Abstract
Breast cancer is a group of diseases with various subtypes and leads to high mortality throughout the globe. Various conventional techniques are in practice to cure breast cancer but these techniques are linked with various shortcomings. Mostly these treatments are not site directed and cause toxicity towards normal cells. In order to overcome these issues, we need smart system that can deliver anticancer drugs to specific sites. Targeted drug delivery can be achieved via passive or active drug delivery using nanocarriers. This mode of drug delivery is more effective against breast cancer and may help in the reduction of mortality rate. Potentially used nanocarriers for targeted drug delivery belong to organic and inorganic molecules. Various FDA approved nano products are in use to cure breast cancer. However, body’s defense system is main limitation for potential use of nano systems. However, this can be overcome by surface modification of nanocarriers. In this review, breast cancer and its types, targeted drug delivery and nanocarriers used to cure breast cancer are discussed. By progressing nanotechnology, we will be able to fight against this life threatening issue and serve the humanity, which is the basic aim of scientific knowledge.
Introduction
The utmost devastating disease related with progressive alterations or variations in the cellular, genetic and epigenetic physiognomies that leads to uncontrolled cell division, eventually causing the development of a malignant mass is known as cancer [Citation1]. This heterogeneity and complexity encourage the fierce growth of cancer cells resulting insubstantial mortality and morbidity [Citation2]. Owing to the growth and aging of global population and a greater adoption of cancer-causing behaviours, the worldwide burden of cancer endures to intensify largely; within economically developing countries [Citation3]. Through multistep carcinogenesis process, cancer is commonly known to develop many cellular physiological patterns, for example apoptosis and cell signalling, creating unintelligible and complex disease [Citation4]. Resulting malignant tumours (cancer) may occupy other adjacent organs, and beyond the location of its initiation (metastasis) posing the disease life-threatening [Citation5]. Specificity in detecting precancerous conditions or early-stage cancer with a low rate of false positives, insufficient sensitivity, screening technologies, high cost and inability to determine tumour stage are the limitations of conventional cancer treatment practices [Citation1]. This demands for a system which can overcome issues associated with conventional treatment. Nanotechnology offers ground to treat cancer where we direct targeted drug delivery to cancerous cells with the aid of nano-carriers.
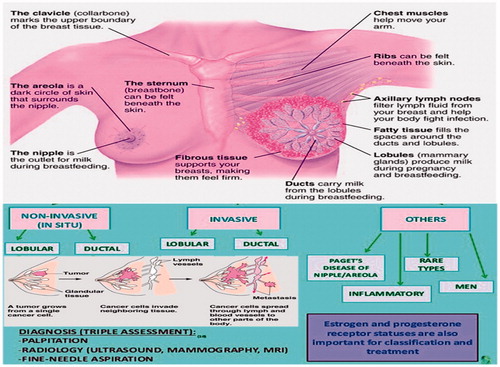
Histo-pathological classification and subtypes of breast cancer (BC)
Breast cancer is a type of malicious tumour originates in the tissues of breast section, most probably from the interior lining of lobules or milk ducts. It is a heterogeneous disease; fifth most common cause of death [Citation1]. Breast cancer may also occur in male but the preponderance occurs in women with probability one out of eight and about one dies in every 35 women [Citation6,Citation7]. Nevertheless, it remains sickly agreed that within the tumour microenvironment, how the alteration from wellbeing to malevolence changes the mechanical properties of cells [Citation8,Citation9]?
Breast cancer has many subtypes with diversified morphologies and clinical extrapolations. The initiation of micro-arrays has resulted in novel prototype breast cancer heterogeneity; on that basis inherent sub typing system using prognostic multi-gene classifiers has been formed. However, overlapping of gene panels to a higher degree, the gain of fresh data and perceptions cause advent of novel subtypes that obscure our insights to heterogeneity [Citation10]. Abide molecular mechanisms understanding, carcinogenesis and advanced targeted therapies, breast cancer is uncontrolled [Citation11]. Breast cancers are of numerous forms (; ) which can be started from different parts of the breast [Citation5].
Table 1. Different types of breast cancer along with site of development and characteristics.
Challenges in conventional treatments for BC
Accelerated partial breast irradiation has been scrutinized for the treatment of early-stage breast cancer as a therapeutic methodology to improve local control rates [Citation7]. The restrictions of the existing cancer therapeutics are non-specific dispersion of drug, inadequate drug concentrations to target site, intolerable toxicity, partial ability to track and control the site-specific therapeutic reactions, adversative possessions and progression of drug resistance [Citation21].
Therapeutic advantages of the nanocarriers for BC
Conventional treatments kill normal cells and are toxic to non-specific targets. Hence, it is more likely to develop such chemotherapeutic strategies that cause less or no toxicity to the patients and affect the cancerous cells passively or actively. Use of nanoparticles may confer targeted drug delivery to tumour cells. Concentration of drug in the cancer cells increases with the use of active or passive targeting. In light of above advantages, if development of nanoparticle therapies is in right direction, the outcomes can be improved in the patients of metastatic cancer. Though, drug resistance is primary hindrance to treat cancer [Citation22]. The novel therapies, i.e. use of nanoparticles are being developed that are implemented to overcome the limitations associated with the chemotherapy.
