Abstract
In terms of the clinical effect of peripheral nerve injury repair, the biological degradable conduit 2 mm small gap tubulization is far better than the traditional epineurial or perineurium neurorrhaphy. The assumption of the bi-directional induction between the central system and the terminal effector during peripheral nerve regeneration is purposed and proved in clinical by our group. The surgical approach of transferring a portion of or the whole contralateral C7 nerve to repair a part of or the whole ipsilateral brachial plexus injury is clinically promoted, in which the most important idea and practice is to use the cone conduit designed by the group to repair thick nerves with fine nerves. Some of the patients suffering from cerebral palsy or cerebral haemorrhage and those who got cerebral infarction yet have not reached recovery after 3–6 months could regain some functions of the ipsilateral upper limb and improve the life quality by transfer of a portion of or the whole contralateral C7 nerve and connection by cone conduit.
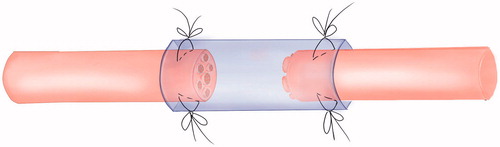
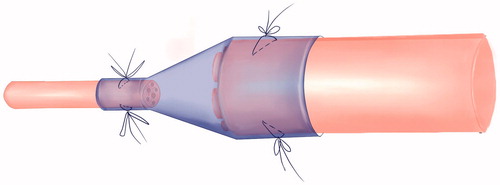
Central nerve and peripheral nerve injury could lead to the corresponding limbs dysfunction. The core of surgical repair after nerve injury is to preserve the greatest extent vitality of neuron, to maintain the effective connection between the central nervous system and peripheral nerve, and to obtain functional recovery of the target organs. It shows that the bidirectional induction and systematic functional remodelling between the central nervous system and peripheral target organs appear after nerve injury. The bi-directional interactive induce remodelling phenomenon between central nerve and peripheral target organs provides a new peripheral nerve repair method to cure limb paralysis which is caused by the central nerve injury [Citation1]. According to WHO data, the mortality rate of stroke was the first in China, and the prevalence rate was 719–745.6 people per 10 million. At present, there are more than 70 million patients suffering limb paralysis caused by stroke and cerebral palsy in China, and the number increased at the annual rate of 2 million. After 6 months physical rehabilitation exercise, patients with stroke and cerebral palsy still remain limp paralysis which the clinician have no way to deal with. The biological degradable conduit 2 mm small gap tubulization had been proved to be more suitable method for peripheral nerve mutilation compared with traditional epineurial neurorrhaphy [Citation2,Citation3] ( and ).
Figure 2. The cone conduit suture tubulization method for specific repair model of repairing thick nerve fibres with fine nerve fibres.
Sleeve suture method by tube has been translated to clinic. On this basis, a release controlled and biodegradable tube, which contains growth factors has been developed and has already been authorized by the Chinese National intellectual property office. These new methods provided us with new chance to comprehend the regeneration and remodelling process and new operation technique for other diseases [Citation4–6].
Directional control during the nerve regeneration
Effective outward nerve regeneration will not occur if the post-injury surgical repair is unsatisfactory because the proximal-distal connection is not re-established. Nerve regeneration needs induction from the distal nerve tissues. More precise regeneration may occur if the various nerve fibres involved are given the appropriate environment [Citation7]. Nerve tissue induction allows the repaired nerve end to regenerate distally and reach the corresponding target organs [Citation4–6,Citation8].
Surgical repair can modify the corresponding relationship between the distal/proximal nerves and the target organs
Patients may present with injuries to multiple peripheral nerves resulting in just distal nerve fibre A and proximal nerve fibre B remaining. Surgeons may have to connect them to achieve any repair. Some examples include the proximal radial nerve used to repair the distal ulnar nerve or the proximal median nerve to the distal ulnar nerves [Citation9–11], the proximal anterior interosseous nerve to the distal ulnar nerves [Citation12], the proximal median nerve to the distal radial nerves or the combined anterior/posterior interosseous nerves to the ulnar nerves [Citation13–15]. A common example of surgery is to use the healthy contralateral C7 nerve root to repair partial or complete branchial plexus injuries. The outcomes showed that the repaired nerves presented various degrees of recovery without affecting the upper limb from which with C7 nerve root was removed [Citation16]. Therefore, surgical repair of the injured nerves, and the subsequent regeneration of function, is considered as train track replacement. This theory has long been ignored. Recent animal models have confirmed that proximal tibial nerves could simultaneously repair distal tibial nerves and common peroneal nerves because the proximal tibial nerves could grow into both to achieve the functional recovery of their target organs.
