?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Magnetic iron oxide nanoparticles (MNPs) were synthesized using Albizia adianthifolia leaf extract as reducing and protecting agent. Colour changing, UV–Vis spectrum, X-ray diffraction (XRD), Fourier transform infrared (FT-IR) spectroscopy and scanning electron microscopy (SEM) confirmed the biosynthesis and characterization of MNPs. The XRD pattern revealed that MNPs are crystalline in nature. FT-IR spectral analysis showed that MNPs was capped with plant constituents. From SEM analysis, the MNPs were generally found to be spherical in shape and the size was ranged 32–100 nm. Free radical scavenging potentials of the MNPs against DPPH were confirmed based on its stable anti-oxidant effects. The synthesized MNPs were used to capture Staphylococcus aureus under the magnetic field effect. Further, it was observed that the MNPs are able to exert cytotoxic effect towards human breast (AMJ-13) and (MCF-7) cancer cells. The anti-proliferative effect of this treatment is due to cell death and inducing apoptosis. Mitochondrial membrane potential, acridine orange-propidium iodide staining assays as well as single cell and DNA gel electrophoresis analyses indicated that MNPs induce cell death only by apoptosis. The findings of present study suggest that the MNPs might be used for medicinal applications particularly for cancer therapeutics.
Introduction
In recent days nanotechnology has induced great scientific advancement in the field of research and technology with developing interest for its various applications ranging from information technologies to medicinal applications. The application of nanotechnology to biomedicine, especially in cancer diagnosis and treatment, promises to have a profound impact on healthcare [Citation1]. The exploitation of the unique properties of nano-sized particles for cancer therapeutics is most popularly known as nanomedicine [Citation2]. The quick development of nanotechnology in new years has created a many of engineered nanomaterials, including magnetic iron oxide nanoparticles (MNNPs) [Citation3].
Magnetic nanoparticles have attracted considerable attention in biomedical applications due to their ability to be handled by an external magnetic field, their great potential in imaging, high uptake by the target tissue, cell separation, magnetic drug and gene delivery, cancer detection via magnetic resonance imaging and improving the treatment in therapeutically optimal doses [Citation4–6]. Modified and unmodified magnetic nanoparticles – Fe3O4 – have been reported to improve the efficiency of anticancer drugs and reverse multidrug resistance [Citation7]. Several studies have showed that magnetic nanoparticles of iron oxide could be phagocytized by cancer cells and interact with constituents inside the cells; hence, those magnetic nanoparticles may act as a good system for drug delivery and offer new perceptions for research and directed therapeutic approaches for cancers [Citation8,Citation9]. Recently, numerous researchers have studied the toxic effects of metal oxide nanoparticles and described the changes in cellular morphology, mitochondrial function and apoptosis [Citation10,Citation11], several metal nanoparticles displayed cytotoxicity after exposure to cells, and iron oxide nanoparticles were found to be more effective [Citation12].
Currently, a large number of chemical, physical, biological and hybrid methods are available to synthesize different types of nanoparticles [Citation13]. The nanoparticles formed using each method show specific properties. However, biosynthesis of metal oxide nanoparticles by plants is currently under development. Green nanotechnology has attracted a lot of attention and includes a wide range of processes that eliminate or reduce toxic substances to restore the environment [Citation14]. The synthesis of metal nanoparticles using inactivated plant tissue [Citation15], exudates [Citation16], plant extracts [Citation17] and other parts of living plants [Citation18] is a modern alternative for their production. Green synthesis of nanoparticles makes use of environmental friendly, safe reagents and non-toxic [Citation19].
Historically, members of the Fabaceae family, particularly Albizia adianthifolia are easily available and grow abundantly in the east coast of South Africa. A. adianthifolia is used in Central and West Africa for the treatment of skin diseases, inflamed eyes, bronchitis, tapeworm, headaches and sinusitis [Citation20]. It is reported that root and bark extracts from A. adianthifolia inhibit acetylcholinestarase and cyclooxygenase activity. These anti-inflammatory properties may be useful in the treatment of human diseases related to oxidative stress [Citation21]. The phytochemistry of this plant has not been elucidated adequately. However, saponins such as prosapogenins and triterpenesaponins were identified; they display a broad range of pharmacological and biological properties [Citation22,Citation23].
The isolated biological properties of A. adianthifolia were considered for the synthesis of a novel iron oxide magnetic nanoparticles using aqueous leaf extract of the plant. It is reported that cancer cell lines are well characterized and extensively used in in vitro nanotoxocity studies [Citation24]. The toxicity against bacteria was also carried out [Citation25]. Understanding the underlying mechanisms of this toxicity induced by iron oxide magnetic nanoparticles especially for cancer therapeutics are need more investigations. However, in present study, the A. adianthifolia leaves extract mediated the synthesis of MNNPs and characterize these nanoparticles using UV–Vis spectroscopy, X-ray diffraction (XRD), Fourier transform infrared (FTIR) and scanning electron microscope (SEM). Moreover, the antioxidant, capturing of Staphylococcus aureus bacteria under magnetic field and cytotoxic roles in human breast (AMJ-13) and (MCF-7) cancer cell lines were also evaluated.
Materials and methods
Cell lines and reagents
The human breast (AMJ-13) and (MCF-7) cancer cell lines were obtained from Iraqi Centre for Cancer and Medical Genetic Research (ICCMGR), AL-Mustansiriyah University, Baghdad, Iraq. Acridine orange, propidium iodide, ethidium bromide, trypsin-EDTA, fetal bovine serum, dimethyl sulfoxide (DMSO), ascorbic acid, 2,2-diphenyl-1-picrylhydrazyl(DPPH),3-(4,5-dimethylthiazal-z-yl)-2,5diphenylterazolium (MTT) and crystal violate stain were purchased from Sigma Chemical Co. (St. Louis, MO); RPMI-1640 medium was purchased from Gibco (Invitrogen, Basel, Switzerland). MitoCapture™ Apoptosis Detection Kit was purchased from CalBiochem (San Diego, CA). KAPA Express Extract Kit for DNA extraction was obtained from (Kapa Biosystems, Inc., Wilmington, MA). Penicillin and streptomycin were used to prevent microbial contamination (Biosource International, Nivelles, Belgium). However, all other chemicals and reagents were used at analytical grade level.
