Abstract
In the regenerative medicine therapies, the availability of engineered scaffolds that modulate inflammatory states is highly required. The aim of this study was to evaluate the efficiency of electrospun nanofibrous scaffolds containing natural substances with anti-inflammatory properties such as Emu oil (EO) to control inflammation and re-polarization of macrophages toward M2 anti-inflammatory phonotype. For this purpose, bead free and smooth EO-blended PCL/PEG electrospun nanofibrous mats were successfully fabricated and characterized using FE-SEM, FTIR, and Universal Testing Machine. GC/MS findings of pure EO revealed the fatty acids composition. MTT results showed that macrophage viability on EO-PCL/PEG nanofibres was higher than on PCL/PEG nanofibres and control (p ≤ .05). Additionally, the presence of EO into nanofibres was found to influence on macrophage morphologies, using FE-SEM. qPCR results showed a reduction in iNOS-2 and an increase in Arg-1 levels of macrophages seeded on EO-PCL/PEG nanofibres, indicating the successfully polarization of the macrophages to M2 phenotype. The change in macrophage phenotype on EO-based nanofibres could suppress the inflammation in LPS/IFN-γ stimulated macrophages as evidenced by a major reduction in pro-inflammatory cytokine levels TNF-α, IL-1β, and IL-6. Conclusively, the results demonstrated that EO-based nanofibres efficiently modulated RAW264.7 macrophage polarity toward an anti-inflammatory M2 phenotype.
Introduction
Macrophages are crucial players in organizing the host innate and adaptive immunity. Most importantly, they are purposefully dispersed throughout the body and involved in host defenxe, initiation and resolution of inflammation, cellular proliferation, and tissue regeneration in wounds [Citation1]. Macrophages are extremely plastic and versatile cells, which are preserved under physiological homeostasis in a continuum of functional polarization states in response to the local environmental cues [Citation2]. After damage, the macrophages recruit to the site of injury and the inflammatory stimulus such as interferon gamma (IFN-γ) and lipopolysaccharide (LPS) drives the macrophage polarization toward M1 phenotype with enhanced secretion of pro-inflammatory cytokines such as IL-1β, TNF-α, and IL-6. M1 macrophages have strong microbicidal activities and stimulate effector T cells in a positive-feedback loop effector. On the one hand, T cells may also inhibit the regenerative capacity of tissue resident stem/progenitor cells via inflammatory cytokines [Citation3,Citation4]. On the other hand, in the late inflammatory stage, the process of efferocytosis (eliminating apoptotic cells) and the alteration in wound microenvironment drive the M1 macrophages toward M2 polarization with high production of anti-inflammatory cytokines IL-4, IL-10, and TGF-β [Citation5]. M2 macrophages participate in regeneration via a crosstalk with regulatory T cells (Tregs), which in turn aid maintain the anti-inflammatory phenotype through production of anti-inflammatory cytokines such as IL-10 [Citation6,Citation7]. Also, Tregs may increase the regenerative power of stem/progenitor cells via secretion of growth factors [Citation1]. Overall, macrophages are chief protagonists in the transition of the damaged tissue from the inflammatory to the resolution or proliferative stage, driving regeneration and marking them as a primary target during designing regenerative strategies.
Recently, several groups exploring tissue-engineering applications aim to shift macrophage type from an M1 to M2 phenotype and achieve a short pro-inflammatory period in which M1 macrophages are recruited to the site, followed by an anti-inflammatory stage where the M2 phenotype dominates. Some strategies can be used to achieve this aim [Citation8]. Among them, the release of natural immunomodulatory agents from scaffolds is an effective strategy to control inflammation and manipulate the M1–M2 response in tissue-engineering applications.
Herein, we hypostasized that Emu oil (EO), an animal-derived lipid composition, with unique anti-inflammatory properties in corporation with proper nano-scaffolds may provide novel platform for the application in tissue-engineering purposes. Hence, we loaded pure EO into electrospun PCL-PEG submicron fibrous scaffolds and evaluated their potential to modulation of functional polarity of macrophages toward anti-inflammatory M2 phenotypes. The sensitivity of cells towards their local environment demands scaffold designs for tissue engineering that optimally mimic the natural extracellular matrix (ECM) [Citation9]. Electrospun nanofibres have gained great interest during the last decade especially as substrates for both tissue engineering and regenerative medicine [Citation10–13].
Materials and methods
Materials
ε-Caprolactone (ε-CL) and polyethylene glycol (PEG, Mn= 4 kDa) and MTT was purchased from Sigma-Aldrich (St. Louis, MO). Dichloromethane (DCM, 99.5%) and Methanol (99.0%) were purchased from Merck Chemical Co. (Kenilworth, NJ). Dulbecco’s Modified Eagle’s Medium (DMEM), foetal bovine serum (FBS), Penstrp, and trypsin were purchased from Gibco (Invitrogen, Carlsbad, CA). All the chemicals were used without further purification.
