Abstract
Silver nanoparticles (AgNPs) were biosynthesized using Bauhinia variegata flower extract (BVFE). The BVF-AgNPs was found to be spherical shaped with the size of 5–15 nm. The phytoconstituents analysis and FTIR spectrum indicated that bioactive compounds like, phenols, flavonoids, benzophenones, nitro compounds, aromatics and aliphatic amines from BVFE might absorb on the surface of BVF-AgNPs. The synthesized BVF-AgNPs showed potent antioxidant property and α-amylase enzyme activity inhibition. The IC50 value of BVF-AgNPs was found to be 4.64 and 16.6 µg/ml for DPPH and ferric reducing power assay, respectively. The IC50 value of BVF-AgNPs for α-amylase inhibition was found to be 38 µg/ml. The Ki value of BVF-AgNPs for α-amylase inhibitory effect was found to be 21 µg/ml with the non-competitive mode of inhibition. These results suggest that BVF-AgNPs might be an effective nano-drug to treat diabetic conditions.
Introduction
Nanosilver synthesis is an emerging research topic in the field of biomedical and environmental applications [Citation1]. Silver nanoparticles (AgNPs) exhibit strong biomedical properties such as antimicrobial, anticancer, antidiabetic, antioxidant and anti-inflammatory activities due to their large surface to volume ratios, high dispersion and crystallographic surface structure [Citation2]. The biosynthesis of AgNPs is a promising alternative to physicochemical methods, which limit the usage of energy ingesting, hazardous and toxic substances. Nowadays, giant literature concerning the biosynthesis of AgNPs using bacteria, fungi, algae and plant extracts has been well documented [Citation3–6]. In particular, the enriched source of plant extract has a considerable attention for the nanoparticles biosynthesis, because they act as both capping and stabilizing agent. Furthermore, it involves the reduction of silver ions (Ag+) to silver atoms (Ag0), which leads to colloidal nanoparticles.
Interestingly, the plant-based biosynthesis of AgNPs is an eco-friendly, biocompatible, and also easiest way in the nanotechnology approach [Citation7–11]. Flowers are enriched with carotenoid pigments and active biomolecules, hence flower-mediated AgNPs biosynthesis are considered advantageous. Bauhinia variegata L. is a species of flowering plant, which belongs to Leguminosae family. Traditionally, B. variegata flower and flower buds are used as astringent, antibacterial, anthelmintic, liver tonic, anti-leprotic and in the treatment of wounds, oedema, eye disease, skin diseases, dysmenorrheal, ulcers, snake bite, haemorrhoids and piles [Citation12,Citation13]. B. variegata flower contains anthocyanins like cynidin-3-glucoside, malvidin-3-glucoside, malvidin-3-diglucoside, peonidin-3-glucoside and peonidin-3-diglucoside [Citation14,Citation15]. Anthocyanins have the bioactive potential to protect against DNA cleavage, anti-inflammatory activity and antioxidant activity to counteract lipid peroxidation [Citation16].
Alpha (α)-amylase is the major enzyme of the pancreas which plays a key role in the breakdown of oligosaccharides to monosaccharides [Citation17]. Increased level of α-amylase activity will lead to hyperglycaemia. In turn, hyperglycaemia is associated with cardiovascular disease, oxidative injury and kidney disorders [Citation18]. Furthermore, the α-amylase activity is directly associated with type II diabetes. In addition, oxidative stress condition plays an important role in the pathogenesis of type II diabetes [Citation19]. Acarbose and miglitol are the currently used α-amylase inhibitors in clinics for short-term glycaemic control. However, these inhibitors are non-specific and target different glycosidases [Citation20]. Also, these inhibitors produce serious side effects that limit their use as a therapeutic drug. Hence, identifying a nature-based therapeutic strategy to overcome diabetes is an important area of investigation.
In this study, B. variegata flower extract (BVFE)-mediated AgNPs (BVF-AgNPs) was biosynthesized and their physicochemical properties were characterized using UV–visible spectrophotometer, transmission electron microscopy (TEM), energy dispersive X-ray spectroscopy (EDX), Fourier transform infrared spectroscopy (FTIR), X-ray diffraction (XRD) and zeta potential analysis. Further, the biosynthesized BVF-AgNPs were analysed for its biomedical applications such as α-amylase inhibitor and antioxidant scavenger.