Collection and extraction of A. adianthifolia
The A. adianthifolia leaves were collected from the locality of Kamb Sara, Al-Karada, and Baghdad, Iraq during September and October 2016. The leaves were washed several times with tap water and three times with distilled water to remove the dust particles and then shade dried to remove the residual moisture. After that, the plant leaves extract was prepared according to a method presented by Ghosh et al. [Citation26].
Synthesis of magnetic nanoparticles
The magnetic nanoparticles were prepared as per slightly modified method presented by Awwad and Salem [Citation27]. In brief, 1.11 g of Ferric chloride hexahydrate (FeCl3·6H2O) and 0.53 g of ferrous chloride tetra hydrate (FeCl2·4H2O) (1/2 molar ratio) were dissolved in 100 mL of sterile deionized distilled water in a 250 mL beaker and heated at 80 °C using magnetic stirrer and under atmospheric pressure. After 10 min, 5 mL of the aqueous solution of A. adianthifolia leaf extract was added to the above solution drop wise. The yellowish colour of the mixture changed to reddish brown colour. After 5 min, 20 mL of 1 M NaOH (0.8 gm) was dissolved in sterile deionized distilled water in a beaker 50 mL and added to the mixture with rate (3 mL min−1) for allowing the magnetite precipitations uniformly. From the first addition of NaOH the reddish brown colour of mixture changed to brownish black suspended particles. The mixture was allowed to cool down to room temperature and the iron oxide nanoparticles were obtained by decantation after that dilution with sterile distilled water and centrifugation to remove heavy biomaterials of plant leaf extract. The iron oxide nanoparticles were purified by dispersing in sterile distilled water and centrifuged three times.
Characterization of MNNPs
The reduction of pure Fe3+ ions was checked first by measuring the UV–Vis spectrum using Hitachi U-2910 Spectrophotometer (Tokyo, Japan). UV–Vis spectroscopic analysis was performed via continuous scanning at the range of 200–900 nm. Then, MNNPs solution was investigated using X-ray diffractometer (XRD-6000, Shimadzu, Japan). The diffraction pattern was obtained using a Cu Kα incident beam (λ = 1.542 Å) at 2θ = 20°–60°. The voltage and current of X-ray tubes were 40 kV and 30 mA, respectively. The size was calculated by using the equation of Debye–Scherrer as follows D = 0.94 λ/β Cos θ. FTIR analysis was carried out by using FTIR spectrometer (8400S, Shimadzu, Japan) in attenuated total reflection mode and spectral range of 4000–400 cm−1 with a resolution of 4 cm−1. Finally, the morphology and the uniformity of MNPs were performed via Scanning Electron Microscopy analysis (Shimadzu AA-7000, Japan).
Capturing S. aureus by MNNPs
In a typical experiment, S. aureus was cultured in nutrient broth for 24 h to reach a concentration of 5 × 107 CFU mL−1. The bacteria were then dispersed in 900 μL PBS and mixed with 100 μL with concentration 1 mg mL−1 MNPs solution and incubated in dark condition at 37 °C for 1 h. External magnetic field with 0.5 Tesla was applied on the mixture for 1 minto observe that S. aureus bacterium can be separated from the solution. The solutions without bacterial growth were used as positive control [Citation28].
Free radical scavenging capacity
The free radical scavenging activities of synthesized magnetic iron nanoparticles were measured with the DPPH assay according to the method introduced by Sulaiman et al. [Citation29]. Ascorbic acid (vitamin C) and plant extract were used as positive and negative controls, respectively. The absorbance of the control (DPPH solution) was also read. The percentage inhibition of the sample was calculated using the following formula:
where AC and AS are the peak intensity for DPPH and test sample solvent, respectively.
Cell viability assay
The AMJ-13 and MCF-7 cells were cultured in RPMI-1640 medium supplemented with 10% FBS, 20 mM Hepes and 2 mM L-glutamine under a humidified atmosphere of 5% CO2 at 37 °C in tissue culture flasks (T 25 cm2; BD Falcon, Bedford, MA). For viability assay, 200 μL of AMJ-13 and MCF-7 cells were seeded in 96-well flat-bottom culture plates (Falcon) at a density of 1 × 105 cells mL−1. After 48 h in the exponentially growing phase, the cells were treated with MNPs at concentrations of 1.9, 3.9, 7.8, 15.6, 31.5 and 62.5 μg mL−1 for 24 h. The same concentrations were also used for plant extract. After incubation, 50 μL of the labelling stain consisting of MTT in phosphate-buffered saline solution was added to each well. Incubation was continued for 10–15 min at 37 °C, and the stain was removed, washed with tap water. A total of 100 μL aliquot of isopropanol was added to each well to dissolve the stain, followed by incubation for 10 min to dissolve air bubbles. The culture plate was placed on a microplate reader (ELx 800, Bio-Tek Instruments Inc., Winooski, VT), and the absorbance was measured at 492 nm. The percentage of inhibition was calculated according to the following equation
where A and B are the optical density for control and test sample, respectively.
Cytotoxicity using crystal violet dye
This assay was performed using crystal violet dye under the same conditions used in MTT assay. Briefly, at the end recovery time, the medium was discarded and, replaced by 50 μL of crystal violet dye. After staining the wells were rinsed with distilled water and air-dried. The morphological aspects of cells were inspected using inverted microscope at a magnification of 200×.
Clonogenicity assay
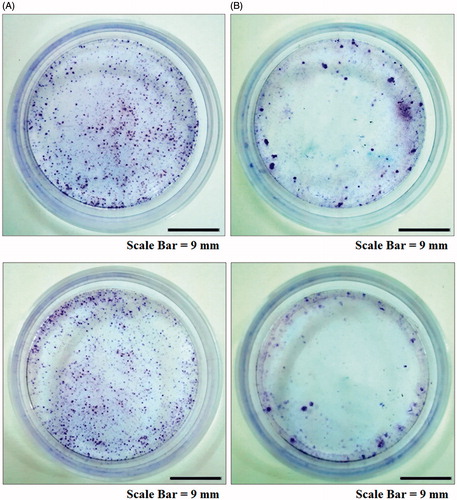
To investigate the inhibition of colony formation, AMJ-13 and MCF-7 cells were seeded at a density of 1000 cells mL−1 in flat-bottom culture plates (Thermo Fisher Scientific, Roskilde, Denmark) and incubated for 48 h. Cells were treated with MNPs (IC50 concentration) of each cell line in three replicates. When the non-treated cells were readily visible under the microscope as a confluent monolayer, the medium was discarded and the wells were washed with 2 mL of filtered PBS. Then, the colonies in each well were stained with 200 μL of crystal violet stain for 2 min. After removing the stain, extensive washing was performed in tap water for 10 min. Then, the plates were tapped on towel paper, dried and photographed.