Fatty acid (FA) composition analysis
We examined three samples of EO that were supplied by an oil producer in East Azerbaijan, Iran. Fat is harvested from the main fat depots from the intra-abdominal (retroperitoneal) and subcutaneous areas of emu and is frozen and stored at −20 °C. Procedures for rendering the fat into oil can vary among producers. Usually, the fat is thawed, ground, or minced, and heated to around 60–70 °C for 30–60 min. The obtained oil is filtered and stored in either nitrogen or argon to avoid oxidation. For GC/MS analysis, total lipids were extracted by chloroform:methanol (2:1 v/v) containing 0.01% butylated hydroxyl toluene as the antioxidant, and were changed to FA methyl esters by transesterification with boron trifluoride in methanol. After extraction with hexane, FA methyl esters were analyzed by a Shimadzu QP-2010 Plus gas chromatograph mass spectrometer (GC/MS) (Shimadzu, Kyoto, Japan) armed with an AOC 20i + s auto injector. An Rtx®-5MS 30 m × 0.25 mm ID ×0.25 µm column was applied for the analysis. The injector was in splitless mode (1 min hold) with 1 µL injection volume at an inlet temperature of 280 °C. Helium was applied as the carrier gas set at constant flow 0.9 ml/min. The oven schedule had an initial temperature of 50 °C, then immediately ramped at 5 °C/min to 260 °C (hold 10 min). Chromatographic peaks were integrated and identified applying the Shimadzu software package (version 7.2.1 SP1, Shimadzu, Kyoto, Japan). Individual FAs are reported as weight percent of total FAs.
Fabrication of nanofibres
First, PCL/PEG (PCL: PEG =90:10, w/w) copolymers was synthesized by ring-opening polymerization of ε-CL initiated by PEG and Sn(Oct)2 as a catalyzer [Citation14,Citation15] and then, PCL/PEG copolymers were dissolved in DCM:methanol (4:1 v/v ratio) to produce a solution of 10% w/v concentration. For the preparation of EO-loaded PCL/PEG solution, 20 wt% concentration of EO (with respect to the PCL/PEG content) were added to PCL/PEG and stirred magnetically at room temperature for 8 h at 25 °C. Carefully, EO was added dropwise to the polymer solutions and kept for stirring for 1 h prior to electrospinning. Each of the prepared solutions was placed into a standard 5 ml plastic syringe with a blunt-ended stainless steel hypodermic needle tip (gauge 22). Electrospun nanofibrous mats were collected on a rotating collector covered with an aluminium foil. All the electrospinning processes were performed at a range of 27–30 KV, a 200 mm needle tip to collector distance, and a 2 ml/h solution flow rate. The mats were dried in a vacuum dryer for 24 h at room temperature to remove the residual solvent, and this sample was applied for further characterizations.
Nanofibre characterization
The morphology of the electrospun fibres was characterized using field emission scanning electron microscopy (FE-SEM) (MIRA3 TESCAN, Czech) at 25 kV. The average diameter and the distribution of the nanofibres were determined from the FE-SEM photographs using image analysis software (Image J, National Institutes of Health, Bethesda, VA) and their mechanical properties were measured using a Universal Testing Machine (1446, Zwick, Ulm, Germany) with a load cell of 10 N), under a crosshead speed of 10 mm/min [Citation19].
Cell culture and seeding
The murine macrophage cell line (RAW264.7) purchased from Pasteur Institute of Iran was cultured on 75 cm2 flask in DMEM supplemented with 10% low-level endotoxin FBS and 1% PenStrep and incubated at 37 °C in humidified atmosphere with 5% CO2. To gain M1 phenotype, the RAW264.7 macrophages were stimulated with both INF-γ (20 ng/ml) and LPS (100 ng/ml) for 48 h. To seeding the macrophages on the nanofibres, first, the nanofibres were cut into circular disks of 15 mm diameter and placed in standard 24-well culture plates with a stainless steel ring to ensure complete contact of the scaffolds with the wells. The fibres were then sterilized under UV radiation overnight for both top and bottom surfaces in a laminar flow hood, washed thrice with PBS to remove any residual solvent, and subsequently immersed in DMEM overnight prior to cell seeding in order to facilitate protein adsorption and cell attachment on the nanofibre surface.