Materials and methods
Chemicals
Silver nitrate, α-amylase, 1, 1-diphenyl-2-picrylhydrazyl (DPPH) and 2, 4, 6-tripyridyl-s-triazine (TPTZ) were purchased from Sigma-Aldrich (St. Louis, MO). All other reagents used in this study were of analytical grade.
Flower extract
Fresh B. variegata flowers () were collected from a nearby area of Periyar University campus, Salem, Tamil Nadu, India. The voucher specimen UT/PUS/093 was identified at ABS Botanical Conservation Research and Training Centre, Salem, Tamil Nadu, India. After washing thoroughly with distilled water to remove the surface contaminants, about 10 g of fresh flower petals was boiled in 100 ml of distilled water for 5 h to obtain BVFE. The crude extract was filtered using Whatman No. 1 filter paper, and the filtrate was stored in the refrigerator until further use.
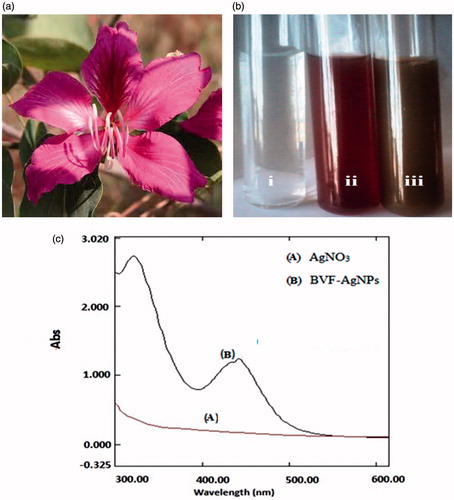
Figure 1. Illustration of the green synthesis of AgNPs using B. variegata flower extract. Descriptive graph showing (a) Bauhinia variegata flower; (b) formation of silver nanoparticles: (i) silver nitrate solution; (ii) B. variegata flower extract (BVFE) without silver nitrate; (iii) BVFE with silver nitrate solution; (c) UV–vis absorption spectrum.
Biosynthesis of AgNPs
About 1 ml of 1 mM silver nitrate solution was added drop-wise into 99 ml of BVFE and mixed well. The reaction mixture was incubated in dark at room temperature for 1 h. The formation of reddish-brown colour indicated the formation of BVF-AgNPs. After incubation, BVF-AgNPs were collected by centrifugation at 5000 rpm for 30 min. Excess of silver ions was removed by washing the BVF-AgNPs three times with deionized water followed by centrifugation at 5000 rpm for 30 min. The biosynthesis of BVF-AgNPs was confirmed using UV–visible spectrophotometer (Shimadzu UV 1800, Torrance, CA) by scanning in the wavelength ranging from 200 to 500 nm with distilled water as a reference.
Phytochemical analysis
Phytochemical screening of both BVFE and BVF-AgNPs was carried out qualitatively for the presence of phenols, flavonoids, glycosides, alkaloids, terpenes, tannins, saponins and steroids according to the method described earlier [Citation21]. Further, the total flavonoids in BVFE and BVF-AgNPs were determined according to the method of Yao et al. [Citation22] and expressed as mg per g.
Characterization of BVF-AgNPs
BVF-AgNPs were characterized using SEM/EDX (HITACHI, Model S-3400N, Tokyo, Japan), TEM (JEM 1101 JEOL, Tokyo, Japan), FTIR (Thermo Scientific, Waltham, MA) in the scanning range of 4000–500 cm−1, XRD (Bruker axs System, D8, Karlsruhe, Germany), and zeta potential using Nano-ZS instrument (Malvern Instruments Limited, Malvern, UK) in order to identify the size, shape, structure, elemental composition and functional group of the particles.