Mitochondrial membrane potential assay
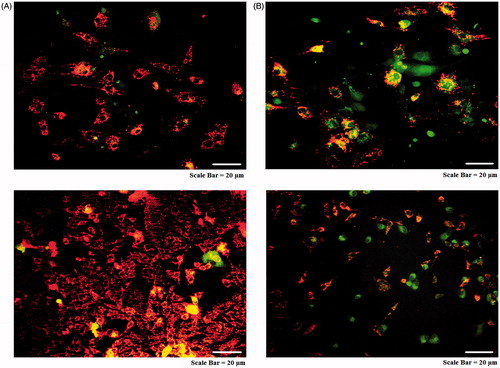
Mitochondrial membrane potential was evaluated using the cationic fluorescent indicator MitoCapture™ stain. MitoCapture™-aggregates of intact mitochondria were fluorescent red with anemission at 590 nm, and MitoCapture™-monomers in the cytoplasm were fluorescent green with emission at 530 nm and anexcitation wavelength of 488 nm. AMJ-13and MCF-7cells treated with (IC50 concentration) MNPs in 96-well plates were incubated for 16 h. Cells were incubated in RPMI containing 10 μM of MitoCapture™ at 37 °C in dark place for 15 min. Then, cells were washed with PBS, and transferred to a clear 96-well plate. Finally, cells were visualized with fluorescence microscopy.
Acridine orange and propidium iodide staining
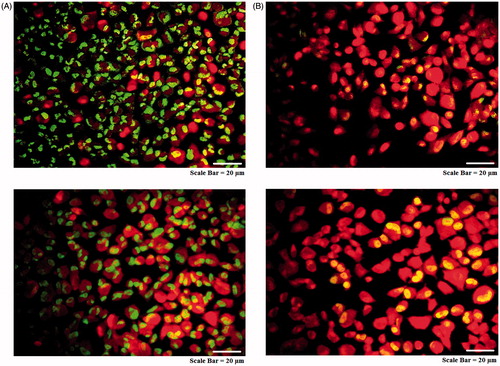
The MNPs induced death of AMJ-13 or MCF-7 cells was performed using acridine orange and propidium iodide dual staining method. Briefly, cells in 96-well plates were treated with MNPs (IC50 concentration) and incubated for 16 h. The cells were detached and washed twice using PBS, and transferred to a clear 96-well plate. A 10 μL of fluorescent dyes containing acridine orange (1 mg mL−1) and propidium iodide (1 mg mL−1) were added into the cells at equal volumes of each. Finally, cells were visualized with fluorescence microscopy.
Single cell gel electrophoresis (comet assay)
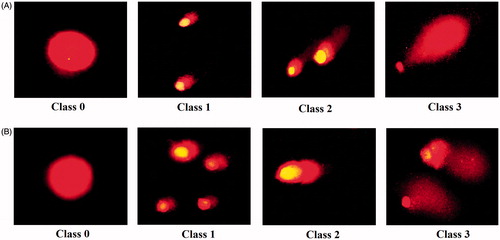
The genotoxicity of MNPs was determined using comet assay, based on the measurement of DNA migration under electrophoresis as technique introduced by Ateeq et al. [Citation30]. AMJ-13 and MCF-7 cells were treated with (IC50 concentration) MNPs for 16 h. Then, the cells were lysed by lysis solution, electrophoresed and the comet images were captured under fluorescence microscope.
Electrophoresis analysis of DNA fragmentation
Analysis of DNA fragmentation was performed according to KAPA Express Extract Kit as manufacturer indicated. AMJ-13 and MCF-7 cells were treated with (IC50 concentration) MNPs for 16 h. Cells at density 1.5 × 106 cell mL−1 were then harvested and suspended in ice-cold PBS. The cell suspension was centrifuged (1200 rpm) at 4 °C for 10 min. The supernatant was discarded and the pellet was frozen (−20 °C) overnight. The DNA was dissolved with DNA loading buffer, and then applied to 0.8% agarose gel electrophoresis. After staining with ethidium bromide, the DNA was visualized by UV irradiation and photographed by gel documentation system (Sci-Plus, UK).
Statistical analysis
The grouped data were statistically performed using ANOVA with SPSS program (SPSS/14.0; SPSS Inc., Chicago, IL). Values were presented as the mean ± SD of the three replicates of each experiment.
Results and discussion
Synthesis of MNNPs
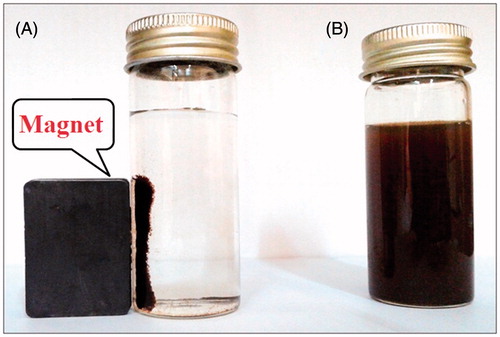
In present study, the MNNPs were successfully synthesized by using A. adianthifolia plant leaves extract. As shown in , the prepared MNPs exhibited a dark black colour in the aqueous is due to collective oscillation of free electrons present in the reduced MNNPs [Citation31]. The addition of ferric chloride solution as iron precursor to the plant extract containing the phenolic and flavonoid compounds may cause the reduction of Fe3+ to Fe2+ and stabilization of the nanoparticles [Citation32]. Decreasing in pH during the formation of MNPs signifies the involvement of the OH group in the reduction process. Initially, ferric chloride hydrolyzes to form ferric hydroxide and releases H + ions thereafter; ferric hydroxide is partially reduced by the A. adianthifolia leaves extract to form MNNPs, while aldehyde groups are oxidized to the corresponding acids [Citation33]. However, the magnetic property of present synthesized MNPs in the presence of a magnetic field was used as described in and exhibited this property. shows the separation of MNPs in deionized distilled water by placing an external permanent magnet near the glass bottle () which proved that the MNPs possessed magnetic properties and when the magnet was removed, the nanoparticles were distributed readily by simple shaking as shown in . The magnetic response of MNNPs synthesized by A. adianthifolia plant extract is qualitatively confirmed by their migration in a few minutes toward a 0.5 T magnet placed in the proximity of the solution. This result indicated successfully synthesized MNPs with ferromagnetic properties and this property is useful for in vitro protein or cell separation [Citation34].