Nanotechnology in targeted drug delivery
Nanotechnology has potential to revolutionize cancer diagnosis and therapy comprising non-invasive therapy, advanced imaging, monitoring response to therapy, improved nodal staging and treatment of metastatic disease [Citation5]. Nanotechnology may modify pharmaco-kinetics and delivery refining effectiveness and lessening side effects [Citation23,Citation24]. The size and surface properties enrich drug portability, mobility, flexibility and thus enhance choosy internment in tumour tissue making them potentially operative tumour conveyance vectors [Citation25]. Nanotechnology including metallic and semiconductor nanoparticles enlarges useful abilities for contemporaneous targeted drug delivery and imaging [Citation26,Citation27].
Targeted drug delivery
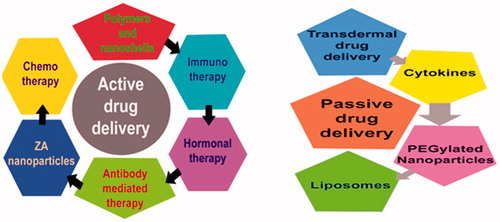
With the development in the imaging, delivery and the sensing techniques; the innovations in field of oncology has breakthrough in the treatment strategies [Citation28]. Targeted drug delivery systems are considered for the bio distribution of specific drugs by macromolecular drug conjugates, chemically or genetically reformed proteins, particulate drug carriers and others [Citation29,Citation30]. Various targeting agents used are targeted drug delivery are shown in . Passive and active approaches for drug delivery are currently in practice.
Passive drug delivery
In passive targeting, the specific features of the cancer cells are exploited that allows drug nanocarriers to accumulate in the tumour cells due to increased retention and permeability (). Leaky vasculature of tumour tissues due to excessive angiogenesis, expresses the basis of passive targeting of the drugs. Brief accounts of these methods are as follows.
Transdermal drug delivery
It is a striking substitute to the conventional drug delivery approaches of oral as well as intravenous systems of drug delivery. Stratum corneum is the only drawback of the complete targeting of transdermal drug that can be modified via application of ultrasound irradiation to the skin and increases permeability of drug [Citation31].
Therapeutics through cytokines (TNF)
Various antitumour effects associate with natural killer cell and lymphocytes necrosis factors, including induction of necrosis and apoptosis, activation of the cells that are cytolytic and ICAM-1expression up regulation. In breast cancer patients, TNF production by monocytes has been found less impaired as compared to the normal persons. Many researches working on metastasis have revealed that interferon IFN-A is the most critical component that is involved in the regulation of the phagocytic response against the breast tumours and cancer. In tumour tissues, significant level of IL-10 mRNA has been observed. IL-10 has noteworthy activity against tumour. In vivo studies suggest that recombinant humanized IL-10 administration to animals significantly inhibits growth due to increase in CD4 positive cells which ultimately increase the Mig and IP-10 production. Both of these are the potent inhibitors of angiogenesis [Citation32].
Anti-metastatic activity has also been demonstrated in IL-10 and it depends on natural killer cell activity. Expression of IL-10 also decreases expression of MHC class 1 in tumour cells which causes increased lyses of natural killers. IL-10 at higher concentration has significant anti-tumour activity. Another factor for the stimulation of natural killer cells is interleukin-12 that is produced by the macrophages and other monocytes, has suppression activity to control tumour size. It also tends to initiate cellular immunity and has significant effects on the natural killer cells and T cells. In recent years, a number of therapeutic strategies have been developed based on the principle of DC (dendritic cell). These cells are able to secrete the stimulator molecules, in addition to cytokines that are considered potent pro-inflammatory [Citation33]. This is effective vaccine to induce cellular immunity. The over expression of IL-24 gene has inhibitory action on tumour growth. In vivo studies have shown that injection of interleukin induces apoptosis in tumour cells. The antitumour effects of the interleukin are mediated in p53-independent manner. The combination therapy of adenovirus vectors and the Herceptin have clear impact on breast cancer. For the gene therapy of cancer, IL-24 has considerable impact. It is under the clinical trials of phase II to determine the effects of the therapy in the cancer patients [Citation34].
PEGylated nanoparticle development
Passive drug delivery systems pave way for the PEGylated nanoparticles; which due to their size and altered chemical structure can be directed to the site of tumour [Citation35]. Long half-life, less immune reactions, good compatibility, ideal targeting, good penetration in the physiological barriers, self-regulation of drug and nearly no side effects are key features of nano vectors. To improve the circulation and selective targeting of drugs, the tools such as the immunolabelling and PEGylation are necessary. PEGylation shielding also enhances stability of drugs. In future, these approaches will be more likely to combine the particles in the hybrid based systems to lessen the limitations and synergistic efficacy [Citation36].
Liposome-mediated drug delivery
Use of liposome assures the delivery of drugs by improving their solubility. In this way, they improve transfer of therapeutics in tumour cells, and also decrease drug toxicity. Among a lot of drug delivery methods, the liposome is in practice or some are in clinical trials after advancements in nanoparticle based delivery systems. While others are still in different stages of clinical development. Liposomes packed drugs are released steadily and have good compatibility. Liposome with the specific features has been developed that are capable to evade the RES system, and have enhanced binding specificity to the tumour cells. Certain studies have also shown promising results with ATP-mediated delivery of liposomes. Due to all these factors, the liposome is considered as the excellent vehicles for the transport of drugs in tumours [Citation37].
