Abstract
Mosquitoes pose a threat to humans and animals, causing millions of deaths every year. Vector control by effective eco-friendly pesticides of natural origin is a serious issue that requires urgent attention. The employment of green-reducing extracts for nanoparticles biosynthesis in a rapid and single-step process represents a promising strategy. In this study, silver nanoparticles (AgNPs) were biofabricated using an essential oil of Aquilaria sinensis (AsEO) and Pogostemonis Herba essential oil of Pogostemon cablin (PcEO) in one step and cost-effective manner. UV–vis spectrophotometry, Fourier transform infrared spectroscopy, scanning electron microscopy, transmission electron microscopy, X-ray diffraction analysis and energy-dispersive X-ray spectroscopy were used to confirm the AgNPs formation and their biophysical characterization. The larvicidal and pupicidal toxicity of AsEO, PcEO and biosynthesized AgNPs were evaluated against larvae and pupae of the dengue and Zika virus vector Aedes albopictus. Compared to the tested essential oils, the biofabricated AgNPs showed the highest toxicity against larvae and pupae of Ae.albopictus. In particular, the LC50 values of AsEO ranged from 44.23 (I) to 166 (pupae), LC50 values of PcEO ranged from 32.49 (I) to 90.05(IV), LC50 values of AsEO-AgNPs from 0.81 (I) to 1.12 (IV) and LC50 values of PcEO-AgPNs from 0.85 (I) to 1.19 (IV). Furthermore, histological analysis of the midgut cells of the control and treated larvae exhibited that the epithelial cells and brush border were highly affected by the fabricated AgNPs compared to the essential oils (AsEO and PcEO). Overall, the A. sinensis and P. cablin essential oils fabricated AgNPs have a potential of application as a biopesticide for mosquito control through safer and cost-effective approach.
Introduction
Mosquitoes (Diptera: Culicidae) are the primary vectors of several serious diseases that affect both animals and humans [Citation1]. They play an important role in the transmission of parasites and pathogens of high public health concerns including malaria, dengue, filariasis, yellow fever, Japanese encephalitis, West Nile and Zika virus [Citation2,Citation3]. Aedes albopictus is considered to be one of the world’s fastest spreading invasive animal species and it originates from the forests of tropical regions of south-east Asia [Citation4]. This mosquito has recently invaded many countries, spreading rapidly to Europe, North and South America, the Caribbean, Africa and the Middle East [Citation5]. The Aedes albopictus is known to have transmitted a number of serious diseases including dengue fever, yellow fever, West Nile fever and Rift Valley fever, Japanese encephalitis [Citation6] as well as Zika virus fever [Citation7].
Mosquito control is a critical element in preventing the outbreaks of mosquitoborne diseases. The application of synthetic insecticides, such as organophosphates and pyrethroids, and insect growth regulators, such as diflubenzuron and methoprene, are currently major tools for mosquito control [Citation8]. However, these options have created many health and environmental issues, such as expanding resistance, disturbance of the ecosystem's natural control mechanisms and non-target organism toxicity and impact on aquatic species [Citation1]. In order to deal with these challenges, effective and eco-friendly control methods for mosquito vectors are urgently required.
In recent years, nanoparticles have emerged as potential pesticides, and currently, both plants and microbes are being used to fabricate metal nanoparticles [Citation5,Citation9]. The synthesis of nanoparticles using plant extracts is rapid, low cost, eco-friendly and a single-step method for biosynthesis process [Citation10]. Essential oils (EOs) are the aromatic oily liquids present in the secretary cavities and glandular hair cells of the plant parts [Citation11]. These oily extracts of plants are becoming increasingly popular as natural products to be used for a variety of purposes including complementary medicine and natural therapeutics, insect repellents, antimicrobial agents and food preservation [Citation12]. The plant genus of Aquilaria (Thymelaeceae) is comprised of approximately 15 species distributed across the rain forests of Southeast Asia [Citation13]. Aquilaria sinensis, the main plant resource in China for agarwood, is chiefly distributed in South China, and is widely cultivated in Hainan and Guangzhou provinces, with the planting area estimated to be covering more than 700 acres [Citation14]. The agarwood plays a role in traditional Chinese medicine (TCM) and a large amount of it is also being consumed by distillation to obtain an essential oil [Citation15]. On the other hand, Pogostemonis Herba is the aerial part of dried Pogostemon cablin (Blanco) and it also plays a vital role in TCM for the treatment of various problems such as to remove dampness, relieve sunstroke, stop vomiting and increase appetite [Citation16]. Patchouli oil is the essential oil of pogostemonis Herba, and it has been widely used by traditional Chinese physicians to treat a wide array of medical conditions such as common cold, nausea, diarrhoea, headache and fever since time memorial. Pogostone is the major chemical constituent of Pogostemonis Herba, and it is largely responsible for the intensive aromatic odour of the essential oil of this herb [Citation17].
In the present work, the larvicidal and pupicidal activity of agarwood essential oil of Aquilaria sinensis (AsEo) and Pogostemonis Herba essential oil of Pogostemon cablin (PcEo) as well as silver nanopartilces (AgNPs) synthesized by both of the essential oils have been tested for acute toxicity against Aedes albopictus mosquito larvae. UV–Vis spectrophotometry, Fourier transform infrared (FTIR) spectroscopy, X-ray diffraction (XRD), scanning electron microscopy (SEM), transmission electron microscopy (TEM) and energy-dispersive X-ray (EDX) spectroscopy have confirmed the rapid and cheap biosynthesis of AgNPs.