Figure 1. Photograph showing colour changing: (A) Aqueous leaf extract of Albizia adianthifolia only (B) FeCl3·6H2O and FeCl2·4H2O without leaf extract of A. adianthifolia (C) Changing colour from reddish brown to black.

Figure 2. Photograph showing (A) separation of biosynthesized magnetic iron oxide nanoparticles from reaction mixture using an external magnet (B) MNPs without external magnet.
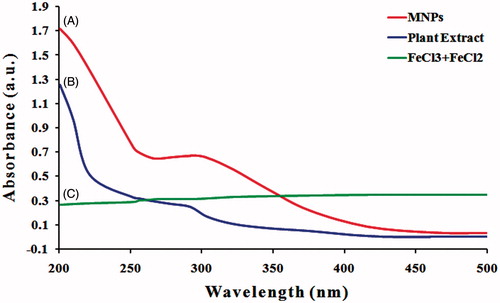
The formation and stability of MNPs in aqueous colloidal solution are confirmed at first using UV–visible spectral analysis of the solution and different wavelengths ranging from 200 to 500 nm were examined and the SPR property of the biosynthesized MNPs revealed a peak at 286 nm (). Njagi et al. [Citation35] and Kumar et al. [Citation36] reported similar UV–Vis spectra for MNPs synthesized using an aqueous Sorghum bran extract and Andean blackberry leaf, respectively. They found that the absorbance peak of iron oxide magnetic nanoparticles synthesized using plant extract ranged from 240 to 360 nm. However, a single SPR band reveals to the spherical shape, while two or more SPR bands correspond to the anisotropic particles. Also, the frequency and width of the SPR absorption band based on the size and shape of the metal nanoparticles, dielectric constant of the metal itself (the composition of the particles) and the dielectric constant of surrounding medium [Citation37]. Thus, the UV–vis results indicated that A. adianthfolia leaves extract can possibly reduce Fe3+ to MNPs, and such finding was further inspected to confirm the synthesis of MNPs from A. adianthfolia.
Characterization of MNNPs
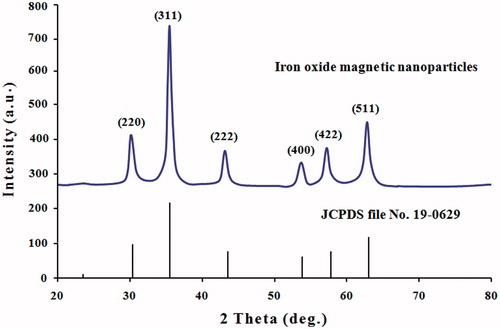
The XRD patterns of MNPs synthesized using the leaves extract of A. adianthifolia was further illustrated and confirmed via the characteristic peaks observed in the XRD spectra as shown in . XRD spectrum analysis showed six different diffraction peaks at 30.22°, 35.60°, 45.60°, 54.10°, 57.68° and 63.10° corresponding to the planes of (220), (311), (222), (400), (422) and (511), respectively. The set of lattice planes can be indexed to pure cubic phase of magnetite and this was compared according to (Joint Committee on Powder Diffraction Standards, JCPDS file, PDF No. 19-0629) and with the findings of previous studies [Citation33,Citation38]. These diffraction peaks illustrate the crystalline nature of the particles and the diameter was found to be 32 nm. No XRD detectable impurities were observed in this sample as well.
Figure 4. XRD spectrum patterns of iron oxide magnetic nanoparticles synthesized by leaf extract of Albizia adianthifolia. The structure of MNPs was matched with the Joint Committee on Powder Diffraction Standards (JCPDS, file No. 19-0629).
FTIR analysis confirmed that the bio-reduction of ferric chloride into MNNPs are due to the reduction by capping material of plant leaf extracts. shows the FTIR spectrum of A. adianthifolia leaves extract. The strong absorption peak at 3412 cm−1 is assigned to O–H stretching of alcohol and phenolic compounds or stretching of the –NH band of amino group. The presence of peak at 2935 cm−1 could be assigned to –CH stretching vibrations of –CH3 and –CH2 functional groups [Citation39]. The peak at 1614, 1406.547 cm−1 is due to stretching vibration of C=C alkene, –CH3 stretching, alkyl halide C–Br respectively, the peaks belonging to 1255, 1114 and 1072 cm−1 are due to COO stretching vibration. The peaks at 758 and 605 cm−1 are due to chloride [Citation40].
Figure 5. FTIR spectroscopy analysis. (A) Spectrum of leaf extract of Albizia adianthifolia and (B) spectrum of MNPs synthesized by leaf extract of A. adianthifolia.
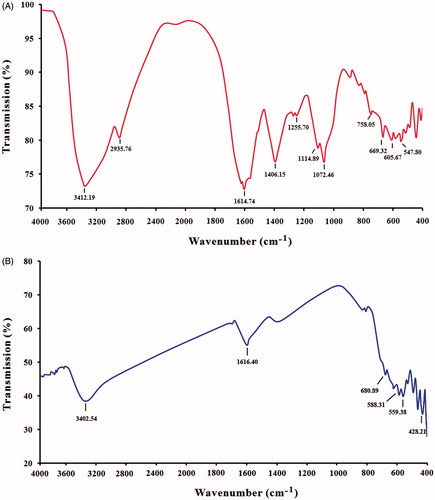
FTIR spectrum of synthesized MNPs is presented in , however, the peaks at 3402 cm−1 (due to overlap of O–H and N–H stretching) indicate –OH stretching of the water in the precursor which disappeared in the nanoparticles caused by the adsorbed water molecules since the nano-crystalline materials exhibit a high surface to volume ratio and thus absorb moisture, 1616 cm−1 are generally assigned to C=O stretching vibration. The formation of MNPs is characterized by the absorption bands from 428 to 686 cm−1. This peak was absent in plant extract which indicates the formation of iron oxide nanoparticles. A similar observation has been reported by several studies [Citation40,Citation41]. From the FTIR result, the soluble elements present in plant extract could have acted as capping agents and thus playing a relevant role in their extracellular synthesis and shaping.