Viability assay
For MTT assay, RAW264.7 cells were seeded drop wise onto the top of the scaffold disks at a cell density of 105 cells per well. Next, the cell seeded scaffolds were kept in a humidified incubator with 5% CO2 at 37 °C and the viability of the cells adhered on the surface of the nanofibrous mats was assessed using the MTT assay on the 1 and 3 day of culture. Appropriate blank samples (PCL/PEG and EO-loaded PCL/PEG nanofibres without cells) were run concurrently.
Scanning electron microscopy analysis
To evaluate morphology of polarized macrophages on the scaffolds, the M1 macrophages were seeded onto the scaffold and incubated for 72 h. At the end of 3 d, the scaffolds were fixed with 2.5% (v/v) glutaraldehyde solution 1 h at 4 °C, and then dehydrated at room temperature in a graded series of ethanol solutions (30, 50, 70, and 90% v/v). The samples were dried overnight at room temperature in a clean bench and morphology was examined using FE-SEM (MIRA3 TESCAN, Brno, Czech).
Quantitative real-time PCR assay
The in vitro efficiency of EO-loaded PCL/PEG nanofibrous mat to modulate macrophage polarization from M1 to M2 phenotype was assessed by determining the alteration in the expression levels of M1 marker (iNOS2) and M2 marker (Arg1), in M1 macrophages seeded on the nanofibres after 3-day incubation using real-time PCR. Also, the expression levels of pro-inflammatory cytokines including TNF-α, IL-1β, and IL-6 was measured using real-time PCR and ELISA. For this purpose, PCL/PEG and EO-loaded PCL/PEG nanofibres (1 cm2) were seeded with M1 macrophages at a seeding density of 200,000 cells/matrix and incubated for 72 h. Then, the supernatants were collected to quantify the levels of IL-6, IL-1β, and TNF-α released into the medium using an ELISA (Sandwich ELISA™), while total RNA of the cells was isolated using Trizol reagent (Invitrogen, Carlsbad, CA), and the RNA concentration was determined by an absorbance at 260 and 280 nm using NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). About 2 μg of RNA was reverse-transcribed into cDNA with RevertAid First Strand cDNA Synthesis Kit (Fermentas, Darmstadt, Germany). Real-time quantitative PCR was carried on the cDNA samples using specific primers and SYBR Green I Master Mix (Invitrogen SYBR GreenERTM qPCR SuperMix Universal, Invitrogen, Carlsbad, CA) in Rotor-Gene 6000 instrument (**Corbett Life Science, Australia). The amplification reaction plan was set at the following steps: 50 °C for 2 min, 95 °C for 10 min, and 40 cycles of 94 °C for 15 s and 60 °C for 1 min. Duplicates were performed for each sample. Relative gene expression levels was normalized by β-actin and relative expression of genes calculated by this formula: (normalized relative ratio =2 − ΔΔCt).
Statistical analysis
All experiments were performed in three independent tests. Results were reported as mean ± SD. All statistical analyses using the software Graph Pad Prism 6.01 and SPSS 16 (SPSS Inc., Chicago, IL) were conducted. Compare data using a two-way ANOVA were performed (p < .05 shows the statistical significance).
Results and discussion
Composition analysis
The emu (Dromaius novaehollandiae) is a large flightless bird, conventionally endemic to Australia, but nowadays farmed throughout the world for its products such as meat, egg, leather, and oil. EO is isolated from the subcutaneous and retroperitoneal fats [Citation16]. As presented in , oleic acid (C18:1n-9), as a monounsaturated omega-9 FA, was the predominant FA in pure EOs tested (52.5%). This was followed by palmitic acid (C16:0), the dominant saturated FA, which constituted 22.2% of the total FAs of the EO samples. Polyunsaturated FAs including linoleic acid (C18:2n-6) and linolenic acid (C18:3n-3) were the constituted 7.3% and 2.1%, respectively. Reports have proved that alterations in diet composition can meaningfully influence the composition of EO and hence possibly impact on oil effectiveness [Citation17]. In present study, we did not find significant difference between the percentages of each FA in the tested samples, due to obtaining from an emu farm with a certain dietary. In addition to triglyceride fraction, EO has a minor non-triglyceride fraction (1–2%) containing variable levels of different compounds with antioxidant and anti-inflammatory properties including carotenoids, flavones, polyphenols, and tocopherol in the, which may confer therapeutic benefits to EO [Citation18].
Table 1. Fatty acid composition (weight % of total fatty acids) of EO in the current study.