Material and methods
Essential oils and chemicals
The agarwood essential oil of Aquilaria sinensis (AsEo) and Pogostemonis Herba essential oil of Pogostemon cablin (PcEo) were purchased from Jiangxi Medicinal Oil Refinery Factory, South China. The silver nitrate (AgNO3) was purchased from Sigma Aldrich (USA). All other chemicals and reagents used were of the highest analytical grade purchased from local agencies.
Insects rearing
The eggs of Ae. albopictus obtained from mosquito colonies reared in the laboratory of Urban Entomology, Institute of Insect Sciences, Zhejiang University were allowed to hatch out under the controlled laboratory conditions at room temperature (RT: 26 ± 2 °C, and 70–85% relative humidity RH) with a naturally prevailing photoperiod of 14:10 h (light/dark). The larvae were maintained in dechlorinated tap water and were fed with finely ground rat food. The different developmental stages of mosquito larvae and pupae were used for the bioassays.
Synthesis of silver nanoparticles
In the present study, the silver nanoparticles (AgNPs) were synthesized using essential oil reduction method. The Aquilaria sinensis essential oil (AsEO) and Pogostemon cablin essential oil (PcEO) were utilized as both reducing and stabilizing agents. In a typical synthesis, the (0.01 g) of each of AsEO and PcEO in 1 ml of DMSO were separately diluted with 10 ml of dechlorinated water. The pH value of the solutions was adjusted to 7 using 0.1 M of sodium hydroxide solution and was dropped slowly into a boiling 100 ml of 2 mM silver nitrate solution. The formation of AgNPs is indicated by the colour change from colourless to light yellow and further to the red.
Characterization of AgNPs
The synthesized nanoparticles were primarily characterized by UV–vis spectroscopy UV-2550 spectra (Shimadzu, Japan) at a resolution of 1 nm in the range of 200–800 nm. Furthermore, the reaction mixture was subjected to centrifugation at 15,000 rpm for 20 min, and the resulting pellet was dissolved in de-ionized water and filtered through a Millipore filter (0.45 μm) and freeze-dried. An aliquot of this filtrate-containing silver nanoparticles was used for scanning electron microscopy (SEM), transmission electron microscopy (TEM), energy-dispersive spectroscopy (EDS), X-ray diffraction (XRD) and Fourier transform infrared (FTIR). The FTIR analysis was accomplished with the aliquot of reduced silver nanoparticles and recorded under identical conditions in the range of 500–4000 cm−1 region at a resolution of 4 cm−1 using Fourier transform infrared spectrometer (Vector 22, Bruker, Germany). The quality and formation of compounds were tested using Siemens X-ray diffractometer (XRD) analysis, where XRD pattern was measured by drop coated film of dried powder of silver nanoparticles onto glass slides. The operation conditions were at a voltage of 45 keV and a current of 20 mA with Cu-Ka radiation as an X-ray source in the range of 20–80 at the 2 h angle. Further, the morphology of the synthesized AgNPs was investigated by scanning electron microscopic (SEM) using TM-1000 (Hitachi, Japan). Thin film of the sample was prepared on a carbon-coated copper grid by simply dropping a very small amount of the sample on the grid. The instrument was equipped with an energy-dispersive X-ray spectrum (EDX) to confirm the presence of silver metal.
The film on the SEM grid was then allowed to dry by putting the grid under a mercury lamp for 5 min. The structural characterization of AgNPs was carried out by transmission electron microscopy (TEM) (JEM-1230, JEOL, Akishima, Japan). The extra sample was removed from carbon-coated copper grid using the cone of a blotting paper and sample was placed on the carbon-coated copper grid to make a thin film of the sample, and then, it was kept in a grid box sequentially.
Larvicidal and pupicidal bioassays
Mosquito larvicidal and pupicidal trials were carried out according to WHO (1996) standard procedures [Citation18], with slight modifications. The AsEO and PcEO were diluted in dimethyl sulfoxide (DMSO) in order to prepare a serial dilution of the test dosages. For experimental treatment, the desired dose of each essential oil in 1 ml DMSO was added to 100 ml of distilled water in a 250-ml beaker and mixed gently to produce a homogeneous test solution (). Twenty Ae. albopictus larvae (I, II, III, IV) instar and pupae were transferred in water into a bowl of the prepared test solution. For the experimental treatment of AgNPs, 20 Ae. Albopictus larvae (I, II, III, IV) instar and pupae were separately introduced in a 250-ml beaker containing 100 ml of dechlorinated tap water and added desired dosages of synthesized silver nanoparticles (). Four duplicate trials were carried out for every sample concentration, and for each trial, a negative control was included using distilled water containing the same amount of DMSO as the test sample. Mortality was determined after 24 h of exposure, during which no food was offered to the larvae.
Table 1. Larval and pupal toxicity of essential oil aquilaria sinensis (AsEO) and pogostemon cablin (PcEO) against zika vector ae. Albopictus.
Table 2. Larval and pupal toxicity of aquilaria sinensis and pogostemon cablin essential oil synthesized silver nanoparticles (AsEO-AgNPs and PcEO-AgNPs) against zika vector ae. Albopictus.