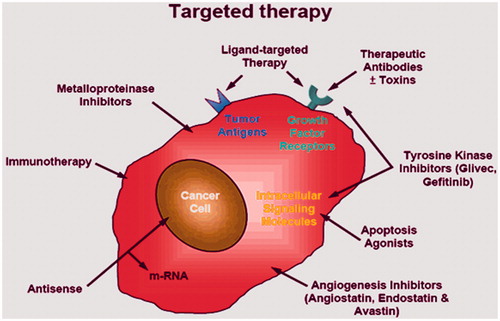
Active drug delivery
Drawbacks and limited efficacy of passive drug targeting methods have urged scientists to move towards active drug delivery to the tumour site. This seems more promising due to ligand interaction with membrane expressed proteins that could effectively concentrate the given drug at the site of action (). Many active drug targeting approaches have been used till now, account of which has been given below.
Formation of smart polymers
Targeted drug delivery via polymers that are sensitive to pH is under consideration. These are designed to deliver drugs to the cells by pH sensitization due to endosomal disruption after endocytosis. An improved efficacy in the delivery process is achieved by the formation of the polymers from different monomers that are made hydrophobic on changing the pKa values and adjusting them in the acidic range. Higher efficacy is achieved because this process mimics the common disruptive peptides behaviour [Citation38].
Nanoshells as active targeting molecules
For the diagnosis and treatment of cancer, the nanoshells have been devised and are considered as promising candidates [Citation39]. Most of the blood vessels in solid tumours have unique properties. These include angiogenesis, high vascular density and extravasations of macromolecules in plasma. The tendency of polymeric drugs to accumulate in the tumour increases due to diffusion of plasma contents facilitated by vascular epidermal growth factor (VEGF). More accumulation of nutrients and oxygen occur due to endothelial factors. These nutrients accumulate in the interstitial space so it allows the components in plasma to penetrate in walls of blood vessels. In this way tumour rapid growth sustain [Citation40]. The applications of nanoshells are versatile but these are limited due to less penetration and tumour sites targeting. The gold nanoshells that lie in the near infrared region are able to heat up the tumours to extent that cause damage to the tumours irreversibly [Citation41]. Bio-recognition sites that are capable to dock to the receptors on the cancer cells have also been included in the nanoshell treatment. These are combined to the endoscopic procedures that help to focus the Near Infrared (NIR) radiation at the increasing depths [Citation42].
Use of transferrin targeted zoledronic acid (ZA) nanoparticles
Transferrin is used to complex with nanoparticles because transferrin receptors over express intra as well as extra cellularly [Citation43]. Tumour has been treated with zoledronic acid and temozolomide. These are the cytotoxic agents that are used to inhibit abnormal proliferation of cell but the problems associated are cytotoxicity to normal cells. They accumulate in the bone marrow, not transported in effective concentration to inhibit cell growth and also cause haemolysis. Studies show that ZA increases apoptosis 1.27 times in U87MG cell lines according to control cells [Citation44]. Recent research has reported that when transferrin is complexed with nanoparticles encapsulating ZA, it increases ability to target tumour cells. The intracellular concentrations are also reported to increase in the tumour cells which indicate increased uptake of the drug by tumour cells. In vivo and in vitro studies have shown that these particles do not cause haemolysis, nor accumulate in bone marrow [Citation45]. Temozolomide is the cytostatic agent that tends to cause cytotoxicity in tumour cells. The cytotoxic effects of temozolomide are potentiated when it is administered along with the transferring targeted nanoparticles [Citation46].
Antibody mediated targeted drug delivery
Among different types of the targeting agents, most common are antibodies, nucleic acids and the ligands of different receptors. Food and Drug Administration (FDA) has approved use of monoclonal antibody after clinical demonstration with the use of 17 antibodies. The use of monoclonal antibody rituximab was done in 1997 for the Hodgkin’s lymphoma. Another antibody, anti-HR antibody has also been approved for breast cancer treatment. Currently about 200 antibodies based delivery systems are under the clinical and preclinical trials. So, due to the advancement in the field of the antibody engineering, chimeric antibodies have been developed that are both human and animal origins [Citation47]. The fragments as well as native form of the antibodies can also be used but the fact is that the use of whole antibody is more effective because binding sites of single antibody provides higher binding capacity and sensitivity of the drug delivery system. Whole antibodies usage also poses an additional advantage as they remain stable during the long-term storage. The use of antibody fragments does not offer stability but they reduce the chances of non-specific binding. Antibodies that are generated by this method bind different epitopes of the same target cells [Citation48].
Hormonal therapy
The growth of the breast cells is regulated by oestrogen receptors. It is fact that when the ovaries of premenopausal females are removed, a regression of the tumours in breast is observed. Oestrogen produced by ovaries diffuses through plasma membrane of the cells where it interacts with ER (oestrogen receptors). On binding of oestrogen to ER, the dimerization of ER causes its translocation in to the nucleus. In nucleus, it binds to Oestrogen responsive element (ERE) that is present in the promoter region to activate downstream gene expression. Scientists have developed the modulators of the oestrogen receptor that act as antagonists through their AF2 domain. In females with ER positive status, a survival of 10-year has been reported. The annual rate of the breast cancer is observed to decrease to 31% by the tamoxifen therapy for continuous 5 years [Citation49].