Characterization of the nanofibres
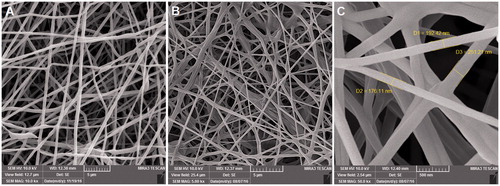
shows the FE-SEM images of electrospun PCL/PEG and EO-PCL/PEG nanofibres. It can be observed that these randomly oriented as-spun nanofibres exhibited bead-free, smooth surface with almost uniform diameters along their lengths. The loading of EO did not meaningfully alter the diameter distribution of the fibres. The diameters of these composite nanofibres were determined to be in the range of 150–300 nm (). The presence of 20 wt% concentration of EO did not significantly alter the morphology or frequency of fibre distribution in relative to fibres loaded with only PCL/PEG. Also, no aggregates of EO were found on the surface of the fibres. The maximum concentration of EO that can be loaded in the PCL/PEG nanofibres under the optimized condition to gaining bead-free electrospun meshes was achieved to be 20% (w/w).
For PCL/PEG electrospun mat, the fibres presented slightly interconnection among the fibres, while the hybrid scaffolds containing EO showed point-bonded fibre structure (). The adhesive property of EO probably provides the connection of two fibres at the points where they were crossing with each other [Citation19].
Since the nanofibrous mats would be potentially applied as a scaffold for tissue engineering, they require a certain mechanical strength. displays the stress strain curves of PCL/PEG and EO-loaded nanofibrous electrospun mats. The PCL/PEG nanofibre mat showed mechanical properties with a tensile strength of 3.7 MPa and elongation at break of 98%. The nonwoven mat of EO-loaded PCL/PEG nanofibres had a tensile strength of 5.8 MPa and elongation at break of 123%. We found that the tensile strength of EO-loaded PCL/PEG nanofibre mat were greater than PCL/PEG mat. As shown in , tensile strength of blended mat was improved with the presence of EO. This improved tensile strength can be supported by considering the adhesive property of EO. As presented in FE-SEM results (), it seems that owing to the formation of hydrogen bonds between PCL/PEG and EO components, more point-bonded sites in the mats provided and therefore the tensile strength of PCL/PEG mats contained EO can increase [Citation19]. These nanofibrous mats were found to have higher mechanical strength than some other scaffolds applied in tissue engineering [Citation20,Citation21]. High-tensile strength may help the scaffold to stay within the wound bed longer during the healing process.
Figure 2. Stress–strain curve of PCL/PEG and EO-PCL/PEG nanofibers using Universal Testing Machine.[Citation19].
![Figure 2. Stress–strain curve of PCL/PEG and EO-PCL/PEG nanofibers using Universal Testing Machine.[Citation19].](/cms/asset/f60d4786-7c89-489c-9588-27f11844dcd4/ianb_a_1367689_f0002_c.jpg)
Macrophage viability
To assess the possible effect of EO-loaded PCL/PEG nanofibrous mat on the viability of RAW264.7 macrophages, we measured the viability of the cells cultured on TCP, PCL/PEG, and EO-PCL/PEG nanofibrous mat after 72 h incubation using MTT assay. As shown in , the presence of EO into PCL/PEG nanofibres caused enhanced cell viability. Although viability of macrophages on PCL/PEG and EO-PCL/PEG nanofibres were similar after 1 d of culture, considerably higher cell viability was observed on EO-PCL/PEG nanofibres in relative to PCL/PEG nanofibres after culture for 3 d.
Figure 3. Cell viability of RAW264.7 macrophages grown on PCL/PEG nanofibres, EO-PCL/PEG nanofibres and tissue culture plates (TCPs) after 1and 3 d of cell culture (*p < .05). Values are expressed as mean ± SD of three parallel measurements.
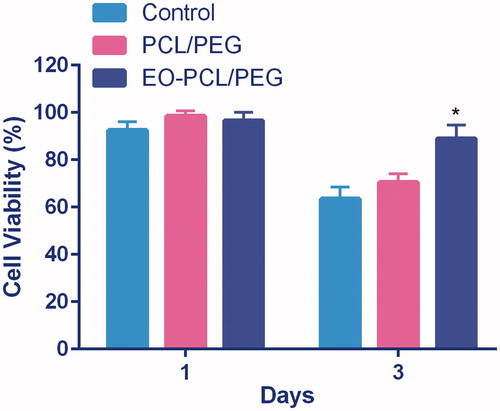
These results demonstrate that EO-PCL/PEG nanofibres have high biocompatibility compared with PCL/PEG nanofibres. Also, using co-polymerized PCL with PEG has provided highly biocompatible, hydrophilic, non-toxic, non-immunogenic, and non-antigenic nanofibres [Citation22,Citation23]. The high viabilities of macrophages on EO-PCL/PEG nanofibres partially reflects biocompatibility of the composite biomaterials and can be benefit for wound healing process and implant survival. Several reports indicated that enhanced recruitment of macrophages occur for improved wound healing.
