Abstract
In the present study, nanoparticles of gold, iron oxide and zinc oxide (ZnO) were studied for cytotoxicity in the colorectal cancer cell HT 29. The metallic nanoparticles in the range of <50 and <100 nm were screened for anticancer activity by MTT assay. The nanoparticles were tested at concentrations ranging from 0.5 to 50 µg/ml. Zinc oxide exhibited significant anti-cancer activity in comparison to other nanoparticles. It had an IC50 value of 17.12 µg/ml. The mechanism of action was studied by fluorescence microscopy with acridine orange, propidium iodide and DAPI staining techniques. The ROS production of ZnO nanoparticles was determined by DCFH-DA. The ZnO nanoparticles were conjugated with novel hydrophobic peptides and evaluated for anticancer activity. It was observed that the nanoparticles peptide complex showed better cytotoxicity than either peptide or nanoparticle alone. Thus, the ZnO nanoparticles tested in our study has anticancer activity against colon cancer cells. It can also be conjugated with peptides and used for targeting cancer cells with higher efficacy.
Introduction
Among the noninfectious diseases, cancer is one of the major cause of mortality globally [Citation1]. The present cancer treatment which involves the use of alkylating agents and antimetabolites, have many side effects and are known for systemic toxicities. Hence, targeted therapy is essential for cancer treatment [Citation2].
One of the novel strategies for the treatment of cancer, is the application of nanomaterials. Nanomaterials and nanostructured devices have been used as carriers of anticancer drugs. The use of nanotechnology in drug delivery has led to the controlled release of the anti-cancer drugs and has enhanced their in vivo efficacy [Citation3].
The nanoparticles with their unique physicochemical properties are known to have intrinsic antitumor effects. The anticancer activity of nanoparticles is either due to the intrinsic features such as their antioxidant action or due to application of external stimuli, such as hyperthermia in response to the application of infrared rays or magnetic fields. On external stimuli, they can generate reactive oxygen species which can kill cancer cells. The nanoparticles can also interact with tumor environment, such as blood vessels or stroma and interfere with the development of tumor mass [Citation4].
Metallic nanoparticles are finding an increased application in the biomedical field. In cancer biology, metal nanoparticles are being designed for both diagnostics and therapeutics. The metal and metal oxide nanoparticles can be synthesized as required and further modified with various chemical functional groups. Their functionalization helps in conjugating them with biological molecules (such as antibodies, nucleic acids and peptides) ligands and anticancer drugs [Citation5].
In the present study, the metal nanoparticles of zinc oxide, iron oxide and gold nanoparticle were studied for the anticancer properties in HT 29 colonic cancer cell. The zinc oxide nanoparticle exhibited significant cytotoxicity in HT29 cells. The mechanism of action as revealed by fluorescence microscopy and DCFH-DA was apoptosis and reactive oxygen species production by zinc oxide (ZnO) nanoparticles. Further, two hydrophobic peptides were conjugated with ZnO nanoparticles and tested for cytotoxicity in HT29. The ZnO peptide conjugate showed enhanced cytotoxicity in HT29 cells than either nanoparticle or peptide alone. The study was performed with an aim to develop anticancer drugs based on nanoparticles and their peptide conjugates.
Materials and methods
Chemicals and reagents
ZnO (<50 nm), Au (<100 nm), Fe (III) oxide (<50 nm) nanoparticles were purchased from Sigma-Aldrich and was used in the study. A stock solution of nanoparticles was prepared by adding 1 mg of nanoparticles to 1 ml of sterile MQ water. Nanoparticle dispersions were obtained by shaking vigorously followed by ultrasonication for 30 min.
The dyes MTT, trypan blue, acridine orange, DAPI and DCFH-DA were obtained from Sigma-Aldrich. The colon cancer cell line HT29 was obtained from NCCS, Pune. Cell culture assay media (DMEM and supplements) were purchased from Sigma-Aldrich.