Immunotherapy
Up regulation of human epidermal growth factor is observed to about 25%in the tumours of breast because of abnormal amplification and over expression of the genes. This is the cause of less survival rates and shortened time. The first anti-kinase therapy was developed based on the genomic research and has been approved by FDA. This treatment is developed for the patients of invasive breast cancer in which Her2 over expresses. This antibody interacts with Her2 extracellular domain and inhibits the proliferation of tumours that are Her2 dependent and they block the formation of dimer [Citation50].
In order to deliver drugs at specific sites of tumour cells, there is a prerequisite of an agent, which is capable to carry a drug and delivered to a destiny. An ideal system for this purpose comprises of nanocarriers, which are now actively used in medical sector.
Nanocarriers: new vehicles of drug delivery
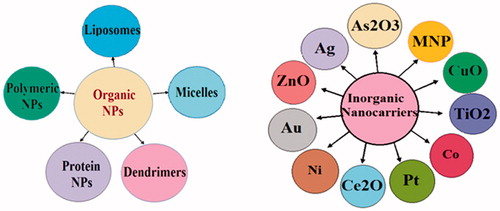
Concept of nanocarriers arose from the observation made by Paul Ehrlich (1854–1915) that if an agent could selectively target a pathogen, then that toxic agent can be delivered with the agent of selectivity. Nanomaterials have been designed to function as a vector for drug delivery [Citation51]. However, site specific targeting through nanomaterials is a pre-requisite to attain diagnostic or therapeutic effects [Citation52]. Nanotechnology based carriers have been employed for high drug load and sustainable drug release [Citation53], avoiding biological obstacles and targeted drug delivery with effective concentration, while sheathing the normal tissues from the toxic effects of these therapeutics [Citation54]. Majority of organic [Citation55] and inorganic [Citation56] biomaterials have been discovered as a drug carrier ().
Organic nanocarriers
Liposomes
Liposomes are aqueous compartment enclosed in spherical vesicles composed of single or multiple membranes of natural or synthetic lipid bilayers [Citation57,Citation58]. Drug can be stacked inside liposomes in watery medium that facilitates diverse medications to be completed [Citation59]. Liposomes as a drug carrier have been used since 1960s [Citation60] and in 1965 were considered as first nanoparticle to be employed in medication [Citation61]. It can enhance drug accumulation in tumour cell while limiting their accumulation in normal tissue [Citation62]. Different drugs i.e. doxorubicin [Citation63], paclitaxel [Citation64], daunorubicin, lurtotecan, annamycin, platinum compounds and genes such as the E1A gene [Citation65], cisplatin [Citation66] etc. have been encapsulated in liposomes. Drugs enclosed in liposomes can be released by altering pH at the target sites [Citation67], induces mechanical stress [Citation68], light [Citation69], temperature [Citation70], redox reactions [Citation71] and by enzymatic reaction on the liposome [Citation72].
Polymeric nanoparticles
These may be employed as the most operative nanocarriers for protracted drug delivery. Natural polymeric substances such as chitosan, heparin, dextran, gelatin, alginate and collagen as well as synthetic polymers as PEG, polyglutamic acid (PGA), polylactic acid (PLA), polycarprolactone (PCL), poly-d,l-lactide-co-glycolide (PLGA) and N-(2-hydroxypropyl)-methacrylamide copolymer (HPMA) have been comprehensively used owing to their biocompatibility, biodegradability, for the preparation of NPs and encapsulate drugs for therapy of cancer [Citation73–76]. Improvement of polymeric nanoparticles encumbered with drugs is in practice since long. Langer and Folkman [Citation77] recognized the first organized release of macromolecules by means of polymers. Couvreur developed polyalkyl cyanoacrylate-based nanoparticles releasing doxorubicin [Citation78]. Langer et al. pronounced nanoparticles self-possessed of poly(lactic acid)/poly (lactic-co-glycolic acid) (PLA/PLGA) and Gref et al. demonstrated PEG block copolymer as “long mixing nanoparticles” due to their stealth properties [Citation79]. The surface of nanoparticle is sterically alleviated by grafting, conjugating or adsorbing hydrophilic polymers such as PEG to its surface, which can also reduce hepatic uptake in addition to improve half-life [Citation80].
Micelles
Micelles are self-assemblies of lipids or other amphiphilic molecules organized into nanoparticles [Citation81]. Two or more polymeric chains with different hydrophilicity systematize into a core-shell assembly in an aqueous medium [Citation82,Citation83]. This leads to design appropriate for drug delivery. Drugs, proteins and nucleic acids can be loaded in the hydrophobic core although the hydrophilic shell is accountable for steric fortification of the nanoparticle [Citation84]. Micelles are considered as an ideal drug delivery vehicles for hydrophobic drugs [Citation85]. Drug released from micelles can be achieved by various means like erosion of biodegradable polymer, diffusion of the drug after polymer swelling or through the polymer matrix, varying external factors like pH and temperature or through surface modification with ligands; help in targeted drug delivery and decrease systemic toxicity and enhance specificities and effectiveness [Citation86]. Drug delivery by micelle can be alienated into four assemblages; these are phospholipid micelles, pluronic micelles, poly (l-amino acid) micelles as well as polyester micelles that demonstrate a comparable molecular design which is not discussed here in detail.
