Abstract
Background: Ovarian carcinoma is the most lethal cancer among all gynaecological malignancies. One of the most chemotherapy drugs used for ovarian cancer is paclitaxel which induces apoptosis. Paclitaxel has been used for many years. Similar to the most cancers this responds to chemotherapy initially but in a long run, drug resistance happens which fails the treatment procedure. Combination of chemotherapy drugs has been suggested to deal with this issue. Silibinin, a plant extraction, has been used from ancient time in traditional medicine and identified to have powerful antioxidant activity.
Aim: The aim of this study was to examine the effect of paclitaxel and silibinin combination on SKOV-3 cancer cell line.
Materials and methods: The human epithelial ovarian cancer cell line, SKOV-3, was cultured and treated with paclitaxel, silibinin and paclitaxel plus silibinin for 48 hours. MTT assay was carried out to determine cell viability. For apoptotic process, we used real-time PCR to study P53 and P21 genes expression after drug treatment and network analysis was performed using Pathway Studio web tool (Elsevier).
Results: Cell growth was inhibited considerably (p < .05) by combination of paclitaxel and silibinin after 48 hours of treatment. Also silibinin and paclitaxel combination induced apoptosis in SKOV-3 cells. Expression analysis by real-time PCR showed the significant up-regulation of two tumour suppressor genes, P53 and P21 in response to combination of silibinin and paclitaxel. In addition, computational network analysis demonstrated the crosstalk between paclitaxel, silibinin and ovarian cancer.
Conclusions: Our results showed that combination of chemotherapy drugs of silibinin and paclitaxel can be more efficient in treatment of ovarian cancer cells.
Introduction
Ovarian cancer is one of the leading causes of mortality in the universe, with 238,700 new cases and more than 150,000 estimated deaths worldwide [Citation1]. It has been considered that more than 90% of ovarian cancers arise from epithelial cells [Citation2]. Through the chemotherapy and surgical procedures, the rate of survival is poor, with 45% in 5 years [Citation3]. Over the half of the patients with cancer receive chemotherapy treatments. Paclitaxel, a Pacific yew tree isolate, is from taxan family and one of chemotherapy drug that has been used against various types of human cancers [Citation4–6]. This agent targets tubulin in cells and block cell cycle growth. Thus, it has developed into imperative component of cancer treatment in both early and advanced stages [Citation4,Citation7,Citation8]; however, paclitaxel and taxane therapies have side effects such as skin reactions, myalgias, arthralgias and peripheral [Citation8]. Subsequently, combination treatment could reduce side effects of paclitaxel and improve clinical endpoints.
Silibinin, a natural flavonolignan, is used for liver diseases in clinical trial around the world [Citation9]. Furthermore, several studies have shown silibinin has anticancer effects in a wide spectrum of human cancers [Citation9–12]. Silibinin is free from adverse effects and has nontoxic consumption. Silibinin induces apoptosis in various human cancer cells such as colon, prostate, lung and bladder [Citation13–16]. Consequently, paclitaxel and silibinin combination could eliminate paclitaxel side effects. In this study, we have explored the toxicity effects of silibinin and paclitaxel on human ovarian cancer cell line (SKOV-3). Then, the mechanisms of silibinin and paclitaxel combination-induced apoptosis of SKOV-3 cells were investigated by expression analysis of two tumour suppressor genes, P53 (TP53: tumour protein P53) and P21 (CDKN1A: cyclin dependent kinase inhibitor 1A) as well as network analysis.
Materials and methods
Cell culture
SKOV-3, human ovarian cancer cell line was purchased from Pasteur Institute Cell Bank of Iran. The cells were grown as monolayer in 25 cm2 flask (Orange Scientific, Braine-l'Alleud, Belgium) with culture medium (RPMI-1640) (Gibgo, Invitrogen, Carlsbad, CA) supplemented with 10% FBS (foetal bovine serum) (Gibgo, Invitrogen, Carlsbad, CA), streptomycin (100 μg/ml), penicillin (100 units/ml) (Sigma, St. Louis, MO) and cultured under standard condition at 37 °C in a 5% humidified CO2 incubator. The medium was replaced twice a week.