The hydrophobic peptides (Peptide 1: Boc-Leu-Aib-Val-dPro-lPro-Val-Aib-Leu-OMe and Peptide 2: Boc-U-Gpn-OMe) were synthesized using conventional solution phase chemistry using a racemization-free fragment condensation strategy. The tert-butoxycarbonyl (Boc) group was used for N-terminal protection and the C-terminal was protected as a methyl ester. Deprotections were performed using 98% formic acid and saponification for the N- and C-terminus, respectively. In the final step, peptide 1 was synthesized by condensation of two fragments Boc-Leu-Ala-Aib-Val-OH and NH-dPro-lPro-Val-Aib-Leu-OMe. A reverse-phase HPLC was performed to purify the crude peptides. Electrospray mass spectrometry revealed that the molecular weight of the Peptide 1 was 921.28 and Peptide 2 was 370.35.
Cytotoxicity assay
MTT assay
MTT assay was performed to evaluate the cytotoxicity and anticancer activity of the nanoparticles on the HT29 cancer cells [Citation6]. Cells were grown in DMEM containing 10% FBS and 1% penicillin–streptomycin and incubated at 37 °C in 5% CO2. 200 µl of suspension of HT29 cells (1 × 105 cell/ml) was added to each well of 96 well microtiter plate and incubated for 24 h. After the incubation period, 0.5 to 50 µg/ml of nanoparticles were added to the cells. 20 μl of MTT dye was added to each well after 24 h of incubation. The media containing MTT dye solution was removed after 4 h. To each well, 200 μl of DMSO was added to solubilize the formed formazan. The absorbance was measured at 570 nm. Percentage cell toxicity and IC50 value were calculated.
Trypan blue exclusion assay
The HT29 cells were grown as described earlier. The cells were treated with nanoparticles (0.5 to 50 μg/ml). After 24 h of incubation, 80 μl of the cell suspension was added to 20 μl of trypan blue stain solution (0.4%) and stained for 2 min [Citation7]. The live and dead cells were counted using a hemocytometer by observing under light microscope. The live cells do not absorb the dye whereas dead cells were stained blue. Percentage of cytotoxicity was calculated by the following formula:
Fluorescence microscopy
Apoptosis detection
The apoptotic effect of nanoparticles was analyzed by using fluorescent nuclear dye Hoechst 33258 [Citation8]. HT29 cells were grown in six well tissue culture dishes and treated with or without nanoparticle. Cells were then washed with PBS and fixed in 4% paraformaldehyde for 10 min. Subsequently the cells were permeabilized with 3% paraformaldehyde and 0.5% Triton X-100 and stained with the dye. After staining the cells were observed with a fluorescence microscope.
Cellular morphological alterations
The cellular morphological alteration after treatment with nanoparticles was analyzed at the end of incubation period (24 h) by bright field and fluorescence microscopy. Further, the cells were washed with PBS (pH 7.4). 100 μg/ml of acridine orange and propidium iodide was added to each well and incubated for 5 min at 37 °C. The cells were washed with PBS. The live and apoptotic cells were visualized with an epifluorescence microscope for membrane damage [Citation9].
Reactive oxygen species analysis
For quantitative ROS analysis, cells (1 × 105 per well) were seeded in black bottom 96-well culture plate and incubated for 24 h in a CO2 incubator at 37 °C [Citation10]. HT29 cells were treated with different concentrations of nanoparticles for 12 h. After exposure, cells were incubated with DCFH-DA (10 mM) for 30 min at 37 °C. Fluorescence intensity was measured at excitation and emission wavelength of 485 and 528 nm, respectively. Values were expressed as the percentage of fluorescence intensity relative to the control wells.
Cytotoxicity of peptides by MTT assay
Two hydrophobic peptides, Peptide 1: Boc-Leu-Aib-Val-dPro-lPro-Val-Aib-Leu-OMe and Peptide 2: Boc-U-Gpn-OMe were screened for cytotoxicity in HT29 cell line by MTT assay. Further, they were conjugated with ZnO nanoparticles by adding them in 1:1 ratio in 1 ml sterile distilled water. The peptide nanoparticle colloidal suspension was ultrasonicated for 10 min and stored at 4 °C until use. The peptide nanoparticle conjugates were tested at 1, 5 and 10 µg/ml in HT29 cells by MTT assay as described in previous section.