Dendrimers
Dendrimers are well defined, regularly branched polymeric complexes surrounding an inner core and physio-chemically similar to macromolecules [Citation87]. Regardless of their large molecular mass [Citation88], they exhibit well-established architecture and functionalities [Citation89]. Glyco, peptide and silicon based dendrimers have been achieved by functionalization of dendrimers [Citation90]. Dendrimers with almost monodispersity in addition to suitable pharmacokinetic possessions are in practice for systemic drug delivery with dissociation of drugs [Citation91]. The polyamidoamine (PAMAM) family of dendrimers is the most frequently studied dendrimers. Due to their biodegradable, biocompatible and fairly soluble nature in aqueous medium made them as a potent agent for drug delivery [Citation92]. In addition, architecture and surface can be altered for intriguing applications including diagnosis and therapy [Citation93]. For pharmaceutical applications, dendrimers are functionalized [PEGylation] most frequently [Citation94]. Various anticancer drugs have been delivered to carcinogenic tissues through PEGylated dendrimers. Drugs release after attainment of target site by enzymatic action, pH changes, etc. [Citation95]. Dendrimers associate with indomethacin [Citation96], fluorouracil [Citation97] and antisense oligonucleotides [Citation98] have been inspected as delivery system. For active drug targeting, dendrimers have been functionalized with folate vector which enhances build-up of anticancer drug in the tumour tissue [Citation99]. Functionalized dendrimers with RGD (arginine–glycine–aspartic acid) and neurotensin peptides got interest in recent research due to their greater stability and affinity to carcinogenic tissues [Citation100].
Protein nanoparticles
Natural polymers such as albumin, heparin, gelatin and collagen, etc. have been widely used in encapsulation of anticancer drugs due to their biocompatibility, biodegradability and regulatory approval for cancer treatment [Citation73]. Albumin is inert in the range of pH [Citation4–9], solubilize in aqueous medium and thermodynamically stable even heated at about 60 °C up to 10 h. In addition, albumin has no immunogenicity and has a longer half-life [Citation101]. Binding of albumin to the glycoprotein gp60 (albondin), surface receptors triggers binding of glycoprotein gp60 to the caveoline-1 (intracellular protein), results in the formation of caveolae (transcytotic vesicles) through membrane invagination. By this mechanism, transportation of albumin is regulated across endothelium into the extra vascular space. Active transportation of plasma proteins occurs in tissues with high metabolism like tumour and inflamed tissues [Citation102]. Three main approaches are in use in drug delivery: (i) encapsulation of low-molecular-weight drugs to endogenous or exogenous albumin; (ii) binding of albumin with bioactive proteins and (iii) loading of drug into albumin NPs [Citation92]. FDA has permitted albumin-bound paclitaxel (Abraxane, ABI-008, January 2005) for metastatic breast cancer treatment, as well as in numerous clinical trials in recent times in evolution for other types of cancer.
Organic materials as nanocarriers also severe some limitations like inadequate chemical and mechanic stability, swelling, liability to microbial attack, insufficient control in drug release [Citation103] and quite expensive. In addition to these problems, polymer NPs also exhibit high polydispersity. During synthesis, particles with a wide size ranges and irregular morphologies limit homogeneous pharmacological properties. Dendrimer can overcome this limitation due to monodispersity and spherical design due to stepwise fabrication and purification [Citation104]. Major shortcoming of dendrimers is high cost. Dendrimers can be quickly removed from the body during circulation in the body via defence mechanisms exhibited by the body remained a challenge.
Inorganic nanoparticles
Inorganic NPs are mostly metallic in nature while some are metalloids and have intriguing applications in many fields.