Antioxidant scavenging activity
Antioxidant activity was determined as the measure of DPPH scavenging activity [Citation23] and ferric reducing antioxidant activity (FRAP) [Citation24]. DPPH assay was carried out in the presence of 0.5–2.5 µg/ml concentration of either BVFE (corresponding to starting plant material) or BVF-AgNPs or ascorbic acid (standard). Briefly, reaction mixture consisted of 100 µM of DPPH and various concentrations of test samples in 2 ml of ethanol. The mixture of test compounds and ethanol served as blank. The control is DPPH solution devoid of any test samples. All the reaction mixtures were incubated in dark for 30 min. The decrease in absorbance was read at 517 nm using UV–vis spectrophotometer (UV-1800, Shimadzu, Torrance, CA).
FRAP assay was carried out in the presence of 10–50 µg/ml concentration of either BVFE (corresponding to starting plant material) or BVF-AgNPs or ascorbic acid (standard). Briefly, in the reaction mixture containing a different concentration of test samples, 20 mM FeCl3, 30 mM acetate buffer and 10 mM TPTZ were added and incubated for 30 min in the dark. After incubation, absorbance was read at 593 nm. The reaction mixture without test samples was used as a control.
Alpha-amylase enzyme activity and inhibitor studies
The α-amylase enzyme activity was determined according to the method of Apostolidis et al. [Citation25] with slight modification. Briefly, the assay mixture consists of 0.25 mg/ml of α-amylase, 0.5% of the starch in 1 ml of 20 mM sodium phosphate buffer (pH 6.9). The reaction mixture was incubated at 25 °C for 1 h and terminated by the addition of 1 ml of the DNS reagent [3, 5 dinitro salicylic acid (1%), Na-K tartrate (12%) in 0.4 M NaOH]. The reaction mixture was placed in a boiling water bath for 15 min and then cooled down to room temperature. The α-amylase activity was determined at 540 nm in UV–vis spectrophotometer.
The inhibition of α-amylase enzyme activity was determined in the presence of various concentrations (10–50 µg/ml) of BVFE or BVF-AgNPs (dissolved in 20 mM sodium phosphate buffer). The α-amylase enzyme was incubated with different concentration of BVFE and BVF-AgNPs at 4 °C for 30 min. After incubation, the α-amylase enzyme activity was determined as given above. A standard drug acarbose was used as positive control.
Kinetics of α-amylase inhibition
The kinetics of interaction between BVF-AgNPs and α-amylase was determined using Lineweaver and Burk [Citation26] double reciprocal plot and Dixon’s plot [Citation27]. α-Amylase enzyme inhibition was determined over a range of sucrose concentrations (0.25–1.5%) in the absence and in the presence of BVF-AgNPs (10–30 µg/ml). All the antioxidant assays and enzyme activity were carried out in three independent sets of duplicates and data were presented as mean ± SD.
Results and discussion
Biosynthesis and characterization of BVF-AgNPs
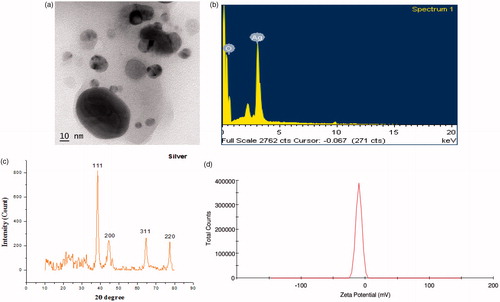
Biosynthesis of AgNPs using BVFE is illustrated in . In the presence of BVFE (magenta colour), the colourless AgNO3 solution is converted into a dark brown colour which indicates the reduction of Ag+ to Ag0. The synthesis of BVF-AgNPs was observed within 10 min and stabilized thereafter. The UV–vis spectra obtained from reaction mixture show absorption bands at 320 nm and 430 nm. The appearance of absorption peaks in this region corresponded to the wavelength of the surface plasmon resonance (SPR) of AgNPs and confirmed the formation of AgNPs. SPR depends on the: size and shape of metal NP; stabilizing molecules on the surface of the NPs; state of aggregation of the NPs; and the dielectric constant of the medium. According to Hartland’s calculation, the 50 nm AgNPs