Abstract
Biogenic synthesis of silver (AgNPs) and gold nanoparticles (AuNPs) using aqueous extract of Euphrasia officinalis has been reported. Stable AgNPs and AuNPs were formed on adding aqueous solutions of silver nitrate and chloroauric acid with E. officinalis leaf extract, in 19 min and 2 min, respectively. The synthesis method used in present study was simple, reliable, rapid, cost effective and ecofriendly. The synthesized nanoparticles were characterized with field emission transmission electron microscopy (FE-TEM), elemental mapping, selected area diffraction pattern (SAED), energy-dispersive X-ray spectroscopy (EDS), X-ray diffractometer (XRD), particle size distribution, zeta potential and Fourier-transform infrared spectroscopy (FTIR). The UV–Vis spectrum confirmed the synthesis of nanoparticles as the absorption band was observed at 450 nm for AgNPs and at 558 nm for AuNPs. The TEM images revealed quasi-spherical shape of AgNPs and AuNPs. The size of nanoparticles was determined to be 40.37 ± 1.8 nm for AgNPs and 49.72 ± 1.2 nm for AuNPs. The zeta potential value demonstrated the negative surface charge and stable nature of nanoparticles. Crystalline nature of the nanoparticles in the face-centred cubic (fcc) structure was confirmed by the peaks in the XRD pattern and SAED pattern. FTIR results showed the functional groups involved in reduction of silver and gold ions to metal nanoparticles. For biomedical application, the nanoparticles have been explored for anticancer, antibacterial and biofilm inhibition activities. It was observed that AgNPs exert anticancer activity against human lung cancer (A549) and human cervical cancer (HeLa) cell lines. On the other hand, AuNPs were able to inhibit only human cervical cancer cells. Furthermore, the AgNPs were active against clinically isolated human pathogens like Pseudomonas aeruginosa, Escherichia coli, Staphylococcus aureus and Vibrio parahaemolyticus. Additionally, AgNPs also showed biofilm inhibition activity against S. aureus and P. aeruginosa.
Introduction
Nanobiotechnology refers to the intersection of nanotechnology and biology. It deals with the development of biogenic and ecofriendly technology for the synthesis of nanomaterials and nanoparticles. Nanoparticles especially silver and gold are of high significance interest because of unique properties and potential applications in the fields of medical nano-engineering, and pharmaceutical for the development of therapeutic agents, chronic disease diagnostics and biosensors [Citation1]. Additionally, silver nanoparticles (AgNPs) and gold nanoparticles (AuNPs) were widely applied to human contact areas such as shampoos, soaps, detergents, shoes, cosmetic products and toothpaste as well as medical and pharmaceutical applications [Citation2].
A number of physical and chemical techniques are available for the syntheses of nanoparticles but unfortunately many of the nanoparticle syntheses methods involve the use of hazardous chemicals or high-energy requirements, which are rather difficult and include waste purifications [Citation3]. Hence, it is becoming a responsibility to emphasize on an alternate as the synthetic route which is not only cost effective but should be environment friendly in parallel. Recently, biological methods become an attractive alternative, because it exploits the natural resources such as plant, fungi and microbes, and are believed to be biocompatible [Citation4]. The usage of plant products for synthesis of nanoparticles has grown tremendously in the recent years as it eliminates the complex steps like intracellular synthesis, several purification steps, long incubation time or maintenance of microbial cell culture, etc.
In order to overcome the issues related to synthesis and stabilization, the present study uses Euphrasia officinalis leaf aqueous extract for the synthesis AgNPs and AuNPs. E. officinalis is an annual, herbaceous, semi-parasitic plant which belongs to the family Orobanchaceae. E. officinalis is known to contain many active compounds like iridoids, flavonoids, phenolic acids or etheric oils [Citation5–7]. The plant exhibits numerous biological activities, including anti-inflammatory, antioxidant, antimicrobial, anticancer, antifungal, antiviral, hypotensive, hepatoprotector, anti-epileptic and anticatarrhal activities [Citation8–11]. Almost every part of this plant exhibits variety of biological actions, for example, the leaf, the stem and small pieces of the flowers are used to make extract, capsule and tea, etc. Consequently, several studies have been done using E. officinalis extract; however, there is no report for the utilization of E. officinalis leaf aqueous extract for nanoparticle synthesis. Therefore, here for the first time we report synthesis of AgNPs and AuNPs using E. officinalis aqueous extract. Further considering the benefits of the plant extract in biomedical field, herein, we explored the application of biologically synthesized AgNPs and AuNPs as an anti-cancer, antibacterial and anti-biofilm agents.