Histological analysis
Histological analysis of the digestive system was performed using fourth instar larvae (treated and control). Twenty-five fourth-instar larvae were exposed to a LC50 concentration for 24 h. The procedures were performed following the method of [Citation19] with a small modification. Briefly, the larvae were fixed in 10% buffered formaldehyde for 24 h, dehydrated through a graded series of ethanol, and cleared with xylene solutions. They were embedded in a lock using melted paraffin at the embedding station. The paraffin blocks were sectioned in 5 µm thickness using a rotary microtome and stained with haematoxylin and eosin. The glass slides were examined for abnormalities using an Olympus BX61 light microscope and photographed by a Canon EOS 1100 D digital camera.
Statistical analysis
The statistical analyses of data from larvicidal and pupicidal experiments were performed by probit analysis calculating LC50 and LC90 and following the method by Finney [Citation20] SPSS software package 16.0 version (SPSS Inc., Chicago, IL) was used. The toxicity data were subjected to one-way ANOVA analysis. Means were separated using Tukey’s HSD test. The acceptance level of statistical significance was p ≤ .05 in all instances.
Results and discussion
Characterization of silver nanoparticles
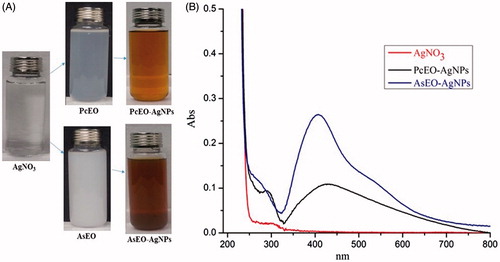
The silver nanoparticles (AgNPs) were synthesized via reduction of silver nitrate using the AsEO and PcEO. This was a one-step silver nitrate reduction process in which the AsEO and PcEO acted as both the reducing and stabilizing agents. The formation of AgNPs was visually confirmed by the change of reaction mixture colours (). When the AsEO and PcEO were separately mixed with AgNO3 solution under constant stirring at boiling degree, the colour of reactant solutions changed from the colourless to light yellow colour and further red colour within one and half hour. Literature reports similar colour changes due to the excitation of surface Plasmon vibrations of the synthesized AgNPs [Citation21]. The UV–vis spectroscopy has always been considered as one of the useful techniques for the preliminary characterization of metal NPs. UV–vis absorption spectra from the reaction solutions in the present investigation were monitored in the 200–800 nm range (). The maximum absorbance of AsEO-AgNPs was found at 408 nm due the excitation of the surface plasmon resonance to the fabricated AgNPs [Citation22]. Whereas absorption peak of PcEO-AgNPs was broader and observed at 430 nm, indicating the formation of AgNPs [Citation23–25]. Similar findings were recently reported by Shibani Basu and his colleagues [Citation26].
Figure 1. Preparation process of AgNPs, and UV-vis spectrum. (A) Reactants before and after the reaction. (B) UV–Vis adsorption spectrum of AgNPs. The maximum absorption peaks of AsEO-AgNP and PcEO-AgNP were 408 and 430 nm, respectively, after 90 min from the reaction.
Moreover, SEM analysis of our synthesized AgNPs was carried out in order to investigate the morphology and size distribution of AgNPs (). The AsEO-AgNPs and PcEO-AgNPs are mainly spherically shaped with the average range of particle size from 15 to 55 nm, and 16 to 87 nm, respectively. And this is a good resemblance with the shape of SPR band observed in the UV–Vis spectra [Citation27].
Figure 2. (A) SEM image of synthesized AgNPs using Aquilaria sinensis essential oil (AsEO). (B) SEM image of synthesized AgNPs using Pogostemon cablin essential oil (PcEO). (C) EDX pattern of synthesized AsEO-AgNPs and (D) EDX pattern of synthesized PcEO-AgNPs.
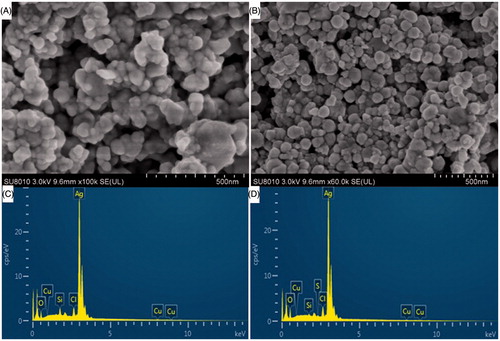
The EDX pattern of AsEO-AgNPs and PcEO-AgNPs were shown in (). The presence of strong peaks of silver element around 3 KeV were observed, which indicate that the Ag is the major element in AgNPs, thus confirming the successful biosynthesis of AgNPs [Citation28]. Similar peak (3 keV) has been reported by other researchers [Citation29].
Further investigations through transmission electron microscpoic (TEM) about the newly fabricated AgNPs using both AsEO and PcEO showed that it is polydispersity in nature. shows the TEM image of AgNPs and it indicates that the synthesized AgNPs are mainly uniform with spherical shape. The transparent organic layer coatings around the AgNPs were shown in the TEM image, It was also found that PcEO-mediated AgNPs, that bound with clearly thin layer of biomolecule coating on their surface which was due to the phytochemicals that served as a capping agent, and therefore, the particles were poly dispersed without direct contact and stable for long period of time to prevent agglomeration [Citation30].