would have an extinction peak at 410 nm with yellow colour, while the 100 nm AgNPs would have a wide extinction peak from 350 to 700 nm, showing black colour [Citation28]. Hence it is clear that the dark brown colour of the BVF-AgNPs sample was due to the size of the aggregate rather than the single NP. Also, in accordance with these notions, experimental observation shows that the formation of SPR band at 430 nm corresponds to spherical shaped BVF-AgNPs with approximately 50 nm in size. In addition, the formation of SPR band at 320 nm might be due to aggregation of BVF-AgNPs with approximately 100 nm size. TEM images () confirmed this observation which shows that BVF-AgNPs are polydispersed with spherical in shape. Also, TEM image established that BVF-AgNPs are in nanoscale range with an average diameter of 5–15 nm and few large-scale aggregates. However, the BVF-AgNPs were spread over the surface without agglomeration. The elemental composition of BVF-AgNPs as EDX spectrum is shown in . The intense signal for silver at 3 keV and weaker signals for oxygen was observed. The presence of oxygen signal indicates the surface binding of biomolecules on the BVF-AgNPs which may be involved in the capping of AgNPs. The XRD analysis shows the presence of Bragg’s reflection peaks at 2θ values of 38.21°, 44.32°, 64.5° and 77.53°, respectively, corresponding to (111), (200), (220) and (311) planes of face-centred cubic crystal structure of metallic silver (). The observed sharp bands represent stable and crystalline nature of BVF-AgNPs with the presence of organic moiety. The interplanar spacing values and the lattice parameters matched well with Joint Committee on Powder Diffraction Standards values (JCPDS, silver file no. 04-0783). Scherrer’s formula estimated the average size of the BVF-AgNPs synthesized in the bioreduction process as 11 nm which is in accordance with the size observed in TEM analysis. The zeta potential value of biosynthesized BVF-AgNPs was found to be –10.1 mV with a peak area of 100% intensity as shown in , which signifies the stability of the synthesized NPs. The large negative potential value could be due to the capping of the bio-organic component present in the BVFE. The coating of AgNPs with the bio-organic component is responsible for electrostatic stabilization of BVF-AgNPs in the colloidal solution.
Figure 2. Structural characterization of BVF-AgNPs. (a) TEM image; (b) energy-dispersive X-ray spectrum; (c) XRD pattern showing crystallinity structure of AgNPs; (d) zeta potential stability measurement obtained for biosynthesized B. variegata flower extract-mediated AgNPs (BVF-AgNPs).
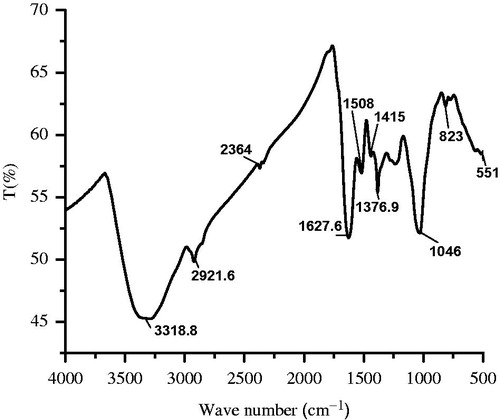
The FTIR spectrum illustrates the functional groups and their possible contribution with biosynthesized AgNPs (). The result reveals that the broad absorbance peaks at 3318 cm−1 correspond to O–H stretch (phenols). The peaks appeared at 2921 cm−1, 1627 cm−1, 1508 cm−1, 1415 cm−1, 1376 cm−1 and 1046 cm−1 represent CH3 stretch, NH2 bend (1° amines), benzene ring in the aromatic compound, CH3 in the aliphatic compound, C–N in primary amide and S=O stretch (sulphate), respectively. These results clearly indicate that bioactive compounds like, phenols, flavonoids, benzophenones, nitro compounds, aromatics and aliphatic amines from BVFE might be absorbed on the surface of BVF-AgNPs. These compounds must have acted as a stabilizing as well as a capping agent and limited the agglomeration of NPs during synthesis. The phytochemical screening result shows that the flavonoids, phenols, terpenoids, anthraquinone, protein, tannins, steroids and glycosides were predominantly present in the BVFE. However, phenolic and flavonoid compounds