Gold NPs
Since 2500–2600 BC various compounds of gold are used as “Swarna Bhasma” by Chinese and Indian in medicines for variety of purposes [Citation105]. Common usage of gold NPs are due to the obvious advantages associated with it like simple, cost effective and dependable methodologies, and various forms generation such as spheres [Citation105], shells [Citation106], rods [Citation107] and cages [Citation108]. AuNPs display strong surface plasmon resonance [Citation109] and dielectric strength [Citation110]. Possession of negative charge and robust binding aptitude of gold with chemical assemblies like thiols, disulfides, phosphine, as well as amines [Citation111] are useful in modification and functionalization with various biological molecules [Citation112]. Besides this, biocompatibility and non-toxicity [Citation113] make them as an attractive tool for medicines especially in cancer therapy [Citation114]. Use of gold NPs as a probe for hyperthermia by means of NIR [Citation115] demonstrates photothermal therapy and treatment of cancer. Recent reports describe use of silica-gold nanoshell with NIR for in vitro and in vivo photothermal therapy of breast cancer [Citation116]. Preliminary (phase I) test for dose intensification studies with use of a CYT-6091, i.e. PEGylated AuNPs with recombinant human tumour necrosis factor alpha have been approved [Citation117] and AuNPs were absent in normal parenchyma of breast cancer patients [Citation118]. Hyperthermia with NIR laser and AuNPs has recognized as an effective in vitro technique against breast cancer [Citation119]. Functionalization of AuNPs with various therapeutic agents can help in drug delivery and controlled drug release in tumour site. So far, AuNPs have been functionalized with many materials, i.e. tumour necrosis factor, antisense DNA, small interfering RNA (siRNA), paclitaxel and plus docetaxel [Citation120]. Lower pH within endosomes assists in the drug release [Citation121] or photothermal therapy from NIR laser also aid in release of drug at target sites [Citation122]. Mode of action of targeted gold mediated hyperthermia can be made easier. Gold NPs can be targeted by passive or active targeting the solid tumours due to their leaky vasculature through enhanced permeability and retention (EPR) [Citation123]. Accumulation and binding of gold NPs first in tumours cells are irradiated with NIR which cause photothermal heating [Citation124]. This procedure prevents side effects linked with conventional cancer treatments due to confined irradiation at the tumour site. Gold nanostars conjugated with trans-activator of transcription (TAT) and cell-penetrating peptide (CPP) showed better response to BT549 cell. CPP help in transportation of NPs across the plasma membrane and boost their delivery within the nucleus by macropinocytosis [Citation112]. Bimetallic NPs of gold-silver functionalized with S2.2 aptamer targeted MUC1 receptors on MCF-7 breast cancer cells but limited binding is observed for HEPG2 or MCF-10 A liver and other breast cancer cells [Citation125]. Another study demonstrated use of functionalized gold nanorods with AS1411 DNA aptamer, coated with mesoporous silica loaded with doxorubicin [DOX] to target breast cancer cells via light stimulated photothermal chemotherapy [Citation126]. Almost 20% cytotoxicity was observed when these conjugated NPs were raised with the cells, representing leakage of more or less DOX . While irradiation with NIR provoked dehybridization of DNA aptamers which commanded in better DOX release expiring up to 90% MCF-7 cells [Citation126]. Same were observed in various other methods such as pH dependent ligands to prompt DOX release commencing gold nanocages [Citation127] or incorporation of gold nanoshells [Citation128]. Thermal imaging of MDA-MB-231 breast cancer tumours in mice via nanomatryoshkas [gold–silica conjugate] are found enough for killing tumour cell after NIR [Citation129]. Plasmonic gold vesicles loaded with chlorine e6 injected in athymic nude mice with breast cancer (MDA-MB-435) tumours and irradiated with laser NIR (671 nm, 2 W/cm2, 6 min) resulted in increase of 10 °C in temperature prohibiting any tumour growth [Citation130]. SK-BR-3 breast cancer cells when incubated with PEGylate AuNSs followed by 820-nm NIR laser resulted in limited survival of breast cancer cells in contrast to cells treated without AuNSs [Citation116]. PEGylated AuNSs conjugated with a HER-2 antibody exhibited higher uptake by SK-BR-3 cells [Citation119]. Use of PEGylated AuNPs loaded with trastuzumab against HER-2 overexpressing SK-BR-3 breast cancer cells with use of radiation [300 peak kV] leads in 5.1 times more breakage in double stranded DNA as compared to PEGylated AuNPs [Citation131].
Iron oxide NPs
Magnetic NPs (MNPs) exists in two types, i.e. magnetite (Fe3O4) and maghemite (Fe2O3). Immense use of these MNPs in oncological studies are due to their tendency to target specific side by limiting systemic circulation, encapsulation of drugs, a slow rate of drug suspension during delivery and improved dissolution while reaching to the target sites, thus letting the usage of lower dosages of cytotoxic drugs and minimizing their side-effects [Citation132–134]. Naked iron NPs can clump forming large clusters which increases particle size in turn reduces innate super paramagnetic character and stimulate opsonization [Citation135]. Surface modifications can overcome clumping and minimizing opsonization. Various agents are in practice for surface modification of NPs like dextran, polyethylene glycol (PEG), poly(vinylpyrrolidone) (PVP), streptavidin, poly-l-lysine (PLL), polyethylene imide (PEI), etc. [Citation136]. Drugs encapsulated in MNPs are mitoxantrone [Citation137], tamoxifen [Citation138], cefradine [Citation139], doxorubicin [Citation140], daunorubicin [Citation141], cisplatin and gemcitabine [Citation142], paclitaxel [Citation143], adriamycin etc. [Citation144]. NPs coated with water-dispersed oleic acid – pluronic resulted in sustained drug (doxorubicin) release and a concentration reliant on anti-proliferative significance experimented on breast and prostate cancer cell lines [Citation145]. Hyperthermia produced by MNPs has been developed as an auspicious therapeutic method in cancer treatment. Iron NPs facilitate destruction of tumour cells by production of effective heat when exposed to an oscillating external magnetic field [Citation146]. In comparison to the conventional hyperthermic techniques, magnetic hyperthermia offers minimal invasive means to carry a therapeutic dose of heat precisely to cancer sites [Citation147]. Hyeon group described the production of uniform-sized ferromagnetic iron oxide nanocubes (FIONs) [Citation148] and have been effectively applied for MRI-based tracing of transplanted pancreatic islets at the single-cell level [Citation149]. Functionalization with trastuzumab targeted HER2 to form tumour-specific radiological nanoprobes have also been used to detect breast xenografts over expressing HER2 receptors [Citation150]. All of the studies discussed above state that iron NPs can be used as a potential carrier or vector for drug delivery and these give new grounds towards cancer treatment.
