum with 15 polymerization cycles. The peak currents are dropped with 20 polymerization cycles. Moreover, at the polymerization cycle of 20, cyclic voltammogram shows the higher peak-to-peak separation (ΔE), indicating slower electron transfer kinetics which would lower the performances of the constructed immunosensor. Then, the optimal thickness was determined regarding the best current response using differential pulse voltammetry by measuring the current response generated by [Fe(CN)6]4-/3- redox couple probe. It was found that 15 cycles of the cyclic voltammetric scan gave the highest current response (see ), which originates from the optimal polymer mass and thickness. Therefore, electrochemical deposition of poly(2-aminobenzylamine) film on screen-printed carbon electrode was achieved with the optimal 15 cycles of electropolymerization using the cyclic voltammetric technique. Scanning electron microscopic images of the screen-printed carbon electrodes without and with the optimized polymer film are shown in , respectively. No significant surface morphology change was observed after electropolymerization. The surfaces showed the irregular shapes of the carbon particles and a small change in the particle size was observed due to covering the particles with our polymer. The micrographs also reveal aggregate particles and agglomerate particles which connect well with each other. The poly(2-aminobenzylamine)-deposited screen-printed carbon electrode is further developed as explained below.
Optimization of the anti-immunoglobulin G immobilization conditions for the immunosensor
Anti-immunoglobulin G immobilization process was performed through amide bond attachment of the antibody to the poly(2-aminobenzylamine) surface on the electrode using 1-ethyl-3–(3-dimethylamino-propyl)carbodiimide/N-hydroxysuccinimide coupling reagents as shown in and the redox response of ferro/ferricyanide system at electrode, which is related to the immobilization step is shown in . Several parameters including antibody concentration, incubation time and incubation temperature of the anti-immunoglobulin G immobilization were optimally fine-tuned, since the number of the anti-immunoglobulin G covalently anchored on the electrode surface profoundly affects the electrochemical signal resulting from the immunoreaction generated by the anti-immunoglobulin G and the immunoglobulin G antigen. Depicted in , the reduction of the current density (J) arising from the differential pulse voltammetric response using [Fe(CN)6]4-/3- as the redox probe is elevated when the anti-immunoglobulin G concentration is increased from 20 to 60 μg mL−1 and constantly remained at 70 μA cm−2, regardless of the higher concentrations of anti-immunoglobulin G. The higher decrease in current density (J) or current density difference (ΔJ) suggests that the poly(2-aminobenzylamine)-deposited screen-printed carbon electrode is immobilized by non-conducting anti-immunoglobulin G. The plateau from anti-immunoglobulin G concentration at 60 μg mL−1 was caused from saturation of anti-immunoglobulin G loading. Thus, 60 μg mL−1 of anti-immunoglobulin G was used for investigating optimal incubation time of anti-immunoglobulin G immobilization.
Figure 6. Effect of (a) concentration of activated anti-immunoglobulin G solution, (b) incubation time for the immobilization of antibody and (c) incubation temperature of antibody attachment on differential pulse voltammetric current responses of the ferro/ferricyanide system at the prepared anti-immunoglobulin G-conjugated screen-printed carbon electrode.
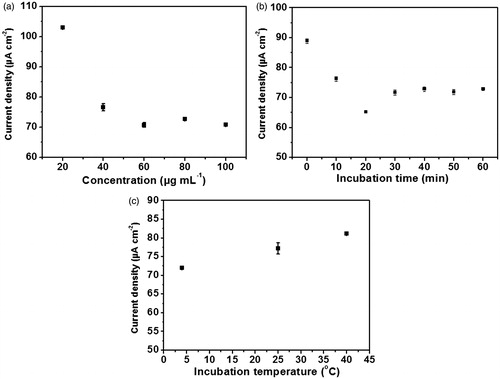
According to , 60 μg mL-1of anti-immunoglobulin G is incubated and current densities are measured at each time point. The result shows that current densities are rapidly decreased and the signal reached the minimum at 20 min; then decline to a constant value starting at 30 min, the point from which current density difference (ΔJ) is time-independent. The unusual decrease in current density (J) at 20 min suggests the unreliable detection of the electrochemical response at this time point; but after that, the current density difference (ΔJ) is diminished and remain constant due to the desorption of non-binding activated anti-immunoglobulin G molecules. Moreover, at this time point anti-immunoglobulin G molecules would be agglomerated due to binding with each other via coupling reaction of their amine and activated carboxylic groups on itself, resulting in instability of the redox response. From the time point of 30 min, the constant response was caused by the saturation of anti-immunoglobulin G loading. Therefore, the anti-immunoglobulin G incubation time of 30 min along with its concentration of 60 μg mL−1 were exploited for the temperature optimization.