were present in the BVF-AgNPs. In particular, total flavonoid contents of the BVFE and BVF-AgNPs were found to be 36.4 ± 0.004 and 32.8 ± 0.002 mg/ml, respectively. The phytochemical analysis is in correlation with FTIR of BVF-AgNPs. It confirms that although different phytochemicals might have synergistically involved in the NPs formation, only phenolics and flavonoids had adsorbed on BVF-AgNPs. The presence of various flavonoids like quercitroside, isoquercitroside, rutoside, taxifoline rhamnoside, kaempferol-3-glucoside, myricetol glycoside, apigenin, cyanidin-3-glucoside, malvidin-3-glucoside, malvidin-3-diglucoside, peonidin-3-glucoside, peonidin-3-diglucoside, 3-galactoside and 3-rhamnoglucoside of kaempferol in flowers of B. variegata are reported by various studies [Citation29,Citation30]. Two mechanisms are postulated for NPs formation by flavonoid: (i) tautomeric transformations from the enol to the keto-form of flavonoids, which may release a reactive hydrogen atom that can reduce metal ions to form NPs; (ii) chelation of metal ions with their carbonyl groups or π-electrons (as reviewed in Makarov [Citation31]). These mechanisms not only explain the ability of flavonoids to reduce the metal ions into NPs but also the ability of flavonoids to be adsorbed onto the surface of a nascent NPs as in case found in BVF-AgNPs through FTIR analysis. In accordance with the results obtained in the present study, previous studies have also shown that isolated pure flavonoids like apigenin [Citation32], apiin (apigenin-7-apiosyl-glucoside) [Citation33] and quercetin [Citation34] were able to form various NPs as well as attached to the NPs through a carbonyl group.
Applications of BVF-AgNPs
The bioactive compounds coated BVF-AgNPs were analysed for its anti-diabetic activity in vitro. Various molecular pathways are targeted to culminate the progression of diabetes; however, two widely studied simple therapeutic strategies are the prevention of oxidative damage caused due to hyperglycaemia with free radical scavengers and inhibition of digestive enzymes such as α-amylase. Hence, BVF-AgNPs were analysed for the antioxidant activity and α-amylase inhibition property.
Antioxidant activity
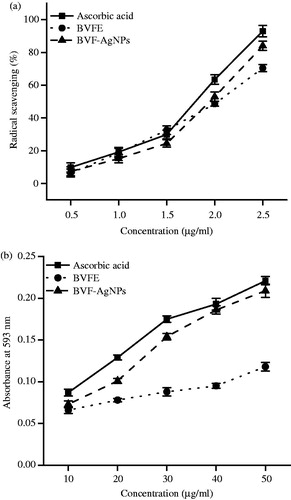
BVFE and BVF-AgNPs showed potent antioxidant activity which was comparable to that of standard ascorbic acid as shown in . The IC50 value of BVFE and BVF-AgNPs for DPPH scavenging activity was found to be 7.19 and 4.64 µg/ml, respectively. The IC50 value of BVFE and BVF-AgNPs for FRAP was found to be 20 and 16.6 µg/ml, respectively.
Figure 4. Antioxidant activities of BVFE and BVF-AgNPs. (a) DPPH radical scavenging activity and (b) FRAP activity in the presence of various concentration of B. variegata flower extract (BVFE) and B. variegata flower extract-mediated silver nanoparticles (BVF-AgNPs) synthesized are shown. BVF-AgNPs showed powerful antioxidant activity than BVFE. Results are expressed as mean ± SD (n = 6).
In vitro α-amylase inhibition assay
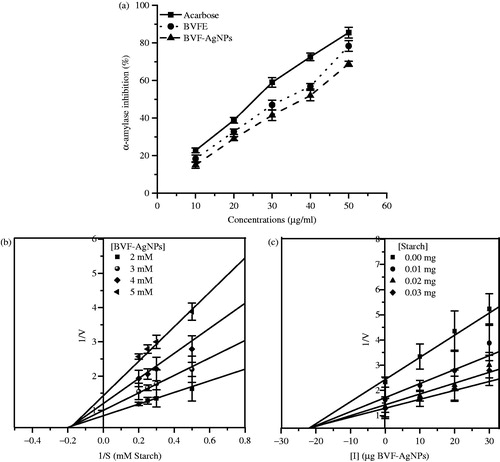
The BVFE and BVF-AgNPs showed a dose-dependent inhibition of α-amylase activity (). At 50 µg/ml concentration, BVFE, BVF-AgNPs and acarbose showed 78.4, 70.0 and 85.5% inhibition of α-amylase activity, respectively. The IC50 value of BVFE and BVF-AgNPs was found to be 33 and 38 µg/ml, respectively. The Lineweaver–Burk (LB) plot in the presence of BVF-AgNPs indicated that the Vmax of the enzyme decreased while the Km value remained constant (). This result indicates that BVF-AgNPs inhibits α-amylase activity in a non-competitive manner. Using Dixon’s plot, the inhibitory constant (Ki) value for BVF-AgNPs was found to be 21 µg/ml against α-amylase activity ().