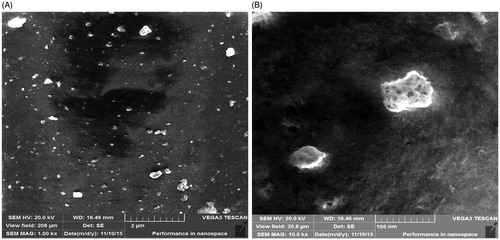
In order to evaluate the surface morphology and uniformity of the MNPs, the analysis using SEM was performed. shows the MNPs synthesized by the plant extract of A. adianthifolia. The nanoparticles assessed at high magnification and reveals that the morphology of the MNPs is roughly irregular spherical with rough surface and relatively crystalline in structure and the particles size was in the range of ∼32–100 nm. The good correlation between particle sizes obtained from Scherrer equation and SEM which supported the crystalline structure of the prepared MNPs and the morphology was mostly appeared to be a porous and spongy. According to the SEM images, the MNPs tend to aggregate and this aggregation may interpret due to the van der Waals force between particles. Also, the agglomeration can be also formed via the repulsive electrostatic forces between attract particles closer to each other and then they aggregate [Citation42].
Antioxidant activity of MNNPs
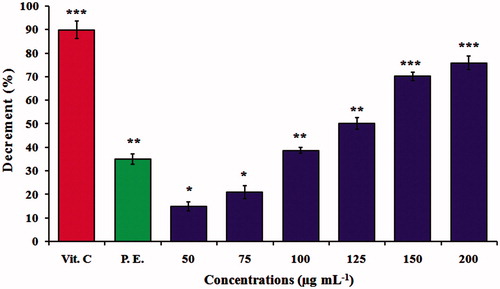
The results obtained for the antioxidant activity showed effective free radical scavenging by MNPs, six different concentrations were monitored as given in . In general, a concentration-dependent inhibition was observed, and 200 μg mL−1 was significantly better than the other concentrations which show 76.3% antioxidant activity. The activity of negative control was 35% while, positive control was 90%. However, the antioxidant property of MNPs could be related with the functional groups adhered to them which were produced from the A. adianthifolia leaves extract. Similar observations was also made by Bhattacharya et al. [Citation43] and Harshiny et al. [Citation44] whom reported that iron oxide nanoparticles exhibited a very high free radical scavenging activity of 89% and 72%, respectively. The identification of antioxidant is useful to biological system against reactive oxygen species (ROS), such as hydrogen peroxide, hydroxyl radical or singlet oxygen within living systems. Antioxidants are beneficial for the management of many deleterious diseases because of their scavenging ability [Citation45].
Figure 7. DPPH free radical scavenging activity. Antioxidant activity of MNPs at different concentrations of 50, 75, 100, 125, 150 and 200 μg mL−1. The values represent the mean ± SD of three experiments. Error bars are *p < .05, **p < .01, ***p < .001. Vit.C, vitamin C (positive control); P.E., plant extract (negative control) of Albizia adianthifolia at 200 μg mL−1.
Capturing S. aureus by MNNPs
represents the capturing of S. aureus by MNNPs dissolved in PBS solutions. This effect can be used to separate S. aureus from solutions in short times. These results are in good agreement with those reported by Ismail et al. [Citation46], who used iron magnetic nanoparticles synthesized by laser ablation method for rapid capture of Gram positive bacteria. Iron oxides are very prominent in biomedicine; not only for their inherent antibacterial potentials, but also for their super paramagnetic nature. Iron ions generate oxygen radicals by converting hydrogen peroxide to the more reactive hydroxyl radical via Fenton reaction. Hydroxyl radicals generated by these iron ions can depolymerize polysaccharides; causing DNA strand breakage, inactivated enzymes and initiate lipid peroxidation. Furthermore, nanoparticles bound to cell membrane or cell membrane proteins, through electro-static interactions are another possible mechanism that could disrupt bacterial function and cause their death [Citation47].
Viability and cytotoxicity assay
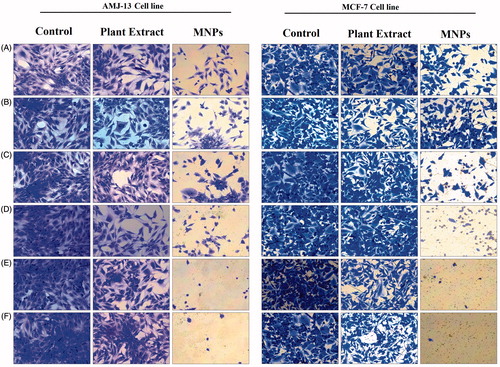
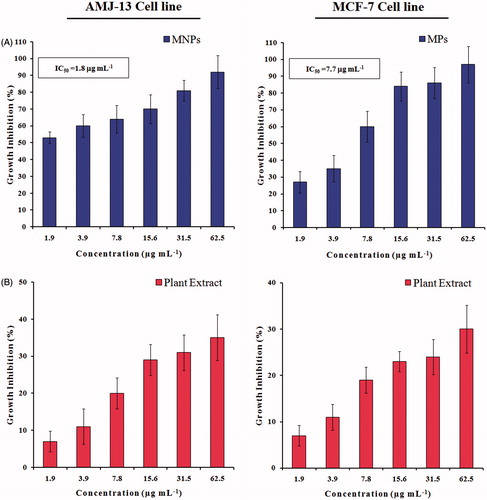
shows the viability results examined by MTT colorimetric assay of AMJ-13 and MCF-7 cancer cells, respectively after 24 h of exposure with different concentrations of MNPs or leaves extract of A. adianthifolia ranged from 1.9 to 62.5 μg mL−1. For AMJ-13 cell line, the results illustrated that treatment with MNPs inhibited the growth cells significantly (p ≤ .05) as compared to those of control cultures and the reduction was concentration dependent. The highest inhibition (92%) was found at concentration of 62.5 μg mL−1 of MNPs, while at 1.9 μg mL−1 concentration 53% cells were dead. The inhibitory concentration value (IC50) was 1.8 μg mL−1 (; upper lane). To confirm the inhibition observed in AMJ-13 cells was related to the synthesized MNPs, the same concentrations of extract were tested. It was found that higher concentration (62.5 μg mL−1) induced inhibitory effects with 35% of cells, while at 1.9 μg mL−1 concentration showed 7% of cells as compared to those of control cultures (; lower lane). However, the proliferation rate was less cytotoxic effects on AMJ-13 cell line treated with A. adianthifolia extract as compared to those cells treated with synthesized MNPs. Almost similar findings were obtained when MCF-7 cell line was tested (), but the IC50 was 7.7 μg mL−1 (; upper lane).