The influence of the incubation temperature during anti-immunoglobulin G immobilization was examined by incubating 60 μg mL−1 of anti-immunoglobulin G for 30 min at these three different temperatures: 4, 25 and 40 °C. Illustrated in , current density is gradually increased as the temperature is mounted to 40 °C. This suggests that at 4 °C the anti-immunoglobulin G is loaded onto the electrode surface at a higher level than the other temperatures, leading to the good capability of blocking the redox process caused by [Fe(CN)6]4-/3- at the electrode surface deposited by poly(2-aminobenzylamine). This implies that coupling of anti-immunoglobulin G via covalent bond with NH2-group of the polymer film requires low temperature. Consequently, the immunosensor for immunoglobulin G detection was fabricated under the optimal condition where 60 μg mL−1 of anti-immunoglobulin G was incubated at 4 °C for 30 min on the poly(2-aminobenzylamine)-electrodeposited screen-printed carbon electrode. This prepared immunosensor is again further exposed to the immunoglobulin G antigen through varying incubation times, temperatures and pH in the purpose of optimizing the condition for immunoglobulin G detection as described below.
Optimization of the conditions for immunoglobulin G detection by the immunosensor
Incubation time, temperature and pH used for immunoglobulin G antigen detection have to be optimized for the immunosensor in order to sensitively capture the immunoglobulin G antigen, because these parameters have an impact on the degree of immunoreaction, which the biological phenomenon converted the differential pulse voltammetric signal by immunosensors. The signal amplification uses recorded charge transfer response of ferro/ferricyanide redox system before and after the immunoreaction. As shown in , when the fabricated immunosensor is incubated with the immunoglobulin G concentration of 25 ng mL−1 from 10 to 60 min, the current density (J) is dramatically decreased and then becomes constant at the incubation time of 30 min. On the other hand, the analytical response (ΔJ) is rapidly increased and reaches the maximum response at 30 min, the point from which the signal starts to be constant. The drop of analytical current (ΔJ) after 30 min indicates that the specific binding of immunoglobulin G to the immunoelectrode occurs. From this point the specifically bound immunoglobulin G via the immunoreaction become saturated on the electrode surface whereas the non-specific adsorption of immunoglobulin G antigen starts desorbing from the electrode surface. Therefore, the immunoglobulin G incubation time of 30 min was adopted in this work, since it is the fastest time point yielding the constant and reliable analytical signal due to the immunoglobulin G specific binding.
Figure 7. Effect of (a) incubation time, (b) temperature and (c) pH on differential pulse voltammetric current responses due to the immunoreaction.
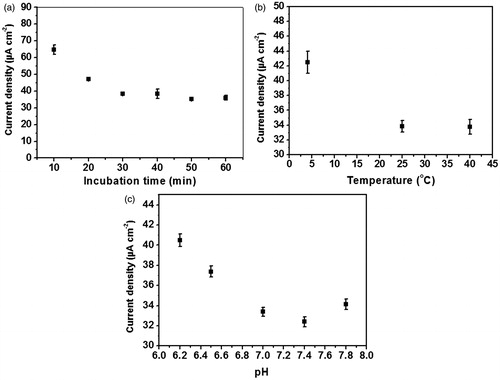
Subsequently, three different temperatures; 4, 25 and 40 °C; are applied to optimize the immunoreaction of immunoglobulin G on the immunosensor. The immunoreactions at 25 and 40 °C leaded to the equally high analytical response (), suggesting that the molecular recognition of immunoglobulin G with anti-immunoglobulin G at 25 °C is as high as that at 40 °C. However, lowest current density difference/analytical response (ΔJ) is observed at 4 °C, probably due to the inappropriate conformer of anti-immunoglobulin G and immunoglobulin G antigen to generate the immunocomplex. Therefore, the incubation temperature of 25 °C was used for allowing the maximum immunoreaction to occur and is as well as practical in clinical use.