MTT assay
The effect of paclitaxel and silibinin inhibition on SKOV-3 cell proliferation was determined by MTT assay. The cells were seeded in 96-well tissue culture plates at a density of 2500 cells per well and incubated at 37 °C in 5% CO2 humidified incubator. After reaching 50% confluence, the cells were treated with paclitaxel and silibinin, and then incubated for 48 h. After that, the upper medium was removed and 0.5 mg/ml of MTT (Sigma, St. Louis, MO) solution (PBS and medium) was added to each well and incubated for 4 h in incubator. Then, the medium was removed and the blue formazan crystals were dissolved in 100 μl of DMSO (Merck, Kenilworth, NJ) and 25 μl Sorensen's glycine buffer. The absorbance was read in a microplate reader (Biotek, Winooski, VT, model Elx808) at 570 nm. Each experiment was repeated in triplicate, and results were reported as means ± SEM.
RNA extraction and c-DNA synthesis
Forty eight hours after treatment, medium was removed from monolayer cancer cells and scrapped in 1 ml RNAX-PLUS (Cinagene, Sima-Ye-Iran, Iran). Total RNA was extracted from samples using Cina gene Kit based on the manufacturer’s instruction (RNX-Plus Solution, Sina Clon, Tehran, Iran). After purification and quantification, RNA was determined by measuring optical density at 260 and 280 nm by nanodrop (NanoDrop-ND-1000). The cDNA synthesis was performing by cDNA synthase kit (Thermo, Waltham, MA).
Real-time PCR
To characterize the relative expressions of P53 and P21 genes, we used real-time PCR with Rotor-Gene 6000 Real-time PCR Detection System (Corbett, UK). For various mRNA, first-strand cDNA was amplified as described before [Citation17,Citation18] using P53 and P21 primers in . β-Actin was used as housekeeping gene. Each experiment was repeated in duplicated format, and results were expressed as means ± SEM.
Table 1. Primers used for real time PCR genes primer sequence (5′–3′).
Network analysis
Big interaction web database of Pathway Studio Mammalian, ChemEffect, DiseaseFXAs (Elsevier) was used for interaction network analysis of paclitaxel, silibinin and their crosstalk with ovarian cancer. Pathway Studio employs text mining through natural language processing to mine the body of literature for extractions of interactions, pathways, functions, etc. [Citation19–21]. In particular, the 2017 web database contains 107,771 entities of Small Molecules, Chemicals and Drugs and 136,226 proteins/genes which provides one of the most enriched datasets of Small Molecules and Drugs interactomes. The total number of relations deposited in 2017 database is 7,211,502 which updates weekly. In addition, Pathway Studio imports the knowledge from big databases such as Gene Ontology Consortium and MiRbase and integrates those information with extracted relationships of literature mining. Furthermore, this tool is enriched with different network construction algorithms such as “Common Binding Partner”, “Network Meta-analysis”, “Union Selected Subnetworks”, “Downstream Target Discovery”, “Upstream Regulator Discovery”, etc.
For interaction network analysis, silibinin and paclitaxel were retrieved from Pathway Studio Mammalian, ChemEffect, DiseaseFXAs database. Then, “Common Binding Partner” algorithm was called to find the proteins/genes which have binding interactions with silibinin and paclitaxel. Then, ovarian cancer was added to network and the interactome members of silibinin and paclitaxel which had crosstalk with ovarian cancer were identified [Citation22,Citation23].
Statistical analysis
Statistical analysis was performed with SPSS version 22.0 software (SPSS Inc., Chicago, IL) and ANOVA test was used to compare between groups. Data are represented mean ± SEM. The differences were considered significant when p < .05.