According our findings, the predominant FAs in triglyceride fraction of EO including oleic acid, palmitic acid, stearic acid, linoleic acid, and linolenic acid might affect macrophage viability. However, further investigation is needed to determine that whether FAs affect macrophage viability significantly or other component of EO in non-triglyceride fraction such as carotenoids and flavonoids also involved. To our knowledge, the effect of FAs on macrophage viability has not been fully described. However, their effects on cell proliferation, gene expression, and protein secretion have been reported for other cell types [Citation24,Citation25].
Macrophage morphology
The influence of biomaterials on macrophage morphology and phenotype played an important role in the assessment of biomaterials immunoregulatory property. Cell shape changes have been reported to be related to different functional states of cells, such as proliferation, nuclear organization, and differentiation [Citation26]. Additionally, cell shape was found to modulate the phenotypic polarization of macrophages.
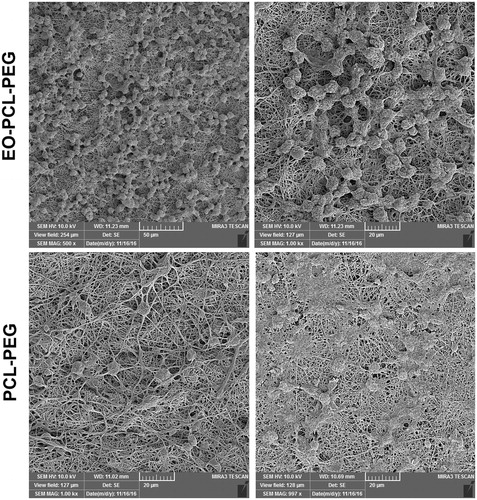
We observed that stimulated RAW264.7 macrophages on PCL-PEG and EO-PCL/PEG nanofibres displayed partially different cell morphologies after 72 h culturing using FE-SEM. As shown in , culturing the stimulated macrophages on PCL-PEG led to cellular elongation and higher surface membrane partly with some observable flopodia. In contrast, almost all stimulated macrophages on EO-PCL/PEG nanofibres were observed to be round, pancake-like shape with few cytoplasmic projections and low spreading within in 3-d culturing. Filopodia are specialized macrophage adhesion structures that can form in the early stages of cell adhesion and consist of punctuate F-actin on plasma membrane extensions, which facilitate attachment and migration [Citation27]. It has been proposed that elongation and the presence of flopodia represents an M1 phenotype, whereas a round shape might indicate a M2 phenotype of macrophages [Citation28]. Therefore, the changing in cell morphologies suggest that macrophages would be more prone to become repolarized in the presence of EO incorporated with PCL/PEG nanofibres, implying that they can exert anti-inflammatory activities and improve wound-healing process. Our results were also consistent with previous reports that M2 macrophages exhibited a more normal round shape compared with M1 macrophages [Citation26,Citation29].
In vitro repolarization study
To investigate the efficiency of EO-PCL/PEG nanofibres to re-polarize RAW264.7 macrophages from M1 to M2 phenotypes, real-time PCR was applied to measure iNOS and Arg-1 gene expression levels in the M1 macrophages cultured on PCL/PEG and EO-PCL/PEG nanofibres for 72 h (.
Figure 5. In vitro polarization study. Expression levels of iNOS-2 (M1 marker) and Arg-1 (M2 marker) in M1 RAW264.7 macrophages seeded on PCL/PEG and EO-PCL/PEG nanofibres for 72 h (*p < .05), Results are mean ± SD (n = 3).
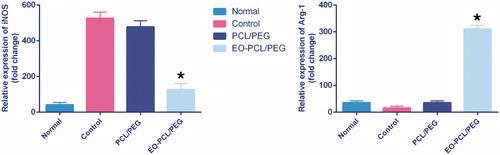
We used iNOS-2, as a M1 marker, and Arg-1, as a M2 marker, to evaluate re-polarization potential of RAW264.7 macrophages on the nanofibres containing EO. Macrophages can switch their polarity to M1 or M2 phenotype with characteristic expression profiles of surface markers as well as cytokines and chemokines secretion depending on the presence of stimuli in the local environment. iNOS-2 and Arg-1 were further established to define a simple system for indicating M1 and M2 macrophages. As shown in , LPS/IFN-γ stimulation enhanced iNOS levels to 525-fold in macrophages cultured in TCPs, indicating the successfully polarization of the macrophages to M1 phenotype. Importantly, it has been found that presence of EO in PCL/PEG nanofibres significantly decreased iNOS level (150-fold) in the macrophages compared with PCL/PEG nanofibres (500-fold) (p < .05). The nanofibres without EO didn’t influence on iNOS expression level (. Furthermore, the expression level of Arg-1 in the macrophages seeded on EO-PCL/PEG significantly was enhanced (300-fold), while nanofibrrs without EO had no effect on the Arg-1 levels of seeded macrophages and the Arg-1 levels in the macrophages seeded on PCL/PEG nanofibre were 40-fold.