Materials and methods
Materials
Silver nitrate (AgNO3), chloroauric acid (HAuCl4, 99.999%), crystal violet solution, MTT, dimethyl sulphoxide (DMSO) and all the other chemicals were purchased from Sigma-Aldrich Chemicals (St. Louis, MO). All the media were purchased from Difco, MB cell (Seoul, Korea). Euphrasia officinalis (dried leaves) was obtained from Mountain Rose herbs (Eugene, OR). RAW 264.7 cells (mouse macrophage), HeLa (human cervical cancer), A549 (human lung cancer) cell lines were obtained from the Korean Cell Line Bank (Seoul, South Korea). Dulbecco’s modified eagle medium (DMEM), Roswell Park Memorial Institute medium (RPMI-1640), foetal bovine serum (FBS), penicillin and streptomycin were purchased from Gibco BRL (Grand Island, NY). The pathogenic bacterial strains were obtained from Korean Agricultural Culture Collection (KACC) and Korean Collection for Type Cultures (KCTC).
Preparation of aqueous extract of Euphrasia officinalis leaf
The dried leaf of Euphrasia officinalis was finely powdered. For preparation of leaf extract, 50 g of leaf powder was boiled in 500 mL deionized water for 30 min. The aqueous extract was subsequently centrifuged at 10,000 rpm for 10 min, and supernatant obtained was filtered through a 0.45 µm PVDF syringe filter (SmartPor, Seoul, Korea). This filtrate was stored at −20 °C and used for further experiment.
Biosynthesis of silver and gold nanoparticles
For the synthesis of silver and AuNPs, 5 mL of leaf extract was mixed with 25 mL of sterile water. AgNO3 and HAuCl4 were added with a final concentration of 1 mM in the corresponding reaction mixtures, separately. The reaction mixture was incubated at 65 °C. The progress of the reaction was routinely monitored by observing colour change, which indicated the formation of silver and AuNPs. The metal nanoparticles were collected by high speed centrifugation at 16,000 rpm for 10 min. The pellet obtained was washed three times by deionized water then air dried and was used for further study.
Characterization of nanoparticles
UV–Vis spectrophotometer (UV–vis; Optizen POP; Mecasys, Daejeon, Korea) was used to confirm the reduction of metal ions and was scanned in the range of 300–800 nm. The morphology of purified nanoparticles was analysed by using field emission transmission electron microscopy (FE-TEM) with a JEM-2100F (JEOL, Tokyo, Japan) instrument operated at 200 kV. Further, the elemental mapping, selected area diffraction pattern (SAED) and energy-dispersive X-ray spectroscopy (EDS) of nanoparticles have been performed using FE-TEM (JEOL, Tokyo, Japan). The sample for FE-TEM was prepared by placing a drop of collected nanoparticles dispersed in water on carbon coated copper grid and subsequently drying at room temperature before transferring it to the microscope. The X-ray diffraction (XRD) pattern for synthesized nanoparticles was recorded using XRD, D8 Advance, (Bruker, Bremen, Germany), operated at 40 kV, 40 mA, with CuKα radiation, at a scanning rate of 6°/min, step size 0.02, over the 2θ range of 20–80°. The particle size distribution and zeta potential of the nanoparticles were studied using zetasizer Nano-ZS90 (Malvern Instruments, Worcestershire, UK). The functional groups capped on surface of the AgNPs were identified using a Fourier-transform infrared (FTIR) spectroscopy (Spectrum One System, Perkin-Elmer, Waltham, MA).