Results
Cell viability of SKOV-3 cells after treatment with silibinin and paclitaxel
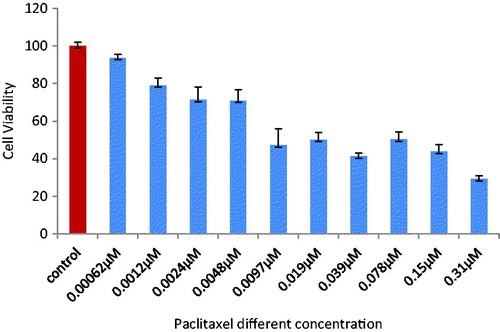
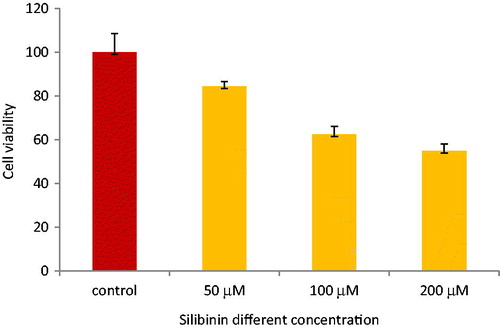
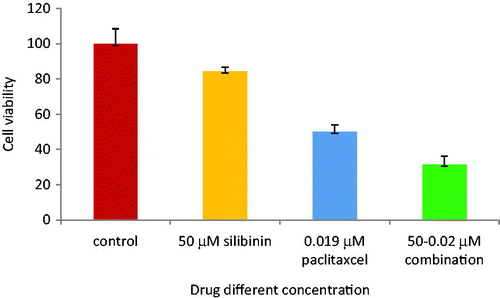
To determine the effect of combined silibinin and paclitaxel treatment on the SKOV-3 cell lines proliferation, MTT assay was used 48 h after drug treatment. The ovarian adenocarcinoma cell line (SKOV-3) was treated with silibinin and paclitaxel alone and their combinations. As shown in , cell growth was sharply inhibited by paclitaxel. The IC50 for paclitaxel treatment was 0.019 μm. illustrates that silibinin has IC50 of 200 μM. Paclitaxel (in its IC50) and silibinin (in low concentration) are used for combination. shows the combination form was more effective in the inhibition compared to each drug alone. As presented in , cell proliferation was decreased to 50% (p < .05) after 48 h of treatment.
Figure 3. Cell viability of SKOV-3 cells after 48 h of paclitaxel and silibinin combinational treatment.
Increased expression of tumour suppressor genes after treatment with silibinin and paclitaxel
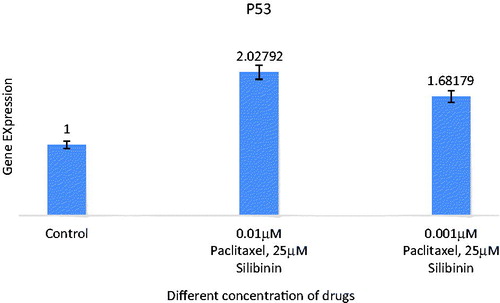
P53 and P21 genes expression in SKOV-3 cells were investigated via real-time PCR analysis ( and ). The genes Ct values were normalized against mRNA level of β-actin as a housekeeping gene and the relative expression for each group was measured. In brief, P53 and P21 genes expression at different concentration of paclitaxel and silibinin combination after 48 h showed significant difference compared to untreated cells as control. This could be associated with the effect of paclitaxel and silibinin combination on the apoptosis.
Figure 4. Expression analysis of combination of silibinin and paclitaxel on expression of P21 (CDKN1A) tumour suppressive gene by real time PCR. All data were normalized to β-actin gene expression: results related to increase in P21 gene expression with paclitaxel and silibinin combination treatment after 48 h.