The results indicated that RAW264.7 macrophages were successfully re-polarized from M1 to M2 type by culturing on the electrospun nanofibrous mats containing 20 wt.% concentration of EO.
Anti-inflammatory effect of EO-PCL/PEG nanofibres
Following macrophage phenotype switching to the M2 type on the EO-loaded nanofibres, the induction (relative mRNA levels) and release (protein expression levels) of three pro-inflammatory cytokines mainly involved in the inflammatory wound process, IL-6 and IL-1β and TNF-α, were investigated in M1 macrophages seeded on PCL/PEG and EO-loaded PCL/PEG nanofibres after 3 d culturing (.
Figure 6. Anti-inflammatory effect of PCL/PEG and EO-loaded PCL/PEG nanofibres evaluated on RAW264.7 cells stimulated with LPS/INF-γ. Histograms show the mean values of mRNA levels of (A) IL-1β, (B) IL-6, and (C) TNF-α and the amounts of (D) IL-1β, (E) IL-6, (F) TNF-α after ELISA evaluation (*p ≤ .05 versus PCL/PEG and control groups). Results are mean ± SD (n = 3).
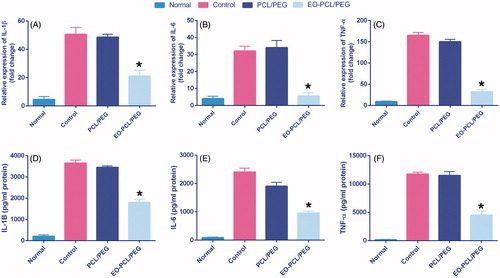
As shown in , the LPS/IFN-γ stimulation significantly upregulated TNF-α, IL-1β, and IL-6 in macrophages seeded on both TCPs and PCL/PEG nanofibres compared with untreated cells. Culturing macrophages on EO-PCL/PEG nanofibres resulted in dramatically reduction in the expression levels of TNF-α, IL-1β, and IL-6 after 3 d. This more pronounced effect indicates that EO could effectively suppress the inflammation caused by IFN-γ/LPS in the macrophages.
According to ELISA results, as shown in reduction of the release of IL-6, IL-1β, and TNF-α was also observed in the cell culture supernatants after LPS/INF-γ stimulation and incubation with the EO-loaded nanofibrous mats.
Consistent with our findings, numerous in vivo and in vitro studies have revealed that EO could decrease the expression level of inflammatory cytokines, such as IL-1β, IL-6, and TNF-α and reduce the production of nitric oxide (NO) [Citation30].
A number of suggestions associate to the potential mechanism by which EO exerts its anti-inflammatory properties. It has been proposed that the n-9 and n-3 FAs existing in EO may be involved in its anti-inflammatory effects. n-3 FAs reduce inflammation both directly, through modulation of the eicosanoid pathways that generate leukotriene B4, prostaglandin E2, and thromboxane B2, and indirectly, by changing the expression levels of inflammatory genes via influences on transcription factor activation, while n-9 FAs hinder macrophage migration. Based on GC/MS findings, oleic acid, a monounsaturated omega-9 FA, is present at a higher amount in EO (about 50%). Previous studies show that oleic acid can inhibit a wide range of inflammatory inducers in vitro, and binds peroxisome proliferator-activated receptor (PPARγ), a transcription factor which promotes M2 macrophage polarization [Citation31–33].
Yoganathan et al. found the strong capability of EO to decrease levels of pro-inflammatory cytokines in a Croton oil-induced auricular swelling in CD-1 mice than other oils recognized to contain higher levels of FAs such as fish oil and olive oil [Citation34]. However, EO contains considerably less anti-inflammatory FAs in comparison with other oils, thus, authors suggested that the anti-inflammatory effects of EO could not be solely ascribed to the FA profile. It is proposed that the effects of EO may be related to the FA ratio and or the synergism between FA fraction and other components in EO. The minor non-triglyceride fraction of EO including natural antioxidants such as carotenoids, flavones, tocopherols, and skin-permeation enhancing factors, are described to induce radical scavenging effects, modulate pro-apoptotic, anti-proliferative, and anti-inflammatory pathways in intestinal epithelial cells and decrease pro-inflammatory cytokine expression and colonic neutrophil infiltration in a colitis mouse model [Citation18,Citation35]. Besides, the high ratio of unsaturated to saturated FAs (UFA: SFA) in EO composition may confer protection versus oxidative stress damages [Citation18].