Figure 3. (A) TEM image of synthesized AgNPs using Aquilaria sinensis essential oil (AsEO) (B) TEM image of synthesized AgNPs using Pogostemon cablin essential oil (PcEO). (C) XRD pattern of synthesized silver nanoparticles by AsEO and (D) XRD pattern of synthesized silver nanoparticles by PcEO.
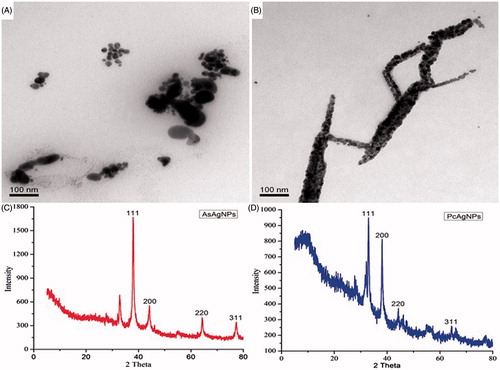
XRD is a rapid analytical method to identify the crystalline structure, the analysis of XRD showed intense peaks at 2 theta. () exhibits four well-defined characteristic peaks at scattering angles (2 h) for each of AsEO-AgNPs and PcEO-AgNPs where the angles (2 h) of 38, 44.28, 64.38, 77.36 corresponded to the lattice planes (111), (200), (220) and (311) in AsEO-AgNPs and the angles (2 h) of 33.28, 38.38, 44.08, 64.44 corresponded to the lattice planes (111), (200), (220) and (311) in PcEO-AgNPs. The peak corresponding to the (111) is more intense than the other planes [Citation31]. Thus, XRD highlighted that AgNPs formed by the reduction of AgNO3 with AsEO and PcEO were face-centred cubic (fcc) and crystalline in nature [Citation9].
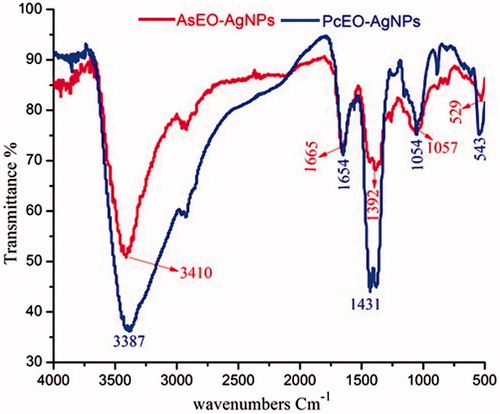
FTIR analysis of the AsEO-AgNPs and PcEO-AgNPs was carried out to identify the molecules which may be responsible for reducing Ag ions and stabilizing AgNPs in the mixture. The FTIR spectrum of AgNPs mediated using AsEO were shown in (). The absorption bands due to vibration of chemical molecules at 3410, 1665, 1392, 1057 and 529 cm−1 were recorded in the spectrum of wave number between 4000 and 500 cm−1. The very strong broad absorption peaks at 3410, (O–H stretch and bending); strong and sharp peaks at 1665 cm−1 (carboxylic acid) and some variable stretching and bending peaks were observed for AsEO-AgNPs, which may be from AgNO3 solution, the metal precursor involved in the AgNP synthesis process [Citation32]. Also, IR spectrum of synthesized AgNPs using PcEO was shown in (). The spectrum shows transmittance peaks at 3387, 1654, 1431, 1054 and 543 cm−1. The strong broad absorption peak at 3387 represents the presence of hydroxyl group (stretch and bending) from polyphenols, proteins, enzymes and/or polysaccharides [Citation33]. The sharp absorption peak close to 1654 cm−1 might be linked with stretching vibration C = O of carbonyl group, amide I and nitro groups [Citation30]. The peak at 1431 arises from the C-N stretching mode of the Aromatic amine group [Citation23]. Overall, these different functional groups from biomolecules of both essential oils may be responsible for reduction and capping of AgNPs [Citation34].