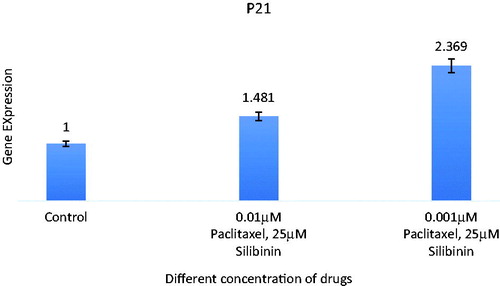
Figure 5. Expression analysis of combination of silibinin and paclitaxel on expression of P53 (TP53) tumour suppressive gene by real time PCR analysis. All data were normalized to β-actin gene expression: results related to increase in P53 gene expression with paclitaxel and silibinin combination treatment after 48 h.
Crosstalk between ovarian cancer with silibinin and paclitaxel protein interactome
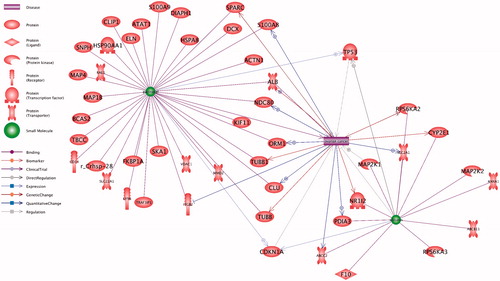
The protein interactome of paclitaxel and silibinin and its crosstalk with ovarian cancer is presented at . The underlying relationships of network and their references are provided at Supplementary 1. As it can be inferred from , combination of paclitaxel with silibinin targets more ovarian cancer associated genes. As example, S100A8, ALB, NDC80, ORM1 and CLU upregulate in ovarian cancer that can be targeted by paclitaxel (, Supplementary 1). On the other hand, silibinin can target the upregulation of SLC2A1, PDIA3 and MAP2K1 in ovarian cancer.
Discussion
In this study, we describe the paclitaxel, silibinin and their combination effects on the ovarian cancer cell line (SKOV0–3). It has been revealed that paclitaxel as anti-microtubule agent prevents cell division and induces apoptosis [Citation24–27] and can be used in clinical trial as anti-tumour agent. Our findings confirm that paclitaxel inhibits cell growth in ovarian cancer cell line with different concentration. On the other hand, the side effect of paclitaxel is definitely disturbing potions of the life quality of patients who receive paclitaxel as chemotherapy treatment. Silymarin and its main component, silibinin, are obtained from the medicinal plant Silybum marianum (milk thistle) and have conventionally been applied for the treatment of liver diseases. Recently, these agents have also been presented to have major anti-neoplastic effects in a diversity of cancer models such as skin, breast, colon, prostate and kidney carcinomas [Citation28]. Recently, we showed anticancer properties of other medicinal plant in ovarian cancer cell line (SKOV-3) and its effect on apoptotic genes [Citation29], so we considered silibinin as an another antitumour agent and nontoxic products with apoptosis properties [Citation13,Citation14] which could reduce paclitaxel side effects. Consequently, we used paclitaxel and silibinin combination for human ovarian cancer cell line to show drug combination is more efficient than separate treatment. Our results strongly suggest that silibinin inhibits cell proliferation in SKOV-3 cells and its combination with paclitaxel is more effective than paclitaxel alone. Also, the P53 and P21 expressions as apoptosis gens, were studied. The results presented that investigated genes were over expressed in paclitaxel and silibinin combination treated cells compared with non-treated group. The previous studies have confirmed that silibinin has potential sensitivity for apoptosis induced by paclitaxel and other antitumour agents [Citation30–33]. In addition, interactome protein analysis of silibinin and paclitaxel in relation to ovarian cancer showed that combination of paclitaxel with silibinin targets more players in ovarian cancer. Moreover, various studies illustrated nanoparticle has a high drug-loading efficiency and could be worthy candidate for drug and gene delivery [Citation34–36], therefore, we suggest the usage of nanoparticles with Silibinin and paclitaxel to achieve excellent result for anticancer effects.
Conclusions
Paclitaxel and silibinin combination is more efficient than sole application of paclitaxel or silibinin in treatment of human ovarian cancer cells.
Supplementary_material.csv
Download Comma-Separated Values File (88.3 KB)Acknowledgements
The authors thank School of Advanced Medical Science, Tabriz University of Medical Sciences, Tabriz, Iran.