Characterization of peptides ZnO nanoparticle conjugate
UV spectra of the peptide alone and peptide ZnO nanoparticle complex in sterile distilled water was studied using UV-Visible spectroscopy (Tecan Infinite 200Pro UV/Vis Spectrophotometer) to observe the interaction of peptides with ZnO nanoparticle. The spectra were obtained in the range of 230–980 nm using water as a blank.
Size, polydispersity index and zeta potential of peptide and peptide ZnO nanoparticle colloidal suspension was measured by using dynamics light scattering (DLS) and a Zetasizer (Microtrac-Zetatrac, Microtrac, Montgomeryville, PA).
Statistical analysis
The experiments were performed in triplicate and results are expressed as mean ± SD. Student’s t-test was used to determine statistical significance. Differences for values of p < .05 were considered significant.
Results and discussion
Cytotoxicity assay
MTT assay
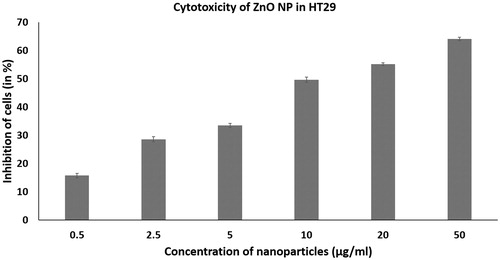
The nanoparticles gold, zinc oxide and iron oxide were screened for anticancer activity in HT 29 cell line. The nanoparticles were tested for activity in the concentration ranging from 0.5, 2.5, 5, 10, 20 and 50 µg/ml ().
Figure 1. Anticancer activity of iron oxide (Fe2O3 NP), gold (AuNP) and zinc oxide (ZnO NP) nanoparticles in HT 29 cells determined by MTT assay at concentrations ranging from 0.5 to 50 µg/ml. The error bar indicates means ± standard deviations from three independent experiments performed in triplicate.
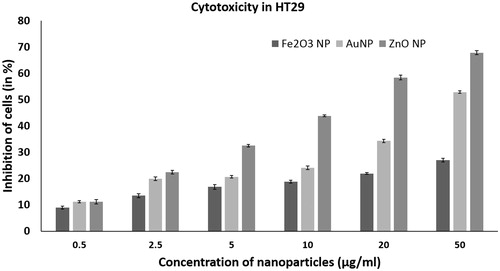
Of the tested nanoparticles, iron oxide nanoparticle didn’t show significant anticancer activity. The iron oxide nanoparticles did not significantly inhibit the HT29 cells with increasing concentration (p < .05). At a concentration of 50 µg/ml, it exhibited a cytotoxicity of 26.98% (). Nanoparticles of iron oxide can kill tumor cells by production of reactive oxygen species or hyperthermia. The paramagnetic and supermagnetic iron oxide nanoparticles develop antitumor activity on the application of near-infrared (NIR) or oscillating magnetic fields (MF) [Citation11]. It has been reported that the antitumor effect of the anticancer drug doxorubicin increases with the use of iron oxide nanoparticles. In such a nano-drug complex, the synergy of magnetic fields and an anticancer magneto-sensitive nanocomplex leads to increased antitumor activity [Citation12]. Spherical iron oxide nanoparticles have been approved by EU for application in magnetic tumor hyperthermia in brain and prostate cancer along with radiotherapy or chemotherapy [Citation13–16]. Thus, though magnetic nanoparticles have cytotoxicity to cancer cells, its anticancer activity can be enhanced by use of external magnetic field and in combination with other cancer therapeutics.