Larvicidal and pupicidal activity of AsEO, PcEO and AgNPs against aedes albopictus
In laboratory conditions, the larvicidal activity of aqueous essential oils of AsEO and PcEO, as well as the synthesized silver nanoparticles were evaluated against larval stages and pupae of Ae. albopictus mosquito. The 24-h exposure of AsEO and PcEO were moderately toxic against larval instars (I–IV) and pupae of Ae. albopictus (). The LC50 values of AsEO and PcEO ranging from 44.23 to 166 ppm and from 32.49 to 90.05 ppm, respectively, are shown in . Mortality was proportional to the tested concentrations and this was in agreement with a number of previously reported plant-borne extracts [Citation35,Citation36]. Earlier Hye-Mi Park, and his colleagues studied the larvicidal activity of PcEO and showed significant effect against Culex pipiens pallens [Citation37]. Also, PcEO showed toxicity and repellency against urban ant’s species [Citation38]. Our present study reported that the PcEO essential oil did not cause any mortality against pupae of Ae. albopictus (). As mentioned above, the 24-h exposure of AsEO was moderately effective against dengue and Zika vector mosquito. To the best of our knowledge, this is the first investigation of mosquitocidal activity of AsEO essential oil. Recently, the mosquitocidal potential of a number of plant essential oils have been reported. For example Zahran, H.E et al. [Citation39] observed pronounced larvicidal activity of A. monosperma, S. terebinthifolius and O. vulgare oils after 24 h of treatment against fourth instar larvae of Cx. Pipiens. Further Benelli, G. et al. [Citation40] reported acute toxicity of montana essential oil and four other plant essential oils on C. quinquefasciatus larvae. Similarly, it has been reported that EO extracted from the leaves of B. eriantha showed high toxicity against third instar larvae of Anopheles, Aedes and Culex species, with LC50 ranging from 41.61 to 61.33 μg/ml [Citation41]. The susceptibility of the Asian tiger mosquito larvae to the Cannabis sativa and Humulus lupulus essential oils with LC50 values of 302 and 331 μg/l respectively were observed [Citation42]. The possible overall toxicity of the both essential oils might be due to the presence of a wide range of phytochemicals and volatile compounds such sesquiterpenes, oxygenated sesquiterpenes, oxygenated phenyls, carboxylic and carbonyl hydrocarbons [Citation15]. While the Pogostone and Patchouli alcohol are the major chemical constituents of Pogostemonis Herba [Citation17]. These bioactive substances may be responsible to cause injury in the midgut of the larvae, and the intake of peroxide compounds could lead to cytotoxicity [Citation43].
Figure 5. Larvicidal and pupicidal efficacy of (A) Aquilaria sinensis essential oil (AsEO), Pogostemon cablin essential oil (PcEO) and (B) green-synthesized silver nanoparticles using AsEO and PcEO against I-IV instar larvae and pupae of dengue and zika vector Aedes albopictus. Mortality was recorded after 24 h of exposure. No mortality was observed in the control. Different letters above each bar indicate significant differences among treatments (ANOVA, Tukey’s HSD test, p < .05).
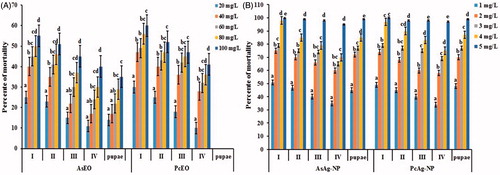
Notably the AgNPs synthesized using AsEO and PcEO were highly toxic against Ae. albopictus larvae and pupae () with LC50 ranging from 0.81 to 1.12 ppm and from 0.90 to 1.19 ppm, respectively (). In the recent years, the green-synthesized AgNPs showed comparable larvicidal and pupicidal toxicity against a number of mosquito vectors [Citation40,Citation44,Citation45]. For instance, the S. maritima-synthesized AgNPs were highly toxic against Ae. aegypti. LC50 ranging from 8 to 17 ppm [Citation9]. Another study by Kumar, P.M. et al [Citation5] reported that AgNPs synthesized from the leaf extract of B. tinctoria were highly effective against Ae. albopictus young instars, with LC50 ranging from 4.97 ppm to 14.87 ppm. In addition, the M. emarginata leaf extract-fabricated AgNPs revealed high toxicity against A. stephensi, Ae. aegypti and Cx. quinquefasciatus with LC50 values of 8.36, 9.20 and 10.02 μg/ml, respectively [Citation46]. In the present investigation, the larvicidal and pupicidal activity of both fabricated AgNPs showed highest toxicity and at low concentrations compared to AsEO and PcEO essential oils against Ae. albopictus mosquito. We hypothesize that the high toxicity rates exerted by the synthesized AgNPs on Ae. albopictus larvae and pupae may be due to the small size of AgNPs, which easily penetrate across the insect cuticle and even into individual cells of the digestive tract, where they interfere with physiological processes [Citation9]. Vector control is one of the major tools due to the development of resistance against the usual insecticides. Therefore, the development of new alternative insecticides is highly needed and it is of great significance [Citation47].
Histological studies
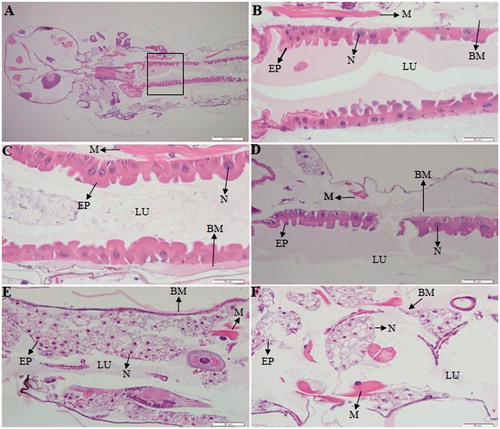
The LC50 exposure at 24-h period and control 4th-stage larvae of Ae. Albopictus were used for histological analysis. Our investigations revealed histological structure alterations in the midgut epithelial cells from treated and control larvae (). Our observations are compatible with the findings of by Kalimuthu, K. et al [Citation24] which noted that the midgut cells of 4th-stage larvae of Ae. Aegypti showed various degrees of apical swelling into the gut lumen, and reducing intercellular contacts, in addition to degeneration of nuclei and brush border after treatment of rhizome-fabricated AgNPs. In the present study, our data showed that the midgut area, including the epithelial cells and brush border, were highly affected by the fabricated AgNPs as compared to the essential oils (AsEO and PcEO). The midgut epithelium of control larvae exhibited normal cytoplasmic characteristics with regular microvilli lining (). The essential oils treated larvae were shown histological alterations in the midgut, such as elongation of epithelial cells protruding into the lumen and disintegration of the brush border (). The treatment of PcEO essential oil on the larvae was partially destructed the epithelial cells in the posterior regions of the midgut (). Similar results were reported by Nunes, F.C. et al [Citation48]. Also, morphological changes in the midgut cells of C. quinquefasciatus have been reported after exposure of essential oil of Peumus boldus Molina and its ascaridole-enriched fraction [Citation43]. Notably, the AgNPs-treated larvae were highly affected, exhibiting severe damage in the midgut epithelial cells and it showed cytopathological variations, such as the destruction of epithelial cells and degradation of nuclei (). Also severe lesions were seen in the midgut epithelium cells, including rupture of the epithelial cells, broken membranes, brush border damage and appearance of vacuolization. Our results support earlier reports by Al-Mekhlafi, F.A [Citation49]. Furthermore, the B. Sundararajan and his colleague observed histological alterations in the digestive tract and midgut of 3rd and 4th instar larvae of Ae. aegypti after exposure of gold nanoparticles [Citation50].