Peripheral target organs reversely induce the structural and functional remodelling of various levels of the proximal peripheral nervous system, spinal cord and upper central nerves according to their own functional needs
The various peripheral nerve transfer operations derived from the surgical repair mentioned above, such as the use of a proportion of or the whole C7 to contra-laterally repair the C5–8 branchial plexus injuries, allowed the patient’s normal C7 to contralaterally control elbow flexion, elbow extension and wrist extension after a period of rehabilitation exercise [Citation16]. The limb functions on the injured side recovered and the upper limb functions were almost unaffected, even if the C7 input was removed [Citation17]. MRI results of the cerebral motor cortex showed that: the motor cortex activation region on the healthy side increased with stronger signals, whereas the corresponding region on the injured side decreased with weaker signals [Citation18]. These results indicated that changes in the peripheral target organs on the injured side induced the positioning and differentiation at various levels up to the central nerves that originally controlled the normal C7 effectors, causing the establishment of new connections between the peripheral nerves and the injured target organs and the consequent effective re-control of the peripheral target organs [Citation2]. The tibial nerves that originally controlled the flexion muscles could effectively control the back extensor muscle group with the induction of those muscles. Those muscles were originally controlled by the distal common peroneal nerves. In the model of using ulnar nerves to repair ulnar nerves and musculocutaneous nerves, the proximal end of the ulnar nerves could simultaneously grow into the distal ulnar nerves and the distal musculocutaneous nerves to achieve partial functional recovery of the corresponding target organs. Those clinical cases and animal models all showed that the nerve cross transfer after the surgical repair allows the controlling nerves to function as the controlled effector. This suggests there is structural and functional remodelling of the corresponding proximal nerves.
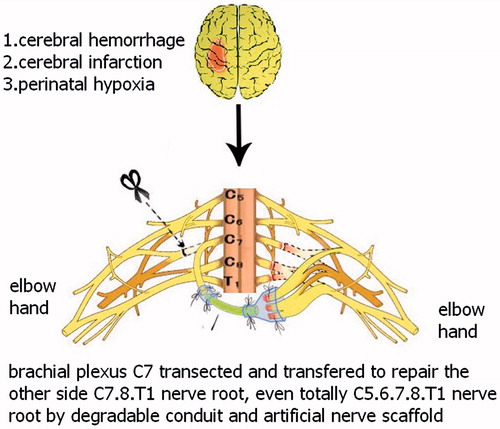
Currently, China has over 70 million patients with limb paralysis resulting from stroke or cerebral palsy, and the patient number increases by 20 million each year. If patients with the limb paralysis, resulting from stroke or cerebral palsy, do not recover after systematic rehabilitation exercise, there is little more doctors can do to help. Peripheral nerve transposition is a new strategy for repairing the injured nerves (brachial plexus injury) and treating the patient with limb paralysis resulting from the stroke or cerebral palsy. Unilateral brachial plexus avulsion can repair upper limb function by transposing a portion of or the whole contralateral C7 nerve. Similarly, unilateral paralysis resulting from the stroke (cerebral haemorrhage or cerebral infarction) or cerebral palsy (perinatal hypoxia) can be treated using transposition of a portion of or the whole contralateral C7 nerve, which would repair the whole brachial plexus at the dysfunctional upper limb side, with potentially partial recovery of the functions of shoulder, elbow, wrist and hands ( and ).
Figure 3. This new operation technique for brachial plexus injury to restore the function of distal elbow and hand.
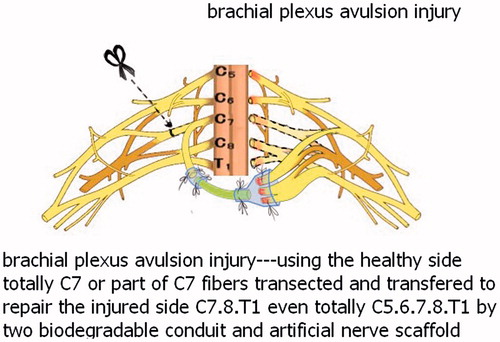
Figure 4. This new operation technique for cerebral haemorrhage or infarction cerebral palsy to restore the function of distal elbow and hand.