In addition, pH also affects the immunoreaction as shown in . When the immunoelectrode is incubated with 25 ng mL−1 immunoglobulin G antigen at 25 °C for 30 min at different pH values (), the highest analytical response (ΔJ) is observed at pH 7.4. Unsurprisingly, the immunoglobulin G antigen and anti-immunoglobulin G assume their native conformations at this pH; so that the highest reduction of the current density is obtained. At this point, the optimal condition for the immunoglobulin G immunoreaction with anti-immunoglobulin G includes: the incubation time of 30 min, the incubation temperature of 25 °C and pH 7.4.
Performance of the immunosensor
Linear response and limit detection of the immunosensor
The linear range of the fabricated immunosensor was assessed by the incubation of the sensor with different immunoglobulin G concentrations at 25 °C and pH 7.4 for 30 min, which is the optimal condition for immunoglobulin G detection. Depicted in , the observed redox responses tend to gradually decrease whereas the immunoglobulin G concentration is elevated. Apparently, increased concentration of immunoglobulin G molecules prevents a [Fe(CN)6]4-/3- redox couple from carrying out the redox reaction at the electrode surface. In turn, the calibration curve was plotted with the logarithm of immunoglobulin G concentration against the current density difference (ΔJ) derived from the current densities (J) before and after binding with immunoglobulin G. As shown in , the analytical response (ΔJ) linearly agrees well with logarithm of immunoglobulin G concentration, giving a linear regression by an equation, current density difference (ΔJ, μA cm−2) = 11.297log [immunoglobulin G concentration (ng mL−1 )] + 5.9107. From the calibration curve (see ), the immunosensor exhibits good linear dynamic range for immunoglobulin G detection from 1.0 to 50 ng mL−1. The sensor also offers low limit of detection of 0.15 ng mL−1, limit of quantitation of 0.50 ng mL−1 and high sensitivity (11.297 μA cm−2), which are sufficient for the detection of immunoglobulin G at the physiological level. shows the detection performances for detecting immunoglobulin G using our proposed immunosensor as compared to other immunosensors reported in the literatures [Citation32–39]. Entries 1–4 are of sandwich structured immunosensors whilst entries 5–8 are of label-free immunosensors. The table verifies that our strategy provides a comparable detection limit and good linear range for the determination of immunoglobulin G. Furthermore, our immunosensor has been demonstrated with less complexity, less expensive chemical consumption, less time-consuming and good analytical performances and stability. Interestingly, the proposed immunosensor can be reused or regenerated several times while maintaining good analytical performances as described in next section.
Figure 8. (a) Differential pulse voltammetric responses of the immunosensor for the detection of immunoglobulin G at different concentrations and (b) calibration curve of the immunosensor.
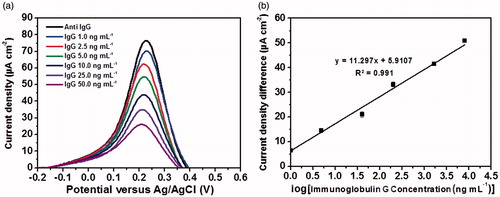
Table 1. Analytical performances comparison of proposed electrochemical immunosensor with other immunoglobulin G immunosensors reported in the literature.