Figure 6. Longitudinal sections of the midgut of an Aedes albopictus 4th instar larvae, magnification: 40×. (A, B) control, (C) a larva under treatment with Aquilaria sinensis essential oil, (D) a larva under treatment with Pogostemon cablin essential oil, (E) a larva under treatment with AsEO-AgNPs, (F) a larva under treatment with PcEO-AgNPs. EP: Epithelial cells; BM: basement membrane; M: muscles; N: nucleus; LU: gut lumen.
Conclusions
In this study, by adopting eco-friendly synthetic route for the fabrication of nanomaterials, we synthesized Ag nanoparticles using Aquilaria sinensis essential oil (AsEO) and Pogostemon cablin essential oil (PcEO) as reducing and capping/stabilizing agents. Biosynthesized Ag nanoparticles were mostly spherical in shape, crystalline in nature with face-centred cubic geometry and their mean size ranged between 15 and 87 nm. The fabricated Ag nanoparticles showed significant larvicidal and pupicidal toxicity against Ae. albopictus mosquito even at low doses. Histological studies confirmed that Ag nanoparticles exert potential damage regarding the digestive tract and midgut cells of mosquito larvae. Further studies are needed to clarify the exact mechanism of action of Ag nanoparticles against mosquito vectors regarding skin impact and mineral balances and transportation within the cells of insect body.
Ethical statement
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Acknowledgements
The authors are grateful to the authorities of Zhejiang University for providing the necessary facilities to carry out this research work.
Disclosure statement
The authors declare that they have no conflict of interest.
Additional information
Funding
References
- Govindarajan M, Rajeswary M, Senthilmurugan S, et al. Larvicidal activity of the essential oil from Amomum subulatum Roxb.(Zingiberaceae) against Anopheles subpictus, Aedes albopictus and Culex tritaeniorhynchus (Diptera: Culicidae), and non-target impact on four mosquito natural enemies. Physiol Mol Plant Pathol. 2017. doi: 10.1016/j.pmpp.2017.01.003
- Benelli G, Mehlhorn H. Declining malaria, rising of dengue and Zika virus: insights for mosquito vector control. Parasitol Res. 2016;115:1747–1754.
- Benelli G, Iacono AL, Canale A, et al. Mosquito vectors and the spread of cancer: an overlooked connection?. Parasitol Res. 2016;115:2131–2137.
- Cunze S, Koch LK, Kochmann J, et al. Aedes albopictus and Aedes japonicus-two invasive mosquito species with different temperature niches in Europe. Parasit Vectors. 2016;9:1–12.
- Kumar PM, Murugan K, Madhiyazhagan P, et al. Biosynthesis, characterization, and acute toxicity of Berberis tinctoria-fabricated silver nanoparticles against the Asian tiger mosquito, Aedes albopictus, and the mosquito predators Toxorhynchites splendens and Mesocyclops thermocyclopoides. Parasitol Res. 2016;115:751–759.
- Buhagiar J. A second record of Aedes (Stegomyia) albopictus (Diptera: Culicidae) in Malta. Eur Mosquito Bull. 2009;27:65–67.
- Ngoagouni C, Kamgang B, Kazanji M, et al. Potential of Aedes aegypti and Aedes albopictus populations in the Central African Republic to transmit enzootic chikungunya virus strains. Parasit Vectors. 2017;10:1–5.
- Govindarajan M, Rajeswary M, Veerakumar K, et al. Green synthesis and characterization of silver nanoparticles fabricated using Anisomeles indica: mosquitocidal potential against malaria, dengue and Japanese encephalitis vectors. Exp Parasitol. 2016;161:40–47.
- Suresh U, Murugan K, Panneerselvam C, et al. Suaeda maritima-based herbal coils and green nanoparticles as potential biopesticides against the dengue vector Aedes aegypti and the tobacco cutworm Spodoptera litura. Physiol Mol Plant Pathol. 2017. doi: 10.1016/j.pmpp.2017.01.002
- Govindarajan M, Rajeswary M, Muthukumaran U, et al. Single-step biosynthesis and characterization of silver nanoparticles using Zornia diphylla leaves: a potent eco-friendly tool against malaria and arbovirus vectors. J Photochem Photobiol B. 2016;161:482–489.