Our finding revealed that efficiently modulation of RAW264.7 macrophages toward an anti-inflammatory phenotype by EO-loaded nanofibres in comparison nanofibres without EO. However, efficient macrophage re-polarization and the anti-inflammatory effects of EO-PCL/PEG nanofibres in an in vitro system may not be comparable with an in vivo situation. Therefore, besides evaluation of EO-mediated macrophage re-polarization in other sources of macrophages and exploring possible repolarization mechanisms, in vivo model studies essential to be established for further validation of the efficiency of EO-PCL/PEG nanofibres in repolarization of macrophages.
Conclusion
In summary, we successfully fabricated bead-free and smooth PCL-PEG nanofibrous mat containing EO 20% (w/w) using electrospinning technique. Synthetic polymers such as PCL/PEG could successfully overcome the poor electro spinnability of EO solution through interaction of PCL/PEG with EO via hydrogen bonding. In this work, we demonstrated that the electrospun EO-PCL/PEG nanofibrous mats could powerfully polarize RAW264.7 macrophages toward anti-inflammatory phenotype. Thus, EO may potentially serve as a useful component for the fabrication of novel bioactive nanofibrous scaffolds to develop an ideal scaffold with anti-inflammatory properties for skin bioengineering and wound-dressing applications.
Correction Statement
This article has been republished with minor changes. These changes do not impact the academic content of the article.
Disclosure statement
The authors announce that there are no conflicts of interest.
Additional information
Funding
References
- Julier Z, Park AJ, Briquez PS, et al. Promoting tissue regeneration by modulating the immune system. Acta Biomater. 2017;53:13–28.
- Martinez FO, Gordon S. The M1 and M2 paradigm of macrophage activation: time for reassessment. F1000Prime Rep. 2014;6:13.
- Wynn TA, Vannella KM. Macrophages in tissue repair, regeneration, and fibrosis. Immunity. 2016;44:450–462.
- Varol C, Mildner A, Jung S. Macrophages: development and tissue specialization. Annu Rev Immunol. 2015;33:643–675.
- Novak ML, Koh TJ. Macrophage phenotypes during tissue repair. J Leukoc Biol. 2013;93:875–881.
- Soroosh P, Doherty TA, Duan W, et al. Lung-resident tissue macrophages generate Foxp3+ regulatory T cells and promote airway tolerance. J Exp Med. 2013;210:775–788.
- Saraiva M, O'garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 2010;10:170–181.
- Alvarez MM, Liu JC, Trujillo-de Santiago G, et al. Delivery strategies to control inflammatory response: modulating M1–M2 polarization in tissue engineering applications. J Control Release. 2016;240:349–363.
- Deldar Y, Pilehvar-Soltanahmadi Y, Dadashpour M, et al. An in vitro examination of the antioxidant, cytoprotective and anti-inflammatory properties of chrysin-loaded nanofibrous mats for potential wound healing applications. Artif Cells Nanomed Biotechnol. 2017[June 9];[1–11]. Forthcoming. doi: 10.1080/21691401.2017.1337022
- Pilehvar-Soltanahmadi Y, Akbarzadeh A, Moazzez-Lalaklo N, et al. An update on clinical applications of electrospun nanofibers for skin bioengineering. Artif Cells Nanomed Biotechnol. 2016;44:1350–1364.
- Zarghami N, Sheervalilou R, Fattahi A, et al. An overview on application of natural substances incorporated with electrospun nanofibrous scaffolds to development of innovative wound dressings. Mini Rev Med Chem. 2017;17.
- Nejati-Koshki K, Pilehvar-Soltanahmadi Y, Alizadeh E, et al. Development of Emu Oil-loaded PCL/collagen bioactive nanofibers for proliferation and stemness preservation of human adipose-derived stem cell: possible application in regenerative medicine. Drug Dev Ind Pharm. 2017[Aug 10];[1–11]. Forthcoming. doi: 10.1080/03639045.2017.1357731
- Dadashpour M, Pilehvar-Soltanahmadi Y, Mohammadi SA, et al. Watercress-based electrospun nanofibrous scaffolds enhance proliferation and stemness preservation of human adipose-derived stem cells. Artif Cells Nanomed Biotechnol. 2017[Jul 11];[1–12]. Forthcoming. doi: 10.1080/21691401.2017.1345925
- Mohammadian F, Pilehvar-Soltanahmadi Y, Zarghami F, et al. Upregulation of miR-9 and Let-7a by nanoencapsulated chrysin in gastric cancer cells. Artif Cells Nanomed Biotechnol. 2017;45:1201–1206.