Cytotoxic effect of AgNPs and AuNPs
Murine macrophage cell lines (RAW 264.7), lung cancer cell lines (A549) and cervical cancer cell line (HeLa) were maintained at 37 °C in an incubator with a 5% CO2 humidified atmosphere in DMEM (Gibco, Grand Island, NY), supplied with 10% FBS and 1% (v/v) P/S, respectively. The cells were detached by trypsinization when they reached 80% confluency during subculture. MTT assay was performed to determine the AgNPs and AuNPs cytotoxic effect at various concentrations against the RAW 264.7, A549 and HeLa cell lines. Briefly, 200 µL of cell suspension containing approximately 1 × 105 cells/well was added into each well of a 96-well culture plate and incubated for 24 h at 37 °C in a 5% CO2 humidified atmosphere. After 24 h, the growth medium was removed. The cells were treated with different concentration (1, 5, 10 and 100 µg/mL) of nanoparticles. After 24 h of treatment, the medium was gently removed and 100 µL (1 mg/mL) of MTT added. The plates were further incubated at 37 °C in a humidified 5% CO2 incubator for 4 h, following which the plate medium was then carefully removed and 100 µL DMSO was added to each well to dissolve the formazan crystals. The absorbance was read at 570 nm using a microplate reader (Molecular Devices E09090, San Francisco, CA). Cells without nanoparticles were used as a control. Cell viability for a well was calculated by the following equation: CV = (the OD value of treated well/the OD value of non-treated control well) × 100%.
Antibacterial activity of AgNPs
Antibacterial activity of AgNPs was determined by disc diffusion method. The bacterial strains were grown-up in nutrient broth (NB) at 28 °C for overnight. The inoculum (100 μL, Optical density 0.5) of the Pseudomonas aeruginosa [KACC 14021], Escherichia coli [CCARM 0237], Vibrio parahaemolyticus [KACC 15069] and Staphylococcus aureus [KCTC 3881] was spread on to the Müller-Hinton agar plates in order to make sure a uniform thick lawn. Then, paper discs (8 mm) impregnated with 30 μL of a 500 ppm and 1000 ppm solution of AgNPs was placed over each plates. Then the plates were incubated for 24 h at 28 °C. After 24 h, the zone of inhibition (ZOI) was measured, which appeared as a clear area around the discs. Three replicates of experiments were carried out and ZOI was expressed in millimetres.
Biofilm inhibition activity of AgNPs
Biofilm inhibition activity was determined against S. aureus and P. aeruginosa as described previously by Du et al. [Citation12]. In brief, the log phase of S. aureus and P. aeruginosa (Optical density 0.1) was cultured in a 96-well plate. After culturing for 24 h, different concentrations of AgNPs (1–10 µg/mL) were added. After 24 h of treatment with AgNPs, CV assay was performed. The solution was removed from the wells and washed with sterile water, and then 200 µL of 1% crystal violet solution was added in each well. After 30 min, the CV solution was removed and the wells were washed with sterile water. Thereafter, 200 µL of DMSO was added to each well. The absorbance was read at 575 nm using a FilterMax F5 Microplate Reader (Molecular Devices, Sunnyvale, CA).
Results and discussion
Synthesis and characterization of nanoparticles
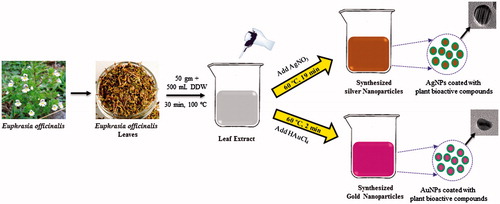
In recent years, several reports suggested the focus on greener approach for the synthesis of nanoparticles [Citation13,Citation14]. A brief overview of synthetic method of AgNPs and AuNPs is represented in . Synthesis of AgNPs and AuNPs by Euphrasia leaf was observed when the extract was incubated with the AgNO3 and HAuCl4 solution. First, the nanoparticles were confirmed by visual observation with the appearance of colour change in the reaction mixture. For AgNPs, the colour was changed from yellow to light brown within 19 min, which resembles the synthesis of AgNPs, as the particles cause surface plasmon resonance (SPR) due to which light brown colour appears. Similarly, for AuNPs synthesis the medium colour turns to deep pink colour after the incubation period of 2 min. The AuNPs exhibit ruby red to dark purple colour in aqueous solutions due to excitation of surface plasmon vibrations by AuNPs. Thus, the appearance of deep pink colour in the reaction mixture indicated the formation of AuNPs. The result is in line with the previously reported work by Singh et al. [Citation15,Citation16].