Titanium dioxide
Titanium dioxide nanoparticles (TiO2 NPs) belongs to semiconductor have been extensively used in biomedical applications such as drug delivery and cancer therapy [Citation151] due to inert, strong oxidizing potential, photoreactivity, eco-friendly, stable, cost efficient and biocompatibility with low or no toxicity [Citation152]. Among rutile, anatase and brookite crystallograph of TiO2, anatase shows enhanced biocompatibility while other have some toxicity [Citation153]. Adriamycin halts the growth of tumour cells and has been used since 1970s to cure a wide variety of tumours [Citation154]. Titanium is a second metal in clinical trials that uses Ti (IV) which binds with DNA through covalent bonds [Citation155]. Budotitane, is a titanium based inorganic complex proved as anti-tumour agent but instability attributes to Ti (IV) species [Citation156]. Recently, titanium salan complexes belong to titanium (IV) complexes, proved to have higher stability in solution with enhanced anti-tumour activity [Citation157]. Scientist has developed a conjugation of doxorubicin and TiO2 NPs. These nanocomposites have enhanced accumulation of DOX in tumour cells led to increase anticancer potential by inducing apoptosis in a caspase-dependent manner [Citation158]. All of the above findings speculate that TiO2 NPs act as potential agent against many cancers especially breast cancers.
Platinum NPs
Use of platinum in Pharmaceutical industries is not well recognized rather their compounds are famous for anticancer potential [Citation159]. Almost 50% of chemotherapeutic techniques comprise of platinum compounds [Citation160]. In recent years, platinum based products are in clinical use due to their widespread applications against various tumours [Citation54]. Common use of platinum NPs in medical sector are as a composite material, i.e. Yolk-shell nanoparticles [FePt@CoS2] being more well-organized in obliteration of HeLa cells associated to cis-platin [Citation161]. Platinum conjugated with multi-walled carbon nanotubes [MWCNTs] displays sensitivity of catalytic exposure of the DNA [Citation162]. FePt NPs linked with luteinizing hormone-releasing peptide (LHRH) hinders tumour growth in a pH-dependent manner [Citation163]. Au-Fe3O4 NPs with dumbbell morphology lodge platinum based anticancer drugs through herceptin antibody to embattle distribution of platin complexes to breast cancer cells [SK-BR-3] [Citation164]. Cisplatin an anti-tumour drug, induces apoptosis through the binding of DNA [Citation165]. FDA has approved drug “Oxaliplatin” that exhibit similar therapeutic results as cisplatin, with no expressive acquisition of multidrug resistance due to immunogenic response dependent of the toll-like receptors [Citation166]. Satraplatin, being more lipophilic has enhanced anticancer pharmacokinetic properties, is in advanced clinical trial phases. Another advantage of Satraplatin is the production of adducts which are not identified by DNA mismatch repair mechanism, which lower the drug resistance [Citation167].
Cerium oxide NPs
Cerium belongs to lanthanide series discovered in 1803 and thereafter used in medicine [Citation168,Citation169]. Cerium oxide NPs [CeO2 NPs], also known as nanoceria, have intriguing applications in biomedicine due to antioxidant potential [Citation170]. Nanoceria have gained remarkable attention due to oxidation state from Ce3+ to Ce4+ [Citation171]. CeO2 NPs have emerged as an antioxidant and anti-inflammatory agent [Citation172] can also induce lipid peroxidation, lung damage, generation of reactive oxygen species, autophagy and shelter normal cells against radiation induced injuries [Citation173,Citation174]. This prevents the subsequent production of ROS which induces apoptosis in normal cells [Citation175]. CeONPs also exhibit distinctive cytotoxicity to cancer cells. Hence, CeONPs have wide prospective as a therapeutic mediator for the management of cancer, as well as additional diseases in which ROS are involved [Citation176]. CeONPs can be used as a nanocarriers for transporting anticancer drugs with less side effects [Citation171–177].
Nickel NPs
Nickel nanoparticle synthesized from different routes are broadly used in catalysis and sensing [Citation178]. Current studies highlight potential use of Ni NPs in biomedicines, comprising cancer diagnosis as well as therapy. Ni nanowire induces ROS which results in apoptosis in human pancreatic adenocarcinoma cancer cells [Citation179], and MCF-7 cells of human breast cancer. Caspase-3 enzyme activation and enhancement of mRNA level is observed after exposure to NPs [Citation180]. Ni NPs conjugated with daunorubicin showed effective cytotoxicity against leukaemia cancer cells [Citation141,Citation181]. Verbascoside; an anticancer drug associated with Ni NPs induces apoptosis in K562 cells averting tumour growth in model organism [Citation182]. MCF-7 human breast cancer cells also showed cytotoxicity after 24 h treatment with Ni NPs [Citation183].