- Vilas V, Philip D, Mathew J. Biosynthesis of Au and Au/Ag alloy nanoparticles using Coleus aromaticus essential oil and evaluation of their catalytic, antibacterial and antiradical activities. J Mol Liq. 2016;221:179–189.
- Manju S, Malaikozhundan B, Vijayakumar S, et al. Antibacterial, antibiofilm and cytotoxic effects of Nigella sativa essential oil coated gold nanoparticles. Microb Pathog. 2016;91:129–135.
- Dahham SS, Hassan LEA, Ahamed MBK, et al. In vivo toxicity and antitumor activity of essential oils extract from agarwood (Aquilaria crassna). BMC Complement Altern Med. 2016;16:11.
- Chen H, Yang Y, Xue J, et al. Comparison of compositions and antimicrobial activities of essential oils from chemically stimulated agarwood, wild agarwood and healthy Aquilaria sinensis (Lour.) Gilg trees. Molecules. 2011;16:4884–4896.
- Zhang Z, Han XM, Wei JH, et al. Compositions and antifungal activities of essential oils from agarwood of Aquilaria sinensis (Lour.) Gilg induced by Lasiodiplodia theobromae (Pat.) Griffon. and Maubl. J Braz Chem Soc. 2014;25:20–26.
- Zhang R, Yan P, Li Y, et al. A pharmacokinetic study of patchouli alcohol after a single oral administration of patchouli alcohol or patchouli oil in rats. Eur J Drug Metab Pharmacokinet. 2016;41:441–448.
- Chen H, Liao H, Liu Y, et al. Protective effects of pogostone from Pogostemonis Herba against ethanol-induced gastric ulcer in rats. Fitoterapia. 2015;100:110–117.
- WHO. 1996 Report of the WHO Informal Consultation on the (Evaluation and Testing of Insecticides), Geneva.
- Kjanijou M, Jiraungkoorskul K, Kosai P, et al. Effect of Murraya paniculata leaf extract against Culex quinquefasciatus larva. Asian Jo F Biological Sciences. 2012;5:201–208.
- Finney D. Probit analysis. 3rd ed. London (UK): R Cambridge University Press; 1971. p. 68–72.
- Vilas V, Philip D, Mathew J. Essential oil mediated synthesis of silver nanocrystals for environmental, anti-microbial and antioxidant applications. Mater Sci Eng C. 2016;61:429–436.
- Ramanibai R, Velayutham K. Synthesis of silver nanoparticles using 3, 5-di-t-butyl-4-hydroxyanisole from Cynodon dactylon against Aedes aegypti and Culex quinquefasciatus. J Asia Pac Entomol. 2016;19:603–609.
- Suman T, Elumalai D, Kaleena P, et al. GC–MS analysis of bioactive components and synthesis of silver nanoparticle using Ammannia baccifera aerial extract and its larvicidal activity against malaria and filariasis vectors. Ind Crops Prod. 2013;47:239–245.
- Kalimuthu K, Panneerselvam C, Chou C, et al. Control of dengue and Zika virus vector Aedes aegypti using the predatory copepod Megacyclops formosanus: Synergy with Hedychium coronarium-synthesized silver nanoparticles and related histological changes in targeted mosquitoes. Process Saf Environ Prot. 2017;109:82–96.
- Fouad H, Hongjie L, Hosni D, et al. Controlling Aedes albopictus and Culex pipiens pallens using silver nanoparticles synthesized from aqueous extract of Cassia fistula fruit pulp and its mode of action. Artif Cells Nanomed Biotechnol. 2017. [Epub ahead of print]. doi: 10.1080/21691401.2017.1329739
- Basu S, Maji P, Ganguly J. Rapid green synthesis of silver nanoparticles by aqueous extract of seeds of Nyctanthes arbor-tristis. Appl Nanosci. 2016;6:1–5.
- Manjamadha V, Muthukumar K. Ultrasound assisted green synthesis of silver nanoparticles using weed plant. Bioprocess Biosyst Eng. 2016;39:401–411.
- Rokade AA, Kim JH, Lim SR, et al. A novel green synthesis of silver nanoparticles using Rubus crataegifolius Bge fruit extract. J Clust Sci. 2017;28:2017–2026.
- Deepak P, Sowmiya R, Ramkumar R, et al. Structural characterization and evaluation of mosquito-larvicidal property of silver nanoparticles synthesized from the seaweed, Turbinaria ornata (Turner) J. Agardh 1848. Artif Cells Nanomed Biotechnol. 2016;44:990–998.
- Yuan CG, Huo C, Gui B, et al. Green synthesis of silver nanoparticles using Chenopodium aristatum L. Stem extract and their catalytic/antibacterial activities. J Clust Sci. 2017;28:1319–1333.
- Krishna IM, Reddy GB, Veerabhadram G, et al. Eco-friendly green synthesis of silver nanoparticles using salmalia malabarica: synthesis, characterization, antimicrobial, and catalytic activity studies. Appl Nanosci. 2016;6:681–689.
- Gopinath K, Devi NP, Govindarajan M, et al. One-pot green synthesis of silver nanoparticles using the orchid leaf extracts of Anoectochilus elatus: growth inhibition activity on seven microbial pathogens. J Clust Sci. 2017;28:1541–1550.