Figure 1. Schematic procedure of synthesis of silver and gold nanoparticles using Euphrasia officinalis leaf extract.
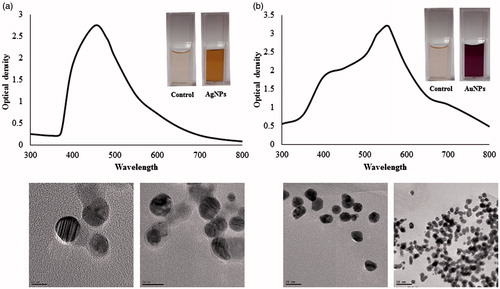
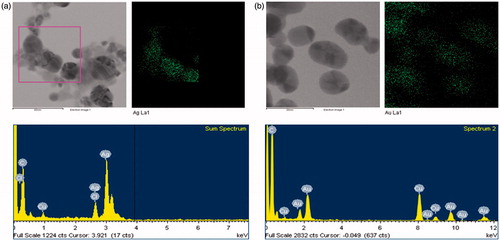
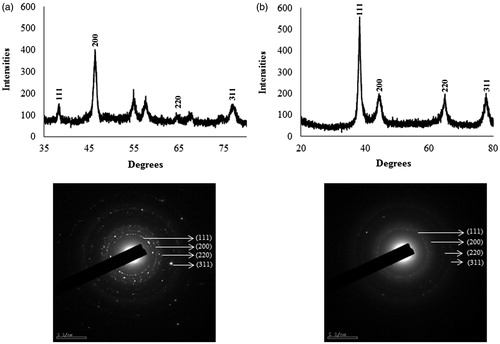
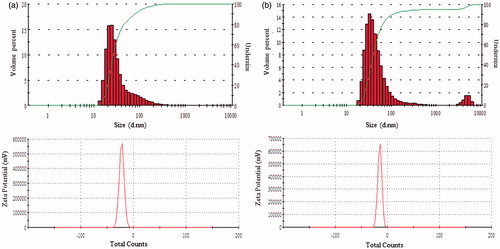
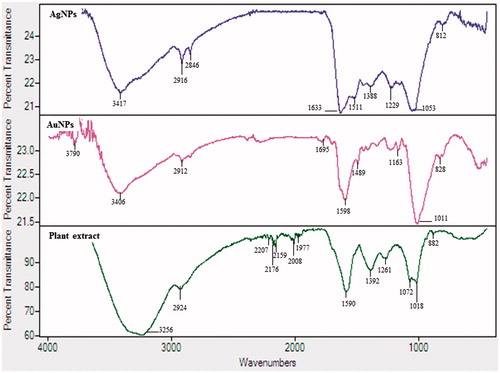
UV–vis spectroscopy is a valuable tool for identifying different types of nanoparticles [Citation15]. Metal nanoparticles strongly interact with specific wavelengths of light and show different peaks. The UV–vis spectra confirmed the AgNPs and AuNPs synthesis (). The spectrum showed maximum absorption band at 450 nm for AgNPs and at 558 nm for AuNPs caused due to SPR. The size and morphology of AgNPs and AuNPs are determined by TEM images as shown in . TEM images reveal the quasi-spherical shapes of both nanoparticles. The elemental mapping results indicated the maximum distribution of silver () and gold elements (), suggested that silver and gold were the predominant elements in the respective product. The chemical compositions of nanoparticles were further analysed by EDX (). The EDX spectrum of nanoparticles showed highest peak at 3 keV for AgNPs and 2.15 keV for AuNPs. The other metal ions group like carbon and copper also appeared in the EDX spectrum which corresponds to the TEM grid utilized for study [Citation16]. Crystallinity of nanoparticles was investigated by XRD and SAED. The XRD and SAED patterns of the nanoparticles are shown in . In the XRD pattern, four prominent peaks are observed at 2θ = 38°, 44°, 64° and 77°, which corresponds to (111), (200), (220) and (311) reflection planes of the face-centred cubic (fcc) structure of Ag planes and Au planes. All these distinct diffraction peaks correspond to the fcc phase of silver and gold standard (JCPDS Card no. 04-0783 and JCPDS card no. 04-0784). The ring pattern in SAED further confirms the crystalline nature the AgNPs and AuNPs. demonstrates the particle size distribution and zeta potential of nanoparticles in the reaction mixture, respectively. The average particles size of synthesized AgNPs was 40.37 ± 1.8 nm with PDI 0.382 and for AuNPs was 49.72 ± 1.2 nm with PDI 0.484, respectively. The zeta potential value was determined to be –22.4 ± 2.1 mV for AgNPs and –15.0 mV ±1.5 for AuNPs. The zeta potential values give us the information regarding the surface charge of nanoparticles which appeared to be negative here. Additionally, it gives us an idea about the stability of nanoparticles and the zeta potential value obtained for AgNPs and AuNPs lays within the stable range, signifying the nanoparticle are stable in aqueous