Gold nanoparticles showed concentration dependent anticancer activity in HT29. It had an IC50 value of 48 µg/ml. The gold nanoparticles were able to inhibit growth of HT29 at as low as 0.5 µg/ml (). Previously, it has been reported that gold nanoparticles have antitumor activity against breast cancer cells MCF 7 [Citation17], lung cancer cells Hep-G2 and liver cancer cells A549 [Citation18]. Recent studies have shown that gold nanoparticles are able to inhibit angiogenesis, a process which plays a crucial role in growth and spread of cancer cells. The anticancer activity was due to the intrinsic property of gold nanoparticle wherein it interacts selectively with heparin-binding glycoproteins and inhibits it activity. The ability of gold nanoparticles to specifically bind to vascular permeability factor/vascular endothelial growth factor (VPF/VEGF)-165 and basic fibroblast growth factor, endothelial cell mitogens and mediators of angiogenesis led to inhibition of endothelial/fibroblast cell proliferation and angiogenesis [Citation19]. Thus, the studies on anticancer activity of gold nanoparticles, shows that gold nanoparticles are cytotoxic to cancer cells due to thermal ablation, photodynamic property and angiogenesis.
ZnO NPs of <50 nm showed significant anticancer activity in HT29 (p < .05). The ZnO nanoparticle had an IC50 value of 17.12 µg/ml. It showed a concentration dependent activity from 0.5 to 50 µg/ml (). ZnO nanoparticles have previously been shown to have selective cytotoxic effect on the various human glioma, breast and prostate cancer cell lines with no major effect on the normal cell lines [Citation20]. ZnO nanoparticles have also shown higher cytotoxicity in human myeloblastic leukemia cells HL60 cells than the normal peripheral blood mononuclear cells PBMCs. The ZnO nanoparticles were capable of increasing lipid peroxidation in the liposomal membrane by reactive oxygen species (ROS) production [Citation21]. ZnO nanoparticles have also shown selective anticancer activity against human hepatocellular carcinoma HepG2, human lung adenocarcinoma A549 and human bronchial epithelial BEAS-2B by inducing apoptosis. It had no cytotoxic effect in case of normal astrocytes and hepatocytes [Citation22]. It has also been reported that zinc oxide nanoparticles have cytotoxicity towards highly proliferating cells due to increased ROS production [Citation23]. Thus, the present and previous study shows that ZnO nanoparticles have selective and high cytotoxicity towards cancer cells of colon, glioma, lung, breast and prostate with less effect on normal cells. Hence, ZnO nanoparticles can find application as a selective cytotoxic agent for the killing of cancer cells.
ZnO nanoparticles had higher cytotoxicity towards HT29 cells than the gold and iron oxide nanoparticles. Hence, our further studies were focused on studying the mechanism of action of ZnO nanoparticles cytotoxicity in HT29.
Trypan blue exclusion assay
The trypan blue assay is used to evaluate cytotoxicity as dead cells absorb the dye into the cytoplasm due to loss of membrane selectivity and live cells remain unstained [Citation7]. In the study, a dose dependent inhibitory effect on HT29 cancer cell lines treated with the nanoparticles was observed at increasing concentrations. At 50 µg/ml of ZnO nanoparticle treatment, the number of dead cells were significantly high (). The results of the trypan blue exclusion assay correlated with the MTT assay.
Fluorescence microscopy
Apoptosis detection
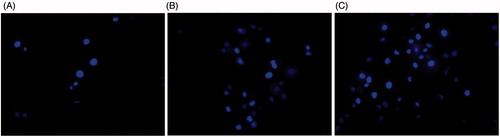
Hoechst 33342 is a nucleic acid blue fluorescent dye which is cell permeable. It is used to visualize chromatin condensation and fragmentation as it stains the condensed nuclei of apoptotic cells [Citation8]. The Hoechst 33258 fluorescent nuclear dye was used to study the apoptotic effect of ZnO nanoparticles in HT 29 cells. At 20 and 50 μg/ml of ZnO nanoparticle treatment, there were more number of apoptotic bodies than in the control (). The control cells were intact and oval shaped whereas, the ZnO nanoparticle treated cells showed apoptosis features including shrinkage of cells, highly condensed and fragmented nuclei, formation of high number of apoptotic bodies, fragmentation and decrement of cells. Apoptosis has been suggested to be the major mechanism of cell death due to cytotoxic effect of ZnO nanoparticles [Citation24]. It could be seen in our study that, exposure of HT29 cells to ZnO nanoparticles induces apoptosis in colon cancer cells.