Based on the application of biodegradable conduit 2 mm small gap tubulization for peripheral nerve mutilation and the deeply comprehension of bi-directional induction and systematic remodelling between central system and target organ, this new technique has brought new recovery hope for the patients with limb paralysis [Citation3]. This surgical approach has been successfully applied to 20 patients in clinic and has a greater extent of improvement in the life quality of patients [Citation19]. This continuous research had accomplished translation from basic research to clinical application.
Disclosure statement
The authors report no declarations of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Jiang BG, Feng Yin X, Xun Zhang P, et al. Hypothesis of peripheral nerve regeneration induced by terminal effectors. Artif Cells Nanomed Biotechnol. 2014;42:92–94.
- Peixun Z, Na H, Baoguo J. Repair, regeneration and remodeling of injured peripheral nerves. Neural Regen Res. 2015;10:1790–1791.
- Zhang P, Han N, Wang T, et al. Biodegradable conduit small gap tubulization for peripheral nerve mutilation: a substitute for traditional epineurial neurorrhaphy. Int J Med Sci. 2013;10:171–175.
- Zhang PX, Li-Ya A, Kou YH. Biological conduit small gap sleeve bridging method for peripheral nerve injury: regeneration law of nerve fibers in the conduit. Neural Regen Res. 2015;10:71–78.
- Zhang P, Yin X, Kou Y, et al. Neural regeneration after peripheral nerve injury repair is a system remodelling process of interaction between nerves and terminal effector. Neural Regen Res. 2015;10:52.
- Zhang P, Yin X, Kou Y, et al. Peripheral nerve mutilation through biodegradable conduit small gap tubulisation: a multicentre randomised trial. Lancet. 2015;386:S40.
- Jiang BG, Yin XF, Zhang DY, et al. Maximum number of collaterals developed by one axon during peripheral nerve regeneration and the influence of that number on reinnervation effects. Eur Neurol. 2007;58:12–20.
- Zhang P, Kou Y, Yin X, et al. The experimental research of nerve fibers compensation amplification innervation of ulnar nerve and musculocutaneous nerve in rhesus monkeys. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:39–43.
- Brown JM, Yee A, Mackinnon SE. Distal median to ulnar nerve transfers to restore ulnar motor and sensory function within the hand: technical nuances. Neurosurgery. 2009;65:966–977.
- Mackinnon SE, Roque B, Tung TH. Median to radial nerve transfer for treatment of radial nerve palsy. Case report. J Neurosurg. 2007;107:666–671.
- Phillips BZ, Franco MJ, Yee A, et al. Direct radial to ulnar nerve transfer to restore intrinsic muscle function in combined proximal median and ulnar nerve injury: case report and surgical technique. J Hand Surg Am. 2014;39:1358–1362.
- Haase SC, Chung KC. Anterior interosseous nerve transfer to the motor branch of the ulnar nerve for high ulnar nerve injuries. Ann Plast Surg. 2002;49:285–290.
- Battiston B, Lanzetta M. Reconstruction of high ulnar nerve lesions by distal double median to ulnar nerve transfer. J Hand Surg Am. 1999;24:1185–1191.
- Novak CB, Mackinnon SE. Distal anterior interosseous nerve transfer to the deep motor branch of the ulnar nerve for reconstruction of high ulnar nerve injuries. J Reconstr Microsurg. 2002;18:459–464.
- Ustün ME, Oğün TC, Büyükmumcu M, et al. Selective restoration of motor function in the ulnar nerve by transfer of the anterior interosseous nerve. An anatomical feasibility study. J Bone Joint Surg Am. 2001;83-A:549–552.
- Gu YD, Chen DS, Zhang GM, et al. Long-term functional results of contralateral C7 transfer. J Reconstr Microsurg. 1998;14:57–59.
- Lu J, Xu J, Xu W, et al. Combined nerve transfers for repair of the upper brachial plexus injuries through a posterior approach. Microsurgery. 2012;32:111–117.
- Xu W, Lu J, Xu J, et al. Full-length ulnar nerve harvest by means of endoscopy for contralateral C7 nerve root transfer in the treatment of brachial plexus injuries. Plast Reconstr Surg. 2006;118:689–693.
- Hua XY, Qiu YQ, Li T, et al. Contralateral peripheral neurotization for hemiplegic upper extremity after central neurologic injury. Neurosurgery. 2015;76(2):187–195.