Regenerability, selectivity, reproducibility and stability of the immunosensor
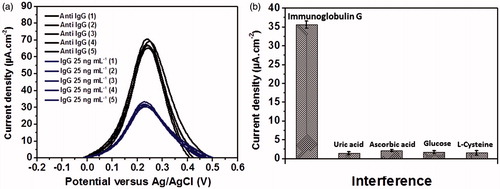
Regenerability of the immunosensor after bonding with incubation in a 25 ng mL−1 immunoglobulin G solution was investigated as shown in . With the appearance of immunocomplex of anti-immunoglobulin G and immunoglobulin G on the electrode surface, the differential pulse voltammetric current of [Fe(CN)6]4-/3- process was decreased from approximately 67.5 ± 2.5 to 30 ± 2 μA cm−2. After surface treatment of the immunosensor with an alkaline base solution, the current response was back to the starting point or baseline current response. The regeneration was examined for five times, which gave no significant change in the analytical signal (ΔJ) in detection of 25 ng mL−1 immunoglobulin G (). Moreover, the electrode surface was successfully regenerated several times throughout construction of the calibration graph (), with inbetween incubation of the immunosensor with different immunoglobulin G solutions. The regenerated electrode surface can rebind with immunoglobulin G. This evidence suggests that our immunosensor is regenerable, giving a stable signal. In addition, the immunosensor has good stability in use for the detection. The selectivity of the immunosensor was tested by common interfering redox biomolecules including L-cysteine, uric acid, ascorbic acid and glucose, since these compounds have the redox property at the window of scanned potential performed by the immunosensor. When the immunosensor is challenged by those four interfering compounds (), the signal from the specific binding of 25 ng mL−1 immunoglobulin G with the immunoelectrode is not significantly interfered by those interferences, albeit the concentrations are 0.10 mM for each compound. This indicates that the optimized immunosensor presents high selectivity to the immunoglobulin G antigen over redox responses of such interferences and interfering non-specific adsorption process. Moreover, the sensor shows good reproducibility after evaluation of electrochemical signals from ten different independent electrodes (data not shown), with the relative standard deviation (RSD) of 2.09 and 1.15% for the immunosensor per se and the immunoglobulin G-bound immunosensor, respectively. According to the result, the high sensitivity, selectivity, precision along with good reproducibility of the immunoelectrode, the developed immunosensor offers a strong potential in the application for disease diagnosis in clinical practice.
Conclusions
From this study, we are the first to fabricate the simple, cost-effective, sensitive, disposable and label-free electrochemical immunosensor based on poly(2-aminobenzylamine) film-modified screen-printed electrode for human immunoglobulin G detection. Poly(2-aminobenzylamine) thin film provided the amine-containing chemical scaffold for the anti-immunoglobulin G antibody to immobilize via strong amide bond linkage. This results in high stability and reproducibility of the immunosensor as compared to those using adsorption and entrapment for antibody immobilization. As a result, the covalent immobilization considerably enhances the immunosensor performances in terms of its sensitivity, selectivity, precision and reproducibility. Furthermore, the advantage of using cheap carbon screened onto the electrode surface together with the avoidance of secondary antibody make our immunosensor affordable. To this end, the proposed immunosensor has the potential applications for further development in clinical diagnostics.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- McDonald JC, Kappblman MD, Mccracken BH. Relative importance of cellular and humoral immunity in human renal transplantation. Ann Surg. 1971;174:602–608.
- Hansen LB, Buus S, Schafer-Nielsen C. Identification and mapping of linear antibody epitopes in human serum albumin using high-density peptide arrays. PLoS One. 2013;8:e68902.
- Villar LM, Casanova B, Ouamara N, et al. Immunoglobulin M oligoclonal bands: biomarker of targetable inflammation in primary progressive multiple sclerosis. Ann Neurol. 2014;76:231–240.
- ten Klooster L, van Moorsel CHM, Kwakkel-van Erp JM, et al. Immunoglobulin A in serum: an old acquaintance as a new prognostic biomarker in idiopathic pulmonary fibrosis. Clin Exp Immunol. 2015;181:357–361.