Figure 9. Growth inhibition of AMJ-13 and MCF-7 cell lines: (A) Cells treated with MNPs and (B) Cells treated with aqueous leaf extract of Albizia adianthifolia only. The values were the mean ± SD from three independent experiments expressed as percent with control. IC50 value of MNPs was 1.8 and 7.7 μg mL−1, respectively.
The cytotoxicity observed in AMJ-13 and MCF-7 cancer cells was also confirmed using a crystal violet method and inspected microscopically after 24 h of exposure with the same concentrations used in MTT assay (, respectively). As mentioned earlier, the effect on both cancer cell lines was concentration dependent and the high concentration was the best in this regard. However, the synthesized MNPs showed potent cytotoxicity toward both cell lines than A. adinthifilia extract. Similarly, previous studies also have shown the concentrations-dependent cytotoxicity of several types of nanoparticles [Citation48–50]. Also, the present results indicated that the AMJ-13 cell line and MCF-7cell line were targeted by the MNPs and induced numerous morphological alterations including cell shape changes, cell clumping and inhibition of cell communication (; upper lane and lower lane, respectively), however, no such alterations were seen in non-treated cells (; upper lane and lower lane, respectively). The mechanism of toxicity of MNPs of cancer cells was suggested by Gupta et al. and Ankamwar et al. through the interactions between surfactant of nanoparticles and proteins on cancer cells through a –NH2 functional group. The magnetic nanoparticles tend to agglomerate and adsorb plasma proteins. After entering the cells, the nanoparticles are able to form necklace-like eccentric circles to the nucleus, caused cells bubbles via a mechanism of endocytosis and then inducing cell death by apoptosis [Citation51,Citation52].
Colony formation assay
The results of clonoginc survival assay of AMJ-13 and MCF-7 cells are presented in . Consistent with the proliferation assays, MNPs significantly reduced the number of colonies of AMJ-13 and MCF-7 cells (; upper lane and lower lane, respectively) as compared to those of control (; upper lane and lower lane, respectively). The rapid loss of clonogenic activity may lead some to believe that the cancer cells in the continuous treatment with MNPs have been killed within the first 24 h of treatment, resulting to the abrupt loss of clonogenic potential. The nanoparticles can suppress cell viability by different mechanisms such as apoptosis or necrosis [Citation53]. Therefore, the present data may show that the MNPs induced cell death through apoptosis or necrosis and thus suggesting that these MNPs had anti-proliferative effects in AMJ-13 and MCF-7 cells.
Mitochondrial membrane potential assay
As shown in , MitoCapture™ exhibited distinct potential-dependent accumulation in mitochondria, indicated by a fluorescence changes from red to green. AMJ-13 cell line (upper lane) and MCF-7 cell line (lower lane) treated with IC50 concentration revealed loss in mitochondrial membrane integrity and apoptotic induction with green nuclei when compared with red nuclei control. The cells with green stain of their nucleus indicating the early stage of apoptosis and this effect were associated with low cell viability. The findings suggest that MNPs may induce apoptosis through the changes in the mitochondrial mediated apoptosis pathway.
Acridine orange and popidium iodide assay
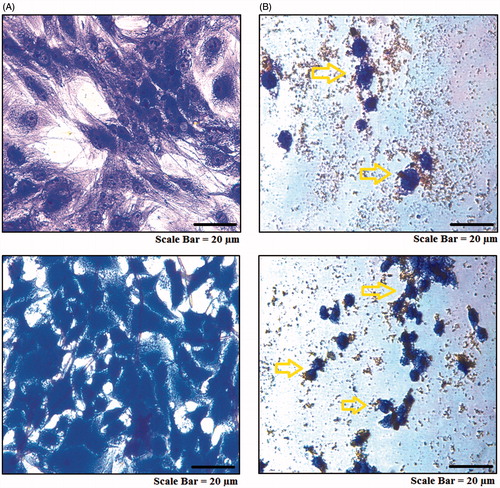
As seen in , the AMJ-13 cell line (upper lane) MCF-7 cell line (lower lane) cells suffered from aggressive membrane disintegration when exposed to MNPs as compared to those of non-treated cells (). The viable cells revealed uniform normal green nuclei with an organized structure and a red staining in cytoplasm were corresponded to RNA and lysosomes. While, treated cells with were significantly enhanced and distinguished according to the fluorescence emission and the morphological aspect of chromatin condensation in the stained nuclei. Late apoptotic cells have orange to red nuclei with condensed or fragmented chromatin. Hence, the observed morphological changes reveal that MNPs induce cell death only through apoptosis and not through necrosis.
Comet assay
DNA damage according to severity of damage and tail length in AMJ-13 cell line () and MCF-7 cell line () were classified to four classes. The microscopic images present the comet in scoring pattern from 0 class to class 3 which indicate the comet length. In controls (Class 0), halo surrounding cell nuclei was clearly visible and microscopic images showed the nuclei were intact and round without any fragmented DNA. In treated cells, fragmented DNA was observed and established an increasing length of DNA migration from nucleus generating significant well-formed comets as shown in Class 1, Class 2 and Class 3, respectively. These findings indicate that MNPs induced DNA fragmentation, which was further evidence of apoptosis, suggesting that MNPs can induce genotoxicity and mutagenicity in DNA of cancer cells. The data of present study were combined with study of Lin et al. who reported that the MNPs induce intracellular ROS generation lead to activation of oxidative stress in HL-7702 cells cause nuclear condensation and chromosomal DNA fragmentation, then leading cells to apoptosis [Citation54].