Cellular morphological alterations
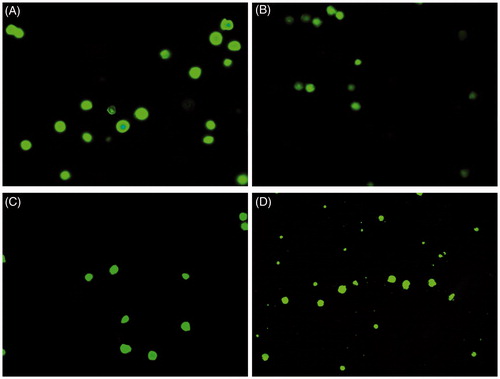
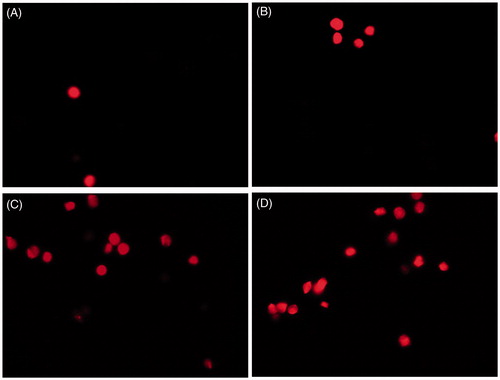
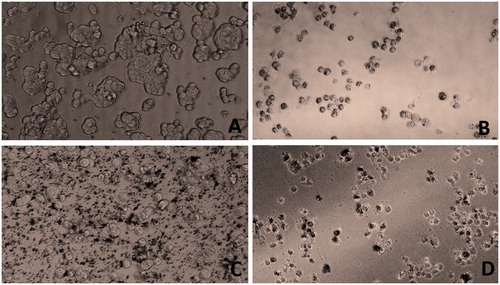
Bright field microscopy was used for preliminary study of cellular morphology on the treatment of HT29 with the gold, iron oxide and zinc oxide nanoparticles. It revealed that at 50 μg/ml, the gold and zinc oxide nanoparticles inhibited cell growth, whereas iron oxide nanoparticles did not have significant toxic effect on HT29 cells (). Further, acridine orange and propidium iodide were used to study the cellular morphological alterations and apoptosis in zinc oxide nanoparticles treated HT 29 cells at 5, 20 and 50 µg/ml. Apoptotic cells were seen as cells having membrane blebs and with apoptotic bodies. Acridine orange stains both the dead and live cell, whereas propidium iodide stains only dead cells, as it cannot cross an intact plasma membrane [Citation9]. In the present study, there was no significant apoptosis in the control or untreated cells ( and ). The study showed that with the increasing concentrations of zinc oxide nanoparticles, there was an increase in the number of early-stage apoptotic cells. The cells appeared damaged and had high morphological alterations. This implies that zinc oxide nanoparticles of <50 nm induces apoptosis and is highly cytotoxic to HT 29 colon cancer cells.
Figure 4. Bright field microscopy of HT29 cancer cells on treatment with nanoparticles at 50 μg/ml. Control (A), gold nanoparticle (B), iron oxide nanoparticle (C) and Zinc oxide nanoparticle (D).
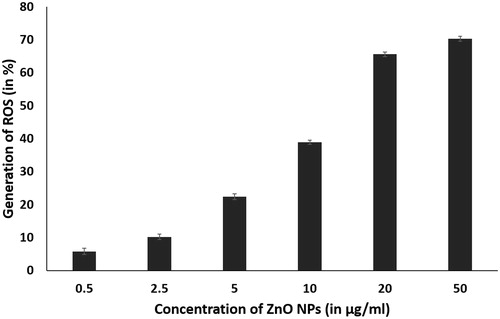
Reactive oxygen species analysis
The in situ detection of ROS production by ZnO nanoparticles was observed by using fluorogenic probe, DCFH-DA. It is a small nonpolar molecule that can easily diffuse into the cell. Within the cell, intracellular esterases enzymatically deacetylate it into a polar non fluorescing compound. The presence of high amount of ROS in the cell can oxidize it to the highly fluorescent green 2,7-dichlorofluorescein (DCF). The study showed that there was an increase in ROS production, with increase in concentration of ZnO nanoparticles from 0.5 to 50 µg/ml (). The ZnO nanoparticles in cancer cells, act as a redox system and react with chemical species in the cell and generate high amount of ROS in the cell, leading to oxidative stress. This in turn causes damage to lipid, protein and nucleic acid of the cell resulting in membrane damage through lipid peroxidation and protein denaturation, causing necrosis and DNA damage, which ultimately results in cell death by apoptosis [Citation24–26].