- Figueiredo A, Vieira NCS, dos Santos JF, et al. Electrical detection of dengue biomarker using egg yolk immunoglobulin as the biological recognition element. Sci Rep. 2015;5:7865.
- Vidarsson G, Dekkers G, Rispens T. IgG subclasses and allotypes: from structure to effector functions. Front Immunol. 2014;5:520.
- Toptygina AP, Pukhalsky AL, Alioshkin VA. Immunoglobulin G subclass profile of antimeasles response in vaccinated children and in adults with measles history. Clin Diagn Lab Immunol. 2005;12:845–847.
- Mashazi P, Tetyana P, Vilakazi S, et al. Electrochemical impedimetric immunosensor for the detection of measles-specific IgG antibodies after measles infections. Biosens Bioelectron. 2013;49:32–38.
- Sobarzo A, Paweska JT, Herrmann S, et al. Optical fiber immunosensor for the detection of IgG antibody to rift valley fever virus in humans. J Virol Methods. 2007;146:327–334.
- Berry NJ, Grundy JE, Griffiths PD. An improved radioimmunoassay method for the detection of IgG antibodies against cytomegalovirus. J Virol Methods. 1986;13:343–350.
- Ksiazek TG, West CP, Rollin PE, et al. ELISA for the detection of antibodies to ebola viruses. J Infect Dis. 1999;179:S192–S198.
- Chevrier MC, Châteauneuf I, Guérin M, et al. Sensitive detection of human IgG in ELISA using a monoclonal anti-IgG-peroxidase conjugate. Hybrid Hybridomics. 2004;23:362–367.
- Türkoğlu EA, Yavuz H, Uzun L, et al. The fabrication of nanosensor-based surface plasmon resonance for IgG detection. Artif Cells Nanomed Biotechnol. 2013;41:213–221.
- Reverberi R, Reverberi L. Factors affecting the antigen-antibody reaction. Blood Transfus. 2007;5:227–240.
- Ricci F, Adornetto G, Palleschi G. A review of experimental aspects of electrochemical immunosensors. Electrochim Acta. 2012;84:74–83.
- Grieshaber D, MacKenzie R, Vörös J, et al. Electrochemical biosensors-sensor principles and architectures. Sensors. 2008;8:1400–1458.
- Tran HV, Yougnia R, Reisberg S, et al. A label-free electrochemical immunosensor for direct, signal-on and sensitive pesticide detection. Biosens Bioelectron. 2012;31:62–68.
- Mao K, Wu D, Li Y, et al. Label-free electrochemical immunosensor based on graphene/methylene blue nanocomposite. Anal Biochem. 2012;422:22–27.
- Xue X, Wei D, Feng R, et al. Label-free electrochemical immunosensors for the detection of zeranol using graphene sheets and nickel hexacyanoferrate nanocomposites. Anal Methods. 2013;5:4159–4164.
- Chauhan R, Solanki PR, Singh J, et al. A novel electrochemical piezoelectric label free immunosensor for aflatoxin B1 detection in groundnut. Food Control. 2015;52:60–70.
- Luppa PB, Sokoll LJ, Chan DW. Immunosensors-principles and applications to clinical chemistry. Clin Chim Acta. 2001;314:1–26.
- Gerard M, Chaubey A, Malhotra BD. Application of conducting polymers to biosensors. Biosens Bioelectron. 2002;17:345–359.
- Yoon H. Current trends in sensors based on conducting polymer nanomaterials. Nanomaterials. 2013;3:524–549.
- Chuekachang S, Janmanee R, Baba A, et al. Electrochemically controlled detection of adrenaline on poly(2-aminobenzylamine) thin films by surface plasmon resonance spectroscopy and quartz crystal microbalance. Surf Interface Anal. 2013;45:1661–1666.
- Wang J, Pamidi PVA, Park DS. Screen-printable sol-gel enzyme-containing carbon inks. Anal Chem. 1996;68:2705–2708.