- Fouad H, Hongjie L, Yanmei D, et al. Synthesis and characterization of silver nanoparticles using Bacillus amyloliquefaciens and Bacillus subtilis to control filarial vector Culex pipiens pallens and its antimicrobial activity. Artif Cells Nanomed Biotechnol. 2016. [Epub ahead of print]. doi: 10.1080/21691401.2016.1241793
- Rajaganesh R, Murugan K, Panneerselvam C, et al. Fern-synthesized silver nanocrystals: towards a new class of mosquito oviposition deterrents? Res Vet Sci. 2016;109:40–51.
- Elemike EE, Onwudiwe DC, Ekennia AC, et al. Phytosynthesis of silver nanoparticles using aqueous leaf extracts of Lippia citriodora: antimicrobial, larvicidal and photocatalytic evaluations. Mater Sci Eng C. 2017;75:980–989.
- Pavithra BV, Ragavendran C, Murugan N, et al. Ipomoea batatas (Convolvulaceae)-mediated synthesis of silver nanoparticles for controlling mosquito vectors of Aedes albopictus, Anopheles stephensi, and Culex quinquefasciatus (Diptera: Culicidae). Artif Cell Nanomed Biotechnol. 2016. [Epub ahead of print]. doi: 10.1080/21691401.2016.1261873
- Park HM, Park IK. Larvicidal activity of Amyris balsamifera, Daucus carota and Pogostemon cablin essential oils and their components against Culex pipiens pallens. J Asia Pac Entomol. 2012;15:631–634.
- Albuquerque EL, Lima JK, Souza FH, et al. Insecticidal and repellence activity of the essential oil of Pogostemon cablin against urban ants species. Acta Trop. 2013;127:181–186.
- Zahran HEDM, Abou-Taleb HK, Abdelgaleil SA. Adulticidal, larvicidal and biochemical properties of essential oils against Culex pipiens L. J Asia-Pac Entomol. 2017;20:133–139.
- Benelli G, Pavela R, Iannarelli R, et al. Synergized mixtures of Apiaceae essential oils and related plant-borne compounds: larvicidal effectiveness on the filariasis vector Culex quinquefasciatus Say. Ind Crops Prod. 2017;96:186–195.
- Benelli G, Govindarajan M, Rajeswary M, et al. Larvicidal activity of Blumea eriantha essential oil and its components against six mosquito species, including Zika virus vectors: the promising potential of (4E, 6Z)-allo-ocimene, carvotanacetone and dodecyl acetate. Parasitol Res. 2017;116:1175–1188.
- Bedini S, Flamini G, Cosci F, et al. Cannabis sativa and Humulus lupulus essential oils as novel control tools against the invasive mosquito Aedes albopictus and fresh water snail Physella acuta. Ind Crops Prod. 2016;85:318–323.
- Jinu U, Rajakumaran S, Senthil-Nathan S, et al. Potential larvicidal activity of silver nanohybrids synthesized using leaf extracts of Cleistanthus collinus (Roxb.) Benth. ex Hook. f. and Strychnos nux-vomica L. nux-vomica against dengue, Chikungunya and Zika vectors. Physiol Mol Plant Pathol. 2017. doi: 10.1016/j.pmpp.2017.05.003
- Govindarajan M, Kadaikunnan S, Alharbi NS, et al. Single-step biological fabrication of colloidal silver nanoparticles using Hugonia mystax: larvicidal potential against Zika virus, dengue, and malaria vector mosquitoes. Artif Cells Nanomed Biotechnol. 2016;1–9.
- Azarudeen RMST, Govindarajan M, AlShebly MM, et al. Size-controlled biofabrication of silver nanoparticles using the Merremia emarginata leaf extract: toxicity on Anopheles stephensi, Aedes aegypti and Culex quinquefasciatus (Diptera: Culicidae) and non-target mosquito predators. J Asia Pac Entomol. 2017;20:359–366.
- Bhuvaneswari R, Xavier RJ, Arumugam M. Larvicidal property of green synthesized silver nanoparticles against vector mosquitoes (Anopheles stephensi and Aedes aegypti). J King Saud Univ Sci. 2016;28:318–323.
- Nunes FC, Leite JA, Oliveira LH, et al. The larvicidal activity of Agave sisalana against L4 larvae of Aedes aegypti is mediated by internal necrosis and inhibition of nitric oxide production. Parasitol Res. 2015;114:543–549.
- de Castro DSB, da Silva DB, Tibúrcio JD, et al. Larvicidal activity of essential oil of Peumus boldus Molina and its ascaridole-enriched fraction against Culex quinquefasciatus. Exp Parasitol. 2016;171:84–90.
- Al-Mekhlafi FA. Larvicidal, ovicidal activities and histopathological alterations induced by Carum copticum (Apiaceae) extract against Culex pipiens (Diptera: Culicidae). Saudi J Biol Sci. 2017. doi: 10.1016/j.sjbs.2017.02.010
- Sundararajan B, Kumari BR. Novel synthesis of gold nanoparticles using Artemisia vulgaris L. leaf extract and their efficacy of larvicidal activity against dengue fever vector Aedes aegypti L. J Trace Elem Med Biol. 2017;43:187–196.