Cytotoxicity of peptides ZnO nanoparticle conjugates
Two hydrophobic peptides: Peptide 1 and Peptide 2 showed concentration dependent anticancer activity in HT29 cell line when tested at 0.5, 5 and 10 µg/ml (). Studies have shown that, as the cell membrane has a hydrophobic environment, the hydrophobicity of the peptides is an important property for its anticancer activity. The bulky hydrophobic amino acids when inserted into the cell membrane hydrophobic core acquire a stable structure which leads to pore formation and as a result cell death occurs either due to apoptosis and/or necrosis. Recent research shows that as there is a correlation between hydrophobicity and anticancer activity, the hydrophobicity of the peptide can be modulated in order to increase its anticancer activity [Citation27]. Our study also confirms that the hydrophobic peptides with toxicity to colonic cancer cells are promising as anti-cancer therapeutics.
Figure 8. Anticancer activity of zinc oxide (ZnO NP) nanoparticles, peptide 1 and peptide 2 and the nanoparticle peptide conjugates in HT 29 cells determined by MTT assay at 0.5, 5 and 50 µg/ml. The error bar indicates means ± standard deviations from three independent experiments performed in triplicate.
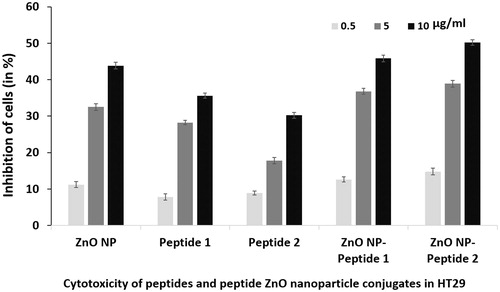
Further, on treating HT 29 cells with peptide ZnO nanoparticle conjugates, there was increased cytotoxicity to HT29 cells in comparison to either ZnO nanoparticle or peptide treatment alone (). Similarly, it has been shown that by loading doxorubicin, an anticancer drug into ZnO nanoparticles of 476 nm size and low PI, higher anticancer activity was observed in comparison to either ZnO nanoparticle or doxorubicin alone. It was hypothesized that the cytotoxic potential of the drug increased, due to the synergism produced by the combination of the anticancer action of both the drug and ZnO nanoparticle [Citation28]. Our study shows that nanoparticles functionalized with peptide have significant enhancement in killing cancer cells and are potential class of nano-drug for cancer therapeutics.