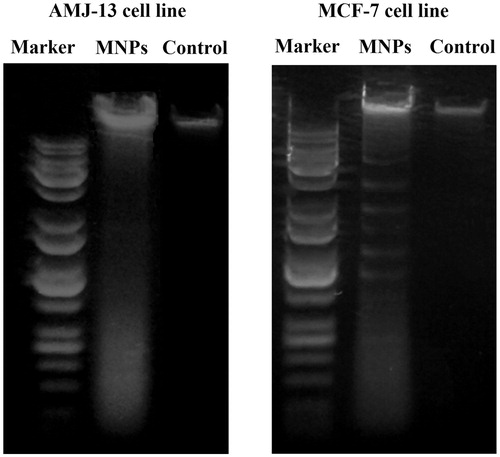
Gel electrophoresis
represents a DNA fragmentation in AMJ-13 cells (left panel) and MCF-7 cells (right panel) after treatment with MNPs and exhibited a cellular apoptotic DNA ladder and smears. However, DNA fragmentation ladders were not observed in non-treated cells (controls), which suggest that MNPs are able to induce fragmentation of DNA by free radicals of ROS produced by MNPs. The results of present study revealed the anticancer potentials of the MNPs, as demonstrated by the apoptotic assay methods. Further, the results clearly showed that the MNPs interacted with the DNA and made some structural or conformational changes which could alter the metabolic function and cause damage to cellular components. Similar observation was made by Sadeghi et al. [Citation50], who reported that the MNPs can initiate the DNA degradation in HepG2 cells that indicated iron oxide magnetic nanoparticles can reached the nucleus and damage DNA or produced ROS can reach to DNA and break it, unrepaired DNA can lead cells to the apoptosis.
Conclusion
Depending on present findings it can be concluded that iron oxide magnetic nanoparticles are useful in the medical applications particularly in cancer research. Several studies showed the ability of the MNPs to deliver the drug to its target site with better therapeutic efficiency than free drugs [Citation55,Citation56]. However, the MNPs can be utilized in designing better and developing more active cancer drugs by chemically modifying with a number of compounds.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Jabir NR, Tabrez S, Ashraf GM, et al. Nanotechnology-based approaches in anticancer research. Int J Nanomed. 2012;7:4391–4408.
- Mousa SA, Bharali DJ. Nanotechnology-based detection and targeted therapy in cancer: nano-bio paradigms and applications. Cancers. 2011;3:2888–2903.
- Zhang XQ, Xu X, Bertrand N, et al. Interactions of nanomaterials and biological systems: Implications to personalized nanomedicine. Adv Drug Deliv Rev. 2012;64:1363–1384.
- Parveen S, Misra R, Sahoo SK. Nanoparticles: a boon to drug delivery, therapeutics, diagnostics and imaging. Nanomedicine. 2012;8:147–166.
- Hilger I, Kaiser WA. Iron oxide-based nanostructures for MRI and magnetic hyperthermia. Nanomedicine. 2012;7:1443–1459.
- Lee J, Yang J, Ko H, et al. Multifunctional magnetic gold nanocomposites: human epithelial cancer detection via magnetic resonance imaging and localized synchronous therapy. Adv Funct Mater. 2008;18:258–264.
- Singh N, Jenkins GJ, Asadi R, et al. Potential toxicity of super paramagnetic iron oxide nanoparticles (SPION). Nano Rev. 2010;1:5358.
- Goya GF, Grazu V, Ibarra MR. Magnetic nanoparticles for cancer therapy. Curr Nanosci. 2008;4:1–16.
- Jain TK, Morales MA, Sahoo SK, et al. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2:194–205.
- Jeng HA, Swan son J. Toxicity of metal oxide nanoparticles in mammalian cells. J Environ Sci Health A. 2006;41:2699–2711.
- Carlson C, Hussain SM, Schrand AM, et al. Unique cellular interaction of silver nanoparticles: size-dependent generation of reactive oxygen species. J Phys Chem B. 2008; 112:13608–13619.
- Gobbo OL, Sjaastad K, Radomski MW, et al. Magnetic nanoparticles in cancer theranostics. Theranostics. 2015;5:1249–1263.
- Mohanpuria P, Rana NK, Yadav SK. Biosynthesis of nanoparticles: technological concepts and future applications. J Nanopart Res. 2008;10:507–517.
- Talebi S, Ramezani F, Ramezani M. Biosynthesis of metal nanoparticles by micro-organisms. Nanocon Olomouc. 2010;10:12–18.
- Mahdavi M, Namvar F, Ahmad MB, et al. Green biosynthesis and characterization of magnetic iron oxide (Fe3O4) nanoparticles using seaweed (Sargassum muticum) aqueous extract. Molecules. 2013;18:5954–5964.
- Lukman AI, Gong B, Marjo CE, et al. Facile synthesis, stabilization, and anti-bacterial performance of discrete Ag nanoparticles using Medicago sativa seed exudates. J Colloid Interface Sci. 2011;353:433–444.
- Shameli K, Ahmad MB, Zamanian A, et al. Green biosynthesis of silver nanoparticles using Curcuma longa tuber powder. Int J Nanomed. 2012;7:5603–5610.
- Parsons JG, Peralta-Videa JR, Gardea-Torresdey JL. Use of plants in biotechnology: synthesis of metal nanoparticles by inactivated plant tissues, plant extracts, and living plants. Develop Environ Sci. 2007;5:463–485.
- Kavitha KS, Baker S, Rakshith D, et al. Plants as green source towards synthesis of nanoparticles. Int Res J Biol Sci. 2013;2:66–76.
- de DieuTamokou J, Mpetga DJS, Lunga PK, et al. Antioxidant and antimicrobial activities of ethyl acetate extract, fractions and compounds from stem bark of Albizia adianthifolia (Mimosoideae). BMC Complement Altern Med. 2012;12:99.
- Eldeen IMS, Elgorashi EE, Van Staden J. Antibacterial, anti-inflammatory, anti-cholinesterase and mutagenic effects of extracts obtained from some trees used in South African traditional medicine. J Ethnopharmacol. 2005;102:457–464.
- Haddad M, Miyamoto T, Lacaille‐Dubois MA. New triterpenoidal saponins acylated with monoterpenic acid from Albizia adianthifolia. Helv Chim Acta. 2004;87:1228–1238.
- Haddad M, Laurens V, Lacaille-Dubois MA. Induction of apoptosis in a leukemia cell line by triterpenesaponins from Albizia adianthifolia. Bioorg Med Chem. 2004;12:4725–4734.
- Lacaille-Dubois MA, Wagner H. Bioactive saponins from plants: an update. Stud Nat Prod Chem. 2000;21:633–687.
- Knasmüller S, Mersch-Sundermann V, Kevekordes S, et al. Use of human-derived liver cell lines for the detection of environmental and dietary genotoxicants; current state of knowledge. Toxicology. 2004;198:315–328.
- Ghosh S, Patil S, Ahire M, et al. Gnidiaglauca flower extract mediated synthesis of gold nanoparticles and evaluation of its chemocatalytic potential. J Nanobiotechnol. 2012;10:1–9.
- Awwad AM, Salem NM. A green and facile approach for synthesis of magnetite nanoparticles. Nanosci Nanotechnol. 2012;2:208–213.