Disclosure statement
The authors declare that they have no competing interests.
References
- Torre LA, Bray F, Siegel RL, et al. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65:87–108.
- Romero I, Bast RC Jr. Minireview: human ovarian cancer: biology, current management, and paths to personalizing therapy. Endocrinology. 2012;153:1593–1602.
- Siegel R, Ward E, Brawley O, et al. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: Cancer J Clin. 2011;61:212–236.
- Priyadarshini K, Keerthi Aparajitha U. Paclitaxel against cancer: a short review. Med Chem. 2012;2:139–141.
- Park S, Kang S, Chen X, et al. Tumor suppression via paclitaxel-loaded drug carriers that target inflammation marker upregulated in tumor vasculature and macrophages. Biomaterials. 2013;34:598–605.
- Ganguly A, Yang H, Cabral F. Paclitaxel-dependent cell lines reveal a novel drug activity. Mol Cancer Ther. 2010;9:2914–2923.
- Sperone P, Gorzegno G, Berruti A, et al. Reversible pancytopenia caused by oral letrozole assumption in a patient with recurrent breast cancer. JCO. 2002;20:3747–3748.
- Cella D, Peterman A, Hudgens S, et al. Measuring the side effects of taxane therapy in oncology: the functional assessment of cancer therapy-taxane (FACT-taxane). Cancer. 2003;98:822–831.
- Saller R, Meier R, Brignoli R. The use of silymarin in the treatment of liver diseases. Drugs. 2001;61:2035–2063.
- Singh RP, Dhanalakshmi S, Agarwal C, et al. Silibinin strongly inhibits growth and survival of human endothelial cells via cell cycle arrest and downregulation of survivin, Akt and NF-kappaB: implications for angioprevention and antiangiogenic therapy. Oncogene. 2005;24:1188–1202.
- Singh RP, Gu M, Agarwal R. Silibinin inhibits colorectal cancer growth by inhibiting tumor cell proliferation and angiogenesis. Cancer Res. 2008;68:2043–2050.
- Bang CI, Paik SY, Sun DI, et al. Cell growth inhibition and down-regulation of survivin by silibinin in a laryngeal squamous cell carcinoma cell line. Ann Otol Rhinol Laryngol. 2008;117:781–785.
- Agarwal C, Singh RP, Dhanalakshmi S, et al. Silibinin upregulates the expression of cyclin-dependent kinase inhibitors and causes cell cycle arrest and apoptosis in human colon carcinoma HT-29 cells. Oncogene. 2003;22:8271–8282.
- Singh RP, Sharma G, Dhanalakshmi S, et al. Suppression of advanced human prostate tumor growth in athymic mice by silibinin feeding is associated with reduced cell proliferation, increased apoptosis, and inhibition of angiogenesis. Cancer Epidemiol Biomark Prevent. 2003;12:933–939.
- Sharma G, Singh RP, Chan DC, et al. Silibinin induces growth inhibition and apoptotic cell death in human lung carcinoma cells. Anticancer Res. 2003;23:2649–2655.
- Tyagi A, Agarwal C, Harrison G, et al. Silibinin causes cell cycle arrest and apoptosis in human bladder transitional cell carcinoma cells by regulating CDKI-CDK-cyclin cascade, and caspase 3 and PARP cleavages. Carcinogenesis. 2004;25:1711–1720.
- Pashaiasl M, Khodadadi K, Richings NM, et al. Cryopreservation and long-term maintenance of bovine embryo-derived cell lines. Reprod Fertil Dev. 2013;25:707–718.
- Gholizadeh-Ghaleh Aziz S, Pashaei-Asl F, Fardyazar Z, et al. Isolation, characterization, cryopreservation of human amniotic stem cells and differentiation to osteogenic and adipogenic cells. PLoS One. 2016;11:e0158281.
- Novichkova S, Egorov S, Daraselia N. MedScan, a natural language processing engine for MEDLINE abstracts. Bioinformatics (Oxford, England). 2003;19:1699–1706.