Characterization of peptides ZnO nanoparticle conjugate
The interaction between ZnO nanoparticle and peptide was studied using UV-Vis absorption spectrophotometer and DLS studies (). UV-Vis studies showed that ZnO nanoparticle showed absorption peak at 305 nm and Peptide 1 and 2 at 255 nm. However, the peptide nanoparticle colloidal solution showed a red shift indicating particle aggregation and interaction between the nanoparticles and peptides. The Peptide 1 and ZnO nanoparticle mixture showed absorption peaks at 255 and 330 nm, whereas the Peptide 2 and ZnO nanoparticle mixture showed absorption peaks at 305 and 355 nm. The DLS studies showed that ZnO nanoparticles had a zeta potential of +3.6 mV and polydispersity index (PDI) of 1.401. The zeta potential of Peptide 1 and 2 were found to be −17.5 and −7.8, respectively. The PDI of both the peptides were high i.e. 6.28 and 4.768 for Peptide 1 and 2, respectively. The zeta potential of Peptide 1-ZnO nanoparticle complex was +4.1 and its PDI reduced by 0.986. The low PDI indicates homogeneity in the dispersion state. In case of, Peptide 2-ZnO nanoparticle complex, zeta potential was found to be −5.0 and PDI was 11.16. In both the peptide nanoparticle conjugates, it could be observed that there was a decrease in negative potential, which probably facilitates better interaction between peptide nanoparticle complex and the cell membrane, resulting in higher toxicity to HT29 cancer cells. It could be hypothesized that, conjugation between ZnO nanoparticle and the hydrophobic peptides is by electrostatic interactions. In a similar study, it was shown that by coupling peptides to nanosilver, an enhanced anticancer activity was observed in MCF-7 breast cancer cell line. It was seen that a high similarity existed between zeta potential of nanosilver and cell membrane of MCF-7, resulting in high degree of repulsion between them leading to decreased cell-particle interactions. However, on coupling of nanosilver with cationic cell penetrating peptides, there was a decrease in the negative potential and reduction in the electrostatic barrier resulting in increased cell-particle interactions, higher uptake and subsequent toxicity to MCF-7 [Citation29]. Previously, it has been reported that surface modification of ZnO quantum dots with silver and PVP for preparation of nanoformulations, have shown promising anticancer activity in MCF7 breast cancer cell lines [Citation30].
Table 1. DLS analysis for zeta potential and polydispersity index of ZnO nanoparticle, peptide and nanoparticle peptide conjugate.
Conclusion
The metallic nanoparticles (gold, iron oxide and zinc oxide) have anticancer activity against colonic cancer cell line HT29. The nanoparticles studied have been in the range of <50 and <100 nm. These have shown activity in the concentrations of 0.5 to 50 µg/ml. Zinc oxide nanoparticles had significant anti-cancer activity in comparison to other tested nanoparticles. Zinc oxide nanoparticles on conjugation with peptides had a higher cytotoxic effect in HT29 colon cancer cells. Further, studies will focus on the molecular mechanism of cytotoxicity of ZnO nanoparticles in HT29 colonic cancer cells and to understand the interaction between the ZnO nanoparticles and the hydrophobic peptides for developing nanoparticle-peptide based anticancer agents against adenorectal carcinoma cell lines.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Vinardell MP, Mitjans M. Antitumor activities of metal oxide nanoparticles. Nanomaterials (Basel). 2015;5:1004–1021.
- Rasmussen JW, Martinez E, Louka P, et al. Zinc oxide nanoparticles for selective destruction of tumor cells and potential for drug delivery applications. Exp Opin Drug Deliv. 2010;7:1063–1077.
- Fernandes E, Ferreira JA, Peixoto A, et al. New trends in guided nanotherapies for digestive cancers: a systemic review. J Control Release. 2015;209:288–307.
- Caputo F, de Nicola M, Ghibelli L. Pharmacological potential of bioactive engineered nanomaterials. Biochem Pharmacol. 2014;92:112–130.
- Mody VV, Siwale R, Singh A, et al. Introduction to metallic nanoparticles. J Pharm Bioallied Sci. 2010;2:282–289.
- McCauley J, Zivanovic A, Skropeta D. Bioassays for anticancer activities. Methods Mol Biol. 2013;1055:191–205.
- He Y, Du Z, Ma S, et al. Biosynthesis, antibacterial activity and anticancer effects against prostate cancer (PC-3) cells of silver nanoparticles using Dimocarpus longan lour. Peel extract. Nanoscale Res Lett. 2016;11:300.
- Afsar T, Trembley JH, Salomon CE, et al. Growth inhibition and apoptosis in cancer cells induced by polyphenolic compounds of Acacia hydaspica: involvement of multiple signal transduction pathways. Sci Rep. 2016;6:23077.
- Liu K, Liu PC, Liu R, et al. Dual AO/EB staining to detect apoptosis in osteosarcoma cells compared with flow cytometry. Med Sci Monit Basic Res. 2015;21:15–20.
- Abruzzo A, Zuccheri G, Belluti F, et al. Chitosan nanoparticles for lipophilic anticancer drug delivery: development, characterization and in vitro studies on HT29 cancer cells. Colloids Surf B Biointerfaces. 2016;145:362–372.