- Hart JP, Crew A, Crouch E, et al. Some recent designs and developments of screen-printed carbon electrochemical sensors/biosensors for biomedical, environmental and industrial analyses. Anal Lett. 2004;37:789–830.
- Palchetti I, Cagnini A, Carlo MD, et al. Determination of anticholinesterase pesticides in real samples using a disposable biosensor. Anal Chim Acta. 1997;337:315–321.
- Hart JP, Wring SA. Recent developments in the design and application of screen-printed electrochemical sensors for biomedical, environmental and industrial analyses. Trends Anal Chem. 1997;16:89–103.
- O'Halloran MP, Pravda M, Guilbault GG. Prussian Blue bulk modified screen-printed electrodes for H2O2 detection and for biosensors. Talanta. 2001;55:605–611.
- Baba A, Mannen T, Ohdaira Y, et al. Detection of adrenaline on poly(3-aminobenzylamine) ultrathin film by electrochemical-surface plasmon resonance spectroscopy. Langmuir. 2010;26:18476–18482.
- Sriwichai S, Baba A, Phanichphant S, et al. Electrochemically controlled surface plasmon resonance immunosensor for the detection of human immunoglobulin G on poly(3-aminobenzoic acid) ultrathin films. Sens Actuators B Chem. 2010;147:322–329.
- Zarei H, Ghourchian H, Eskandari K, et al. Magnetic nanocomposite of anti-human IgG/COOH–multiwalled carbon nanotubes/Fe3O4 as a platform for electrochemical immunoassay. Anal Biochem. 2012;421:446–453.
- Noh HB, Rahman A, Yang JE. Ag(I)-cysteamine complex based electrochemical stripping immunoassay: ultrasensitive human IgG detection. Biosens Bioelectron. 2011;26:4429–4435.
- Ma L, Ning D, Zhang H, et al. Au/Ag nanorods based electrochemical immunoassay for immunoglobulin G with signal enhancement using carbon nanofibers polyamidoamine dendrimer nanocomposite. Biosens Bioelectron. 2015;68:175–180.
- Wang L, Jia X, Zhou Y, et al. Sandwich-type amperometric immunosensor for human immunoglobulin G using antibody-adsorbed Au/SiO2 nanoparticles. Microchim Acta. 2010;168:245–251.
- Zhang L, Liu Y, Chen T. A mediatorless and label-free amperometric immunosensor for detection of h-IgG. Int J Biol Macromol. 2008;43:165–169.
- Wang M, Cao L, Yan P, et al. Fabrication and modeling of ultrasensitive label free impedimetric immunosensor for IgG based on poly(o-phenylenediamine) film modified gold electrode. Int J Electrochem Sci. 2012;7:7927–7934.
- Liu L, Li Y, Tian L, et al. A label-free voltammetric immunoassay based on 3D-structured rGO–MWCNT–Pd for detection of human immunoglobulin G. Sens Actuators B Chem. 2015;211:170–176.
- Li R, Wu K, Liu C, et al. 4-Amino-1-(3-mercapto-propyl)-pyridine hexafluorophosphate ionic liquid functionalized gold nanoparticles for IgG immunosensing enhancement. Anal Chem. 2014;86:5300–5307.

![Figure 3. (a) Cyclic voltammograms and (b) differential pulse voltammograms for [Fe(CN)6]4-/3- process at poly(2-aminobenzylamine) film-modified screen-printed carbon electrodes with different numbers of electropolymerization cycles.](/cms/asset/6e591374-b788-48a2-b8f8-73621f573896/ianb_a_1360322_f0003_c.jpg)
![Figure 5. Differential pulse voltammetric current responses of screen-printed carbon electrode measured in a 5.0 mM [Fe(CN)6]4-/3- solution for each step of the modification. Anti-immunoglobulin G and immunoglobulin G concentrations used in this study are 60 μg mL−1 and 50 ng mL−1, respectively.](/cms/asset/0a30937a-018a-4461-ad49-c933def13c37/ianb_a_1360322_f0005_b.jpg)