Figure 5. Effect of BVF-AgNPs on α-amylase enzyme activity. (a) Percentage inhibition of α-amylase activity by acarbose, B. variegata flower extract (BVFE) and B. variegata flower extract-mediated silver nanoparticles (BVF-AgNPs) synthesized at various concentrations are shown; (b) LB plot to understand the mode of inhibition by BVF-AgNPs; (c) Dixon’s plot for determining the inhibitor constant of BVF-AgNPs.
Only a small number of studies have reported on the effect of biologically synthesized NPs on α-amylase inhibition. The AgNPs synthesized using Lonicera japonica [Citation35], Pouteria sapota [Citation36], Heritiera fomes, Sonneratia apetala [Citation37] and Momordica charantia [Citation38] have shown α-amylase inhibition. Also, the copper NPs synthesized using Dioscorea bulbifera have shown α-amylase inhibition [Citation39]. However, BVF-AgNPs inhibited α-amylase activity significantly at a lower concentration in comparison to these previous reports. The report on mechanism of α-amylase inhibition by NPs is limited, however, it is proposed that (i) NPs can conjugate with either the amine or a carboxyl group present in the N-terminus and C-terminus ends, respectively of α-amylase [Citation40]; (ii) binding of NPs can alter the native conformation of the protein structure [Citation39]. The non-competitive mode of inhibition of BVF-AgNPs in the present study suggests that binding of these NPs may have altered the conformation of the α-amylase. On the other hand, the functional role of bioactive phytoconstituents bound on the BVF-AgNPs in its antioxidant activity and α-amylase inhibition property cannot be ignored. B. variegata flower is rich in flavonoids, in particular, anthocyanins. The flavonoid compounds are effective hydrogen donors which make it better antioxidants. Also, anthocyanin extracts from various plants are reported for its α-amylase inhibition [Citation41,Citation42], in particular, cyandin-3-rutinoside has effectively inhibited pancreatic α-amylase activity in a non-competitive mode [Citation43]. Hence, the organic part of the BVF-AgNPs which are attached on the surface of the NPs might have a significant role in enhancing the efficiency of metal ion part to synergistically impart the biological function. BVF-AgNPs can be used as an alternative potential candidate to understand its therapeutic strategy as an anti-diabetic agent in vivo.
Conclusions
AgNPs have been biosynthesized using BVFE without using any harmful reducing agents. The phytoconstituent present in the flower extract not only catalytically induced the formation of AgNPs but also attached on the surface of the NPs. BVF-AgNPs showed the potent antioxidant property. Also, it efficiently inhibited the α-amylase activity. The phytoconstituents attached on the surface of NPs might have a significant role in the biological application of BVF-AgNPs. The BVF-AgNPs can be further explored for its efficiency to control postprandial hyperglycaemia in in vivo model of diabetes.
Acknowledgements
Ms. J. Preethi acknowledges Periyar University, Tamil Nadu, India for the award of University Research Fellowship to carry out this research successfully. Ms. L. Chitra acknowledges Science and Engineering Research Board, India for the award of National Post-Doctoral Fellowship (Ref. No. PDF/2015/000252). Mr. S. Penislusshiyan is thankful to Department of Science and Technology, India for the award of INSPIRE Fellowship (Ref. No. IF140651).
Disclosure statement
The authors declare no conflict of interest.