- Nikitin A, Egorov S, Daraselia N, et al. Pathway studio–the analysis and navigation of molecular networks. Bioinformatics (Oxford, England). 2003;19:2155–2157.
- Yuryev A, Kotelnikova E, Daraselia N. Ariadne's chemeffect and pathway studio knowledge base. Expert Opin Drug Discov. 2009;4:1307–1318.
- Pashaiasl M, Khodadadi K, Kayvanjoo AH, et al. Unravelling evolution of Nanog, the key transcription factor involved in self-renewal of undifferentiated embryonic stem cells, by pattern recognition in nucleotide and tandem repeats characteristics. Gene. 2016;578:194–204.
- Pashaiasl M, Ebrahimi M, Ebrahimie E. Identification of the key regulating genes of diminished ovarian reserve (DOR) by network and gene ontology analysis. Mol Biol Rep. 2016;43:923–937.
- Jordan MA, Wendell K, Gardiner S, et al. Mitotic block induced in HeLa cells by low concentrations of paclitaxel (Taxol) results in abnormal mitotic exit and apoptotic cell death. Cancer Res. 1996;56:816–825.
- Milross CG, Mason KA, Hunter NR, et al. Relationship of mitotic arrest and apoptosis to antitumor effect of paclitaxel. J Natl Cancer Inst. 1996;88:1308–1314.
- Fan W. Possible mechanisms of paclitaxel-induced apoptosis. Biochem Pharmacol. 1999;57:1215–1221.
- Ling YH, Yang Y, Tornos C, et al. Paclitaxel-induced apoptosis is associated with expression and activation of c-Mos gene product in human ovarian carcinoma SKOV3 cells. Cancer Res. 1998;58:3633–3640.
- Cheung CW, Gibbons N, Johnson DW, et al. Silibinin-a promising new treatment for cancer. Anticancer Agents Med Chem. 2010;10:186–195.
- Pashaei-Asl R, Pashaei-Asl F, et al. The inhibitory effect of ginger extract on ovarian cancer cell line. Appl Syst Biol. 2017;7:241–249.
- Zhou L, Liu P, Chen B, et al. Silibinin restores paclitaxel sensitivity to paclitaxel-resistant human ovarian carcinoma cells. Anticancer Res. 2008;28:1119–1127.
- Deep G, Singh RP, Agarwal C, et al. Silymarin and silibinin cause G1 and G2-M cell cycle arrest via distinct circuitries in human prostate cancer PC3 cells: a comparison of flavanone silibinin with flavanolignan mixture silymarin. Oncogene. 2006;25:1053–1069.
- Tyagi AK, Agarwal C, Chan DC, et al. Synergistic anti-cancer effects of silibinin with conventional cytotoxic agents doxorubicin, cisplatin and carboplatin against human breast carcinoma MCF-7 and MDA-MB468 cells. Oncology Rep. 2004;11:493–499.
- Ho BY, Lin CH, Apaya MK, et al. Silibinin and paclitaxel cotreatment significantly suppress the activity and lung metastasis of triple negative 4T1 mammary tumor cell in mice. J Tradition Complement Med. 2012;2:301–311.
- Gholizadeh-Ghaleh Aziz S, Fathi E, Rahmati-Yamchi M, et al. An update clinical application of amniotic fluid-derived stem cells (AFSCs) in cancer cell therapy and tissue engineering. Artif Cells Nanomed Biotechnol. 2017;45:765–774.
- Motaali S, Pashaeiasl M, Akbarzadeh A, et al. Synthesis and characterization of smart N-isopropylacrylamide-based magnetic nanocomposites containing doxorubicin anti-cancer drug. Artif Cells Nanomed Biotechnol. 2017;45:560–567.
- Pashaei-Asl R, Khodadadi K, Pashaei-Asl F, et al. Legionella pneumophila and dendrimers-mediated antisense therapy. Adv Pharm Bull. 2017;7:179–187.