solution. FTIR spectroscopy was used to investigate the role of possible functional groups of E. officinalis that are responsible for the reduction and stabilization of nanoparticles. The spectrum reveals distinct bands throughout the entire range of observation (). The bands around 3417, 3406, 3256, 2924, 2912, 2916 and 2864 cm−1 correspond to the N–H, C–H and O–H stretching vibrations. The pronounced peaks between the range 2000–1500 cm−1, corresponds to the following C=O, C=N and C=C starching vibration of functional groups. The peaks between the ranges 1400–1000 cm−1 were assigned to the C–F stretching. The bands at 882, 828 and 812 cm−1 were due to alkene. All the above peaks appeared in both leaf extract and the nanoparticles, indicating the capping groups surrounding the AgNPs and AuNPs. Some additional peaks only appeared in E. officinalis leaf extract like 2207, 2176, 2159, 2008 and 1977 cm−1 which indicated the presence of alkyne and thiocyanate functional group. As these peaks only appeared in the leaf extract, it signifies that this functional group was not involved in the formation of nanoparticles.
Cytotoxic effect
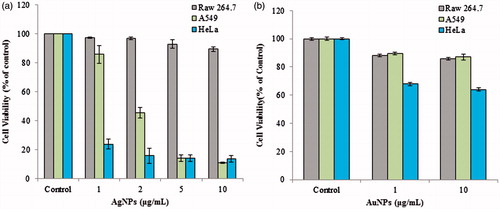
Several investigators have reported that the nanoparticles can induce toxicity to cancer cells and therefore be utilized as potent therapeutic agents for cancers [Citation17–19]. In the present study, the anticancer activity of AgNPs and AuNPs was tested against RAW 246.7 cells (murine macrophage, non-cancerous), A549 (human lung cancer) and HeLa (human cervical cancer) cell line. AgNPs and AuNPs did not show cytotoxicity towards RAW 246.7 cell at the concentration up to 10 μg/mL. However, in case of AgNPs, the growth of A549 cell line was inhibited up to 11 ± 0.5% and growth of HeLa cell line was inhibited by 13.5 ± 2.2% at the concentration 10 μg/mL (). On the other hand, AuNPs did not show any cytotoxic effect against A549 cancer cells, at the concentration 10 μg/mL, the viability was determined to be 87.9 ± 2.3%. But AuNPs exhibited little cytotoxicity towards HeLa cells. The viability was reduced to 67.8 ± 1.1% at the concentration 10 μg/mL (). The MTT analysis is an assessment of the activity of succinate dehydrogenase, so the observed effect could be due to the cytostatic or cytotoxic effect of nanoparticles, although, the activity revealed the significant anticancer activity of AgNPs and AuNPs. Similarly, activity AgNPs synthesized from Styrax benzoin and Borago officinalis has been reported recently [Citation12,Citation20]. However, in vivo study is required to understand the mechanism and mode of clearance of nanoparticles.