- Mohammadian F, Pilehvar-Soltanahmadi Y, Mofarrah M, et al. Down regulation of miR-18a, miR-21 and miR-221 genes in gastric cancer cell line by chrysin-loaded PLGA-PEG nanoparticles. Artif Cells Nanomed Biotechnol. 2016;44:1972–1978.
- Jeengar MK, Kumar PS, Thummuri D, et al. Review on emu products for use as complementary and alternative medicine. Nutrition. 2015;31:21–27.
- Beckerbauer L, Thiel-Cooper R, Ahn D, et al. Influence of two dietary fats on the composition of emu oil and meat. Poult Sci. 2001;80:187–194.
- Abimosleh SM, Tran CD, Howarth GS. Emu oil: a novel therapeutic for disorders of the gastrointestinal tract? J Gastroenterol Hepatol. 2012;27:857–861.
- Pilehvar-Soltanahmadi Y, Nouri M, Martino MM, et al. Cytoprotection, proliferation and epidermal differentiation of adipose tissue-derived stem cells on emu oil based electrospun nanofibrous mat. Exp Cell Res. 2017;357:192–201.
- Bui HT, Chung OH, Cruz JD, et al. Fabrication and characterization of electrospun curcumin-loaded polycaprolactone-polyethylene glycol nanofibers for enhanced wound healing. Macromol Res. 2014;22:1288–1296.
- Suganya S, Venugopal J, Mary SA, et al. Aloe vera incorporated biomimetic nanofibrous scaffold: a regenerative approach for skin tissue engineering. Iran Polym J. 2014;23:237–248.
- Saei Arezoumand K, Alizadeh E, Pilehvar-Soltanahmadi Y, et al. An overview on different strategies for the stemness maintenance of MSCs. Artif Cells Nanomed Biotechnol. 2016[Nov 3];[1–17]. Forthcoming. doi: 10.1080/21691401.2016.1246452
- Rahmani Del Bakhshayesh A, Annabi N, Khalilov R, et al. Recent advances on biomedical applications of scaffolds in wound healing and dermal tissue engineering. Artif Cells Nanomed Biotechnol. 2017[Jul 12];[1–15]. Forthcoming. doi: 10.1080/21691401.2017.1349778
- Kang JX, Wan JB, He C. Concise review: regulation of stem cell proliferation and differentiation by essential fatty acids and their metabolites. Stem Cells. 2014;32:1092–1098.
- Jung YH, Lee S-J, Oh SY, et al. Oleic acid enhances the motility of umbilical cord blood derived mesenchymal stem cells through EphB2-dependent F-actin formation. Biochim Biophys Acta (BBA)-Mol Cell Res. 2015;1853:1905–1917.
- Dai X, Wei Y, Zhang X, et al. Attenuating immune response of macrophage by enhancing hydrophilicity of Ti surface. J Nanomater. 2015;2015:3.
- Trindade R, Albrektsson T, Tengvall P, et al. Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res. 2016;18:192–203.
- McWhorter FY, Wang T, Nguyen P, et al. Modulation of macrophage phenotype by cell shape. Proc Natl Acad Sci. 2013;110:17253–17258.
- Saino E, Focarete ML, Gualandi C, et al. Effect of electrospun fiber diameter and alignment on macrophage activation and secretion of proinflammatory cytokines and chemokines. Biomacromolecules. 2011;12:1900–1911.
- Lindsay RJ, Geier MS, Yazbeck R, et al. Orally administered emu oil decreases acute inflammation and alters selected small intestinal parameters in a rat model of mucositis. Br J Nutr. 2010;104:513.
- Reardon M, Gobern S, Martinez K, et al. Oleic acid attenuates trans-10, cis-12 conjugated linoleic acid-mediated inflammatory gene expression in human adipocytes. Lipids. 2012;47:1043–1051.
- Kliewer SA, Sundseth SS, Jones SA, et al. Fatty acids and eicosanoids regulate gene expression through direct interactions with peroxisome proliferator-activated receptors α and γ. Proc Natl Acad Sci. 1997;94:4318–4323.
- Camell C, Smith CW. Dietary oleic acid increases m2 macrophages in the mesenteric adipose tissue. PLoS One. 2013;8:e75147.
- Yoganathan S, Nicolosi R, Wilson T, et al. Antagonism of croton oil inflammation by topical emu oil in CD-1 mice. Lipids. 2003;38:603–607.
- Ruiz PA, Haller D. Functional diversity of flavonoids in the inhibition of the proinflammatory NF-kappaB, IRF, and Akt signaling pathways in murine intestinal epithelial cells. J Nutr. 2006;136:664–671.