References
- Zaheer ZR. Bio-conjugated silver nanoparticles: from ocimum sanctum and role of cetyltrimethyl ammonium bromide. Colloids Surf B: Biointerfaces. 2013;108:90–94.
- Shedbalkar U, Wadhwani S, Gaidhani S, et al. Microbial synthesis of gold nanoparticles: current status and future prospects. Adv Colloid Interface Sci. 2014;209:40–48.
- Aziz SG, Aziz SG, Akbarzadeh A. Advances in silver nanotechnology: an update on biomedical applications and future perspectives. Drug Res (Stuttg). 2017;67:198–203.
- Sathishkumar P, Gu FL, Zhan Q, et al. Flavonoids mediated ‘Green’ nanomaterials: a novel nanomedicine system to treat various diseases – current trends and future perspective. Mater Lett. 2017;210:26–30.
- Venil CK, Sathishkumar P, Malathi M, et al. Synthesis of flexirubin-mediated silver nanoparticles using Chryseobacterium artocarpi CECT 8497 and investigation of its anticancer activity. Mater Sci Eng C. 2016;59:228–234.
- Maurya A, Chauhan P, Mishra A, et al. Surface functionalization of TiO2 with plant extracts and their combined antimicrobial activities against E. faecalis and E. coli. J Res Updates Polym Sci. 2012;1:43–51.
- Sathishkumar P, Vennila K, Jayakumar R, et al. Phyto-synthesis of silver nanoparticles using Alternanthera tenella leaf extract: an effective inhibitor for the migration of human breast adenocarcinoma (MCF-7) cells. Bioprocess Biosyst Eng. 2016;39:651–659.
- Moteriya P, Chanda S. Synthesis and characterization of silver nanoparticles using Caesalpinia pulcherrima flower extract and assessment of their in vitro antimicrobial, antioxidant, cytotoxic, and genotoxic activities. Artif Cells Nanomed Biotechnol. 2016;30:1–12.
- Saravanakumar A, Peng MM, Ganesh M, et al. Low-cost and eco-friendly green synthesis of silver nanoparticles using Prunus japonica (Rosaceae) leaf extract and their antibacterial, antioxidant properties. Artif Cells Nanomed Biotechnol. 2017;45:1–7.
- Singh H, Du J, Singh P, et al. Ecofriendly synthesis of silver and gold nanoparticles by Euphrasia officinalis leaf extract and its biomedical applications. Artif Cells Nanomed Biotechnol. 2017;8:1–8.
- Vijayan R, Joseph S, Mathew B. Indigofera tinctoria leaf extract mediated green synthesis of silver and gold nanoparticles and assessment of their anticancer, antimicrobial, antioxidant and catalytic properties. Artif Cells Nanomed Biotechnol. 2017;6:1–11.
- Sharma AK, Sharma UK, Pandey AK. Protective effect of Bauhinia variegata leaf extracts against oxidative damage, cell proliferation and bacterial growth. Proc Natl Acad Sci India B Biol Sci. 2017;87:45–51.
- Mishra A, Sharma AK, Kumar S, et al. Bauhinia variegata leaf extracts exhibit considerable antibacterial, antioxidant, and anticancer activities. BioMed Res Int. 2013;2013. doi: 10.1155/2013/915436
- Kirtikar KR, Basu BD. Indian medicinal plant. 3rd ed. Allahabad: Sudhindra Nath Basu; 1991. p. 898–900.
- Sahu K, Gupta PK. Review on Bauhinia variegata linn. Int Res J Pharm. 2012;3:48–51.
- Lila MA. Anthocyanins and human health: an in vitro investigative approach. J Biomed Biotechnol. 2004;2004:306–313.
- Kandra L. α-Amylases of medical and industrial importance. J Mol Struct (Theochem). 2003;666–667:487–498.
- Sharma M. The obese patient with diabetes mellitus: from research targets to treatment options. Am J Med. 2006;119:S17–S23.
- Tangvarasittichai S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. WJD. 2015;6:456–480.
- Cheng YY, Fantus IG. Oral antihyperglycemic therapy for type 2 diabetes mellitus. CMAJ. 2005;172:213–226.
- Harborne AJ. Phytochemical methods. A guide to modern techniques of plant analysis. Netherland: Springer; 2007.