Antibacterial activity
The antibacterial activity of biosynthesized AgNPs was tested against four different pathogenic bacteria, including P. aeruginosa, E. coli, V. parahaemolyticus, S. aureus at the concentration 500 ppm and 1000 ppm (). The average antibacterial activity of AgNPs against bacterial strains ranged from 10.7 mm to 14.3 mm. The results were interpreted in terms of standard deviation of mean diameter of ZOI in . Generally, AgNPs are very efficient as an antimicrobial agent and microbes do not build up resistance against AgNPs. Several reports suggested the use of AgNPs as an effective antibacterial agent [Citation16]. The mechanism of the bactericidal effect of AgNPs against bacteria is not very clear but the major mechanism through which AgNPs manifest antibacterial properties was either by anchoring or penetrating the bacterial cell wall, and modulating cellular signalling by dephosphorylating putative key peptide substrates on tyrosine residues [Citation21]. Reports also suggest that the bactericide effects of ionic silver, the antimicrobial activity of colloid AgNPs are influenced by the dimensions of the particles, the smaller the particles, the greater antimicrobial effect. This suggests the possibility that the AgNPs may also penetrate inside the bacteria and fungi causing damage by interacting with electron phosphorous and sulphur containing compounds such as DNA [Citation22,Citation23]. The method used in the present study for the synthesis of AgNPs resulted in the formation of small size AgNPs thereby resulting in antimicrobial activity.
Figure 8. Zones of inhibition against Pseudomonas aeruginosa [KACC 14021], Escherichia coli [CCARM 0237], Vibrio parahaemolyticus [KACC 15069] and Staphylococcus aureus [KCTC 3881], respectively.
![Figure 8. Zones of inhibition against Pseudomonas aeruginosa [KACC 14021], Escherichia coli [CCARM 0237], Vibrio parahaemolyticus [KACC 15069] and Staphylococcus aureus [KCTC 3881], respectively.](/cms/asset/50b483cd-69f7-4370-80c1-08d6e9f9308b/ianb_a_1362417_f0008_c.jpg)
Table 1. Antimicrobial activity of the AgNPs synthesized from Euphrasia officinalis leaf extract.
Biofilm inhibition activity
Microorganisms attach to surfaces and develop biofilms. There are many bacteria, such as P. aeruginosa and S. aureus which are known to form biofilms for colonization [Citation24]. Microbial biofilms are ubiquitous in nature and result in the study of a number of infectious disease processes from a biofilm perspective. These bacteria are becoming a major threat to the health-care environment, as they become resistant to the antibiotics [Citation25]. Developing some anti-biofilm therapy remains a challenge and essential need with regards to their applicability in the biomedical field. Herein, we studied the effect of AgNPs on the biofilm inhibition of S. aureus and P. aeruginosa. The results suggest that the AgNPs were able to inhibit the biofilm formation in concentration dependent manner. It was found that at 10 µg/mL concentration of AgNPs completely inhibited the biofilm formation of S. aureus and P. aeruginosa ().
Conclusions
The present study demonstrated the ecofriendly, cheap and fast synthesis of AgNPs and AuNPs by using aqueous leaf extracts of E. officinalis. The synthesized nanoparticles were further characterized by FE-TEM, EDX, XRD, SAED, DLS, zeta potential and FTIR. Anticancer activity of nanoparticles was confirmed by MTT assay. AgNPs, demonstrated significantly high cytotoxicity against human lung and cervical cancer cells at 10 μg/mL. Additionally, the AgNPs showed antibacterial and ant-biofilm activity. The biosynthesized nanoparticles are ecofriendly and may be used for biomedical applications. However, further study is needed, regarding in vivo toxicity and to understand the mechanism.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Majdalawieh A, Kanan MC, El-Kadri O, et al. Recent advances in gold and silver nanoparticles: synthesis and applications. J Nanosci Nanotechnol. 2014;14:4757–4780.
- Bhumkar DR, Joshi HM, Sastry M, et al. Chitosan reduced gold nanoparticles as novel carriers for transmucosal delivery of insulin. Pharm Res. 2007;24:1415–1426.
- Ahmed S, Ikram S. Chitosan & its derivatives: a review in recent innovations. Int J Pharm Sci Res. 2015;6:14.
- Faramarzi MA, Sadighi A. Insights into biogenic and chemical production of inorganic nanomaterials and nanostructures. Adv Colloid Interface Sci. 2013;189:1–20.
- Petrichenko VM, Sukhinina TV, Babiyan LK, et al. Chemical composition and antioxidant properties of biologically active compounds from Euphrasia brevipila. Pharm Chem. 2006;40:312–316.
- Stoss M, Michels C, Peter E, et al. Prospective cohort trial of Euphrasia single-dose eye drops in conjunctivitis. J Altern Complement Med. 2000;6:499–508.