- Gu H, Ho PL, Tsang KW, et al. Using biofunctional magnetic nanoparticles to capture vancomycin-resistant enterococci and other gram-positive bacteria at ultralow concentration. J Am Chem Soc. 2003;125:15702–15703.
- Sulaiman GM, Al Sammarrae KW, Ad’hiah AH, et al. Chemical characterization of Iraqi propolis samples and assessing their antioxidant potentials. Food Chem Toxicol. 2011;49:2415–2421.
- Ateeq B, Farah MA, Ahmad W. Detection of DNA damage by alkaline single cell gel electrophoresis in 2,4-dichlorophenoxyacetic-acid- and butachlor-exposed erythrocytes of Clarias batrachus. Ecotoxicol Environ Saf. 2005;62:348–354.
- Noginov MA, Zhu G, Bahoura M, et al. The effect of gain and absorption on surface Plasmon's in metal nanoparticles. Appl Phys B. 2007;86:455–460.
- Sonibare MA, Ayoola IO, Elufioye TO. Antioxidant and acetylcholinesterase inhibitory activities of leaf extract and fractions of Albizia adianthifolia (Schumach) WF Wright. J Basic Clin Physiol Pharmacol. 2017;28:143--148.
- Basavegowda N, Magar KBS, Mishra K, et al. Green fabrication of ferromagnetic Fe3O4 nanoparticles and their novel catalytic applications for the synthesis of biologically interesting benzoxazinone and benzthioxazinone derivatives. New J Chem. 2014;38:5415–5420.
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26:3995–4021.
- Njagi EC, Huang H, Stafford L, et al. Biosynthesis of iron and silver nanoparticles at room temperature using aqueous sorghum bran extracts. Langmuir. 2010;27:264–271.
- Kumar B, Smita K, Cumbal L, et al. Phytosynthesis and photocatalytic activity of magnetite (Fe3O4) nanoparticles using the Andean blackberry leaf. Mater Chem Phys. 2016;179:310–315.
- Narayanan S, Sathy BN, Mony U, et al. Biocompatible magnetite and gold nanohybrid contrast agents via green chemistry for MRI and CT bioimaging. ACS Appl Mater Interfaces. 2011;4:251–260.
- Sun XY, Yu SS, Wan JQ, et al. Facile graft of poly (2‐methacryloyloxyethyl phosphorylcholine) onto Fe3O4 nanoparticles by ATRP: synthesis, properties, and biocompatibility. J Biomed Mater Res A. 2013;101:607–612.
- Venkateswarlu S, Rao YS, Balaji T, et al. Biogenic synthesis of Fe3O4 magnetic nanoparticles using plantain peel extract. Mater Lett. 2013;100:241–244.
- Kumar Das A, Marwal A, Verma R. Bio-reductive synthesis and characterization of plant protein coated magnetite nanoparticles. In Nano Hybrids. 2014;7:69–86.
- Lu W, Shen Y, Xie A, et al. Green synthesis and characterization of superparamagnetic Fe3O4 nanoparticles. J Magn Magn Mater. 2010;322:1828–1833.
- Rosicka D, Sembera J. Changes in the nanoparticle aggregation rate due to the additional effect of electrostatic and magnetic forces on mass transport coefficients. Nanoscale Res Lett. 2013;8:1–9.
- Bhattacharya K, Gogoi B, Buragohain AK, et al. Fe2O3/C nanocomposites having distinctive antioxidant activity and hemolysis prevention efficiency. Mater Sci Eng C Mater Biol Appl. 2014;42:595–600.
- Harshiny M, Iswarya CN, Matheswaran M. Biogenic synthesis of iron nanoparticles using Amaranthus dubius leaf extract as a reducing agent. Powder Technol. 2015;286:744–749.
- Phaniendra A, Jestadi DB, Periyasamy L. Free radicals: properties, sources, targets, and their implication in various diseases. Indian J Clin Biochem. 2015;30:11–26.
- Ismail RA, Sulaiman GM, Abdulrahman SA, et al. Antibacterial activity of magnetic iron oxide nanoparticles synthesized by laser ablation in liquid. Mater Sci Eng C Mater Biol Appl. 2015;53:286–297.
- Kashmiri ZN, Mankar SA. Free radicals and oxidative stress in bacteria. Int J Curr Microbiol Appl Sci. 2014;3:34–40.
- Shafagh M, Rahmani F, Delirezh N. CuO nanoparticles induce cytotoxicity and apoptosis in human K562 cancer cell line via mitochondrial pathway, through reactive oxygen species and P53. Iran J Basic Med Sci. 2015;18:993–1000.
- Krishnaraj C, Muthukumaran P, Ramachandran R, et al. Acalypha indica Linn: biogenic synthesis of silver and gold nanoparticles and their cytotoxic effects against MDA-MB-231, human breast cancer cells. Biotechnol Report. 2014;4:42–49.
- Sadeghi L, Tanwir F, Babadi VY. In vitro toxicity of iron oxide nanoparticle: oxidative damages on Hep G2 cells. Exp Toxicol Pathol. 2015;67:197–203.
- Gupta AK, Curtis AS. Lactoferrin and ceruloplasmin derivatized super paramagnetic iron oxide nanoparticles for targeting cell surface receptors. Biomaterials. 2004;25:3029–3040.
- Ankamwar B, Lai TC, Huang JH, et al. Biocompatibility of Fe3O4 nanoparticles evaluated by in vitro cytotoxicity assays using normal, glia and breast cancer cells. Nanotechnology. 2010;21:075102.
- Lunov O, Syrovets T, Büchele B, et al. The effect of carboxydextran-coated superparamagnetic iron oxide nanoparticles on c-Jun N-terminal kinase-mediated apoptosis in human macrophages. Biomaterials. 2010;31:5063–5071.
- Lin XL, Zhao SH, Zhang L, et al. Dose-dependent cytotoxicity and oxidative stress induced by “naked” Fe3O4 nanoparticles in human hepatocyte. Chem Res Chin Univ. 2012;28:114–118.
- Chertok B, David AE, Yang VC. Polyethyleneimine-modified iron oxide nanoparticles for brain tumor drug delivery using magnetic targeting and intra-carotid administration. Biomaterials. 2010;31:6317–6324.
- Wahajuddin SA. Superparamagnetic iron oxide nanoparticles: magnetic nanoplatforms as drug carriers. Int J Nanomed. 2012;7:1–3445.