- Laurent S, Dutz S, Häfeli UO, et al. Magnetic fluid hyperthermia: focus on superparamagnetic iron oxide nanoparticles. Adv Colloid Interface Sci. 2011;166:8–23.
- Orel V, Shevchenko A, Romanov A, et al. Magnetic properties and antitumor effect of nanocomplexes of iron oxide and doxorubicin. Nanomedicine. 2015;11:47–55.
- Van Landeghem FK, Maier-Hauff K, Jordan A, et al. Post-mortem studies in glioblastoma patients treated with thermotherapy using magnetic nanoparticles. Biomaterials. 2009;30:52–57.
- Silva AC, Oliveira TR, Mamani JB, et al. Application of hyperthermia induced by superparamagnetic iron oxide nanoparticles in glioma treatment. Int J Nanomed. 2011;6:591–603.
- Johannsen M, Thiesen B, Wust P, et al. Magnetic nanoparticle hyperthermia for prostate cancer. Int J Hyperthermia. 2010;26:790–795.
- Maier-Hauff K, Ulrich F, Nestler D, et al. Efficacy and safety of intratumoral thermotherapy using magnetic iron-oxide nanoparticles combined with external beam radiotherapy on patients with recurrent glioblastoma multiforme. J Neurooncol. 2011;103:317–324.
- Kamala Priya MR, Iyer PR. Anticancer studies of the synthesized gold nanoparticles against MCF 7 breast cancer cell lines. Appl Nanosci. 2015;5:443.
- Rajeshkumar S. Anticancer activity of eco-friendly gold nanoparticles against lung and liver cancer cells. J Genet Eng Biotechnol. 2016;14:195–202.
- Mukherjee P, Bhattacharya R, Wang P, et al. Antiangiogenic properties of gold nanoparticles. Clin Cancer Res. 2005;11:3530–3534.
- Ostrovsky S, Kazimirsky G, Gedanken A, et al. Selective cytotoxic effect of ZnO nanoparticles on glioma cells. Nano Res. 2009;2:882–890.
- Premanathan M, Karthikeyan K, Jeyasubramanian K, et al. Selective toxicity of ZnO nanoparticles toward Gram-positive bacteria and cancer cells by apoptosis through lipid peroxidation. Nanomedicine. 2011;7:184–192.
- Akhtar MJ, Ahamed M, Kumar S, et al. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine. 2012;7:845–857.
- Taccola L, Raffa V, Riggio C, et al. Zinc oxide nanoparticles as selective killers of proliferating cells. Int J Nanomedicine. 2011;6:1129–1140.
- Bisht G, Rayamajhi S. ZnO nanoparticles: a promising anticancer agent. Nanobiomedicine. 2016;3:9.
- Manke A, Wang L, Rojanasakul Y. Mechanisms of nanoparticle-induced oxidative stress and toxicity. BioMed Res Int. 2013;2013:15.
- Valko M, Rhodes CJ, Moncol J, et al. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40.
- Gaspar D, Veiga AS, Castanho MARB. From antimicrobial to anticancer peptides. A review. Front Microbiol. 2013;4:294.
- Sharma H, Kumar K, Choudhary C, et al. Development and characterization of metal oxide nanoparticles for the delivery of anticancer drug. Artif Cells Nanomed Biotechnol. 2016;44:672–679.
- Farkhani SM, Fard AA, Zakeri-Milani P, et al. Enhancing antitumor activity of silver nanoparticles by modification with cell-penetrating peptides. Artif Cells Nanomed Biotechnol. 2016;45:1029–1035.
- Abdolmohammadi MH, Fallahian F, Fakhroueian Z, et al. Application of new ZnO nanoformulation and Ag/Fe/ZnO nanocomposites as water-based nanofluids to consider in vitro cytotoxic effects against MCF-7 breast cancer cells. Artif Cells Nanomed Biotechnol. 2017. Forthcoming. [cited 2017 Feb 20]:[9 p.]. DOI: 10.1080/21691401.2017.1290643.