- Blazics B, Alberti A, Kéry A. Antioxidant activity of different phenolic fractions separated from Euphrasia rostkoviana Hayne. Acta Pharm Hung. 2009;79:11–16.
- Citarasu T. Herbal biomedicines: a new opportunity for aquaculture industry. Aquacult Int. 2010;18:403–414.
- Porchezhian E, Ansari SH, Shreedharan NK. Antihyperglycemic activity of Euphrasia officinale leaves. Fitoterapia. 2000;71:522–526.
- Ríos JL, Recio MC. Medicinal plants and antimicrobial activity. J Ethnopharmacol. 2005;100:80–84.
- Trovato A, Monforte MT, Forestieri AM, et al. In vitro anti-mycotic activity of some medicinal plants containing flavonoids. Boll Chim Farm. 2000;139:225–257.
- Du J, Singh H, Yi TH. Antibacterial, anti-biofilm and anticancer potentials of green synthesized silver nanoparticles using benzoin gum (Styrax benzoin) extract. Bioprocess Biosyst Eng. 2016;39:1923–1931.
- Soshnikova V, Kim YJ, Singh P, et al. Cardamom fruits as a green resource for facile synthesis of gold and silver nanoparticles and their biological applications. Artif Cells Nanomed Biotechnol. 2017. [Epub ahead of print]. doi: 10.1080/21691401.2017.1296849
- Huo Y, Singh P, Kim YJ, et al. Biological synthesis of gold and silver chloride nanoparticles by Glycyrrhiza uralensis and in vitro applications. Artif Cells Nanomed Biotechnol. 2017. [Epub ahead of print]. doi: 10.1080/21691401.2017.1307213
- Singh P, Kim YJ, Wang C, et al. The development of a green approach for the biosynthesis of silver and gold nanoparticles by using Panax ginseng root extract, and their biological applications. Artif Cells Nanomed Biotechnol. 2016a;44:1150–1157.
- Singh P, Kim YJ, Wang C, et al. Biogenic silver and gold nanoparticles synthesized using red ginseng root extract, and their applications. Artif Cells Nanomed Biotechnol. 2016b;44:811–816.
- Ahlawat J, Sehrawat AR. Biological synthesis of silver nanoparticles using aqueous leaf extract of Capparis decidua (FORSK.) EDGEW: a better alternative. J Pharm Res. 2015;11:244–249.
- Singh P, Kim YJ, Singh H, et al. Biosynthesis, characterization, and antimicrobial applications of silver nanoparticles. Int J Nanomed. 2015c;10:2567–2577.
- AshaRani PV, Low Kah Mun G, Hande MP, et al. Cytotoxicity and genotoxicity of silver nanoparticles in human cells. ACS Nano. 2008;3:279–290.
- Sanpui P, Chattopadhyay A, Ghosh SS. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl Mater Interfaces. 2011;3:218–228.
- Kim S, Choi JE, Choi J, et al. Oxidative stress-dependent toxicity of silver nanoparticles in human hepatoma cells. Toxicol In Vitro. 2009;23:1076–1084.
- Singh H, Du J, Yi TH. Green and rapid synthesis of silver nanoparticles using Borago officinalis leaf extract: anticancer and antibacterial activities. Artif Cells Nanomed Biotechnol. 2016. [Epub ahead of print]. doi: 10.1080/21691401.2016.1228663
- Shrivastava S, Bera T, Roy A, et al. Characterization of enhanced antibacterial effect of novel silver nonparticles. Nanotechnology. 2007;18:225103.
- Morones JR, Elechiguerra JL, Camacho A, et al. The bactericidal effect of silver nanoparticles. Nanotechnology. 2005;16:2346.
- Baker C, Pradhan A, Pakstis L, et al. Synthesis and antibacterial properties of silver nanoparticles. J Nanosci Nanotechnol. 2005;5:244–249.

![Figure 9. Biofilm inhibition activity of silver nanoparticles against Staphylococcus aureus [KCTC 3881] and Pseudomonas aeruginosa [KACC 14021].](/cms/asset/c60ce06e-5eb3-4d75-90ef-accfed0c68a4/ianb_a_1362417_f0009_c.jpg)