- Yao H, Xu W, Shi X, et al. Dietary flavonoids as cancer prevention agents. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2011;29:1–31.
- Kumaran A, Karunakaran RJ. In vitro antioxidant activities of methanol extracts of five Phyllanthus species from India. LWT-Food Sci Technol. 2007;40:344–352.
- Benzie IF, Strain JJ. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal Biochem. 1996;239:70–76.
- Apostolidis E, Kwon YI, Shetty K. Inhibitory potential of herb, fruit, and fungal-enriched cheese against key enzymes linked to type 2 diabetes and hypertension. Innovat Food Sci Emerg Technol. 2007;8:46–54.
- Lineweaver H, Burk D. The determination of enzyme dissociation constants. J Am Chem Soc. 1934;56:658–666.
- Dixon M. The determination of enzyme inhibitor constants. Biochem J. 1953;55:170–171.
- Hartland GV. Optical studies of dynamics in noble metal nanostructures. Chem Rev. 2011;111:3858–3887.
- Duret S, Paris RR. The flavonoids of several species of Bauhinia. Plant Med Phytother. 1977;11:213–215.
- Saleh NAM, Ishak M. Anthocyanins of some leguminosae flowers and their effect on colour variation. Phytochemistry. 1976;15:835–836.
- Makarov VV. Green nanotechnologies: synthesis of metal nanoparticles using plants. Acta Nat. 2014;6:20.
- Kasthuri J, Veerapadian S, Rajendiran N. Biological synthesis of silver and gold nanoparticles using apiin as reducing agent. Colloids Surf B Biointerfaces. 2009;68:55–60.
- Rajendran I, Dhandapani H, Rajaram A, et al. Apigenin mediated gold nanoparticle synthesis and their anti-cancer effect on human epidermoid carcinoma (A431) cells. RSC Adv. 2015;5:51055.
- Pal R. Characterization of citrate capped gold nanoparticle-quercetin complex: experimental and quantum chemical approach. J Mol Struct (Theochem). 2013;1046:153–163.
- Balan K, Wang Y, Liu X, et al. Antidiabetic activity of silver nanoparticles from green synthesis using Lonicera japonica leaf extract. RSC Adv. 2016;6:40162.
- Prabhu S, Elenchezhiyan C, Rajeswari D. Evaluation of antidiabetic activity of biologically synthesized silver nanoparticles (AgNPs) using pouteria sapota on STZ-induced diabetic rats. J Diabetes. 2017. [Epub ahead of print]. DOI:10.1111/jdb.12553
- Thatoi P, Gouda SO, Das G, et al. Photo-mediated green synthesis of silver and zinc oxide nanoparticles using aqueous extracts of two mangrove plant species, Heritiera fomes and Sonneratia apetala and investigation of their biomedical applications. J Photochem Photobiol B: Biol. 2016;163:311–318.
- Dhivya G, Rajasimman M. Synthesis of silver nanoparticles using Momordica charantia and its applications. J Chem Pharm Res. 2015;7:107–113.
- Ghosh S, Nitnavare R, Jagtap S, et al. Antidiabetic and antioxidant properties of copper nanoparticles synthesized by medicinal plant Dioscorea bulbifera. Nanomed Nanotechnol. 2015;S6:007. doi:10.4172/2157-7439.S6-007.
- Dhobale S, Laware SL, Rode CV, et al. Zinc oxide nanoparticles as novel alpha-amylase inhibitors. 2008;104. DOI:10.1063/1.3009317
- Nickavar A, Amin G. Bioassay-guided separation of an alpha-amylase inhibitor anthocyanin from Vaccinium arctostaphylos berries. Z Naturforsch C J Biosci. 2010;65: 567–570.
- Sui X, Zhou W. In vitro and in silico studies of the inhibition activity of anthocyanins against porcine pancreatic alpha amylase. J Funct Foods. 2016;21:50–57.
- Akkarachiyasit S, Wacharasindhu S, Adisakwattana S. In vitro inhibitory effects of Cyandin-3-rutinoside on pancreatic α-amylase and its combined effect with acarbose. Molecules. 2011;16:2075–2083.