ZnO NPs
Zinc is an essential trace element required to body for more than 300 cellular processes therefore used in numerous biological applications [Citation184,Citation185]. Cytotoxicity of ZnO NPs can be improved further by reduction of size [Citation186]. Researchers have revealed that ZnO NPs show cytotoxic activity against carcinogenic cells with negligible effects on normal cells [Citation187]. These outcomes propose that if selective targeting of cancer cells is done with ZnO NP; it can be used as a potential agent in cancer treatment. Eukaryotic plasma membranes are usually negatively charged [Citation188] while cancerous cells have over expressed negatively charged phospholipids bilayer with respect to normal cells [Citation189] and probably these charged membranes interact with positively charged NPs. One probable way to develop a targeted killing of cancerous cells is to modify/functionalize ZnO NPs with various substances such as polyacrylic acid [PAA], or dextran carboxyl [–COOH], or amine [NH2] groups [Citation190]. ZnO nanocomplex with liposomes facilitates daunorubicin release in response to pH change [Citation191]. The well-studied action of ZnO NPs is due to the production of ROS [Citation192]. ROS act as second messengers and enhance cellular pathways [Citation193] while higher level of ROS activates apoptotic pathways [Citation194] and results in significant cytotoxicity [Citation194]. Superior cancer cell cytotoxicity of ZnO NPs is most probably related to the proliferative cell potential [Citation194]. While another study confirmed higher vulnerability of cancerous T cells towards the ZnO NPs [Citation195].
Cobalt [Co] NPs
NPs of cobalt may be fabricated as cobalt oxide, conjugated with organometallic compounds or biological polymers [Citation196]. Anticancer activity of cobalt oxide NPs can be enhanced through surface modification of these NPs [Citation197,Citation198]. In biomedicines, cobalt is recognized as contrast agent via magnetic resonance imaging [MRI], in conjugation with gold, iron, graphite and platinum [Citation199]. It has also been inspected for cancer treatment especially breast cancer MCF-7 cell line [Citation200]. In addition, eco-friendly nature also marks them as an appropriate nominee for biomedical applications [Citation201].
Copper oxide NPs
Copper is an intriguing element because of its unique electronic configuration and its role as a co-factor for redox reaction in enzymes cycling and induces cellular stresses [Citation202]. Due to this reason, various copper compounds have been scrutinized for anticancer activity [Citation203]. Anticancer potential of copper compounds are due to their selective permeability to cross cancer plasma membrane [Citation204]. Cu2+ has potential to interact with single stranded DNA due to cross linking with adjacent phosphate groups [Citation204]. Although there is still absence of adequate data regarding mode of action of CuO NPs [Citation205], different pathways have been proposed which utilizes involvement of ROS species [Citation206]. Toxicity exhibited by CuO NPs are also due to the production of ROS [Citation207]. In general, CuO NPs induced cellular stress results in apoptosis which act as a basic indicator for researcher to study the therapeutic prospective of CuO NPs [Citation208]. These NPs participate in inhibition of in vitro growth and autophagostic mechanism in MCF-7 cells, demonstrating that CuO NPs in conjugation with autophagy inhibitor are vital in induction of apoptosis in breast cancer cells [Citation209]. MCF-7 cells are usually recognized as resistant to chemical treatments such as adriamycin and paclitaxel [Citation210] and have the possibility to develop responses to cellular stress, such as the MEK5-BMK/Erk5, which may subsidize anti-apoptotic signalling [Citation211]. Regardless of the fact that ROS and cancer are related in a contradictory way; ROS and oxidative stress induce carcinogenesis, but enhance level of ROS induces cell death [Citation212,Citation213].
Silver nanoparticles
Silver NPs have been used in medical sector since long especially well known for antibacterial activity. AgNPs also exhibit in vitro antitumour, antiproliferative and antiangiogenic properties by ROS mediated apoptotic pathway [Citation214]. AgNPs show potential cytotoxicity in breast cancer cells MDA-MB-231, MCF-7 via preventing cell growth by activating production of ROS and caspases-3 which lead to cell death through concentration dependent activation of LDH [Citation215,Citation216]; by disturbing membrane structure, activating different genes involved in apoptosis lead to cell death [Citation217]. AgNPs activates production of ROS and JNK pathway responsible to mitochondrial mediated apoptosis that results in cell death in NIH3T3 cells [Citation218,Citation219]. Various studies highlighted cytotoxicity induced by AgNPs in phagocytosing cells both in mouse peritoneal macrophages and human monocytes [Citation219] due to damages in DNA by generation of ROS [Citation220], increased caspase-3 activity [Citation221]. These findings suggest that AgNPs can be used as a possible alternative agent for human breast cancer therapy by constraining the development of the tumour cells and offers a novel technique to advance molecule for cancer therapy.
Arsenic trioxide
Arsenic trioxide [As2O3] is being employed in medicines for more than 2400 years against cancer and infectious diseases [Citation222]. As2O3 is recognized as a highly reactive molecule and potentially linked with thiol moieties of many proteins [Citation223]. This property of As2O3 has made it an effective candidate against acute promyelocytic leukaemia [APL] [Citation223]. Recent research reveals that As2O3 suppress cell growth and induces apoptosis to breast cancer [Citation224] through inhibition of oestrogen receptor [Citation225]; directive of p53 tumour suppressor protein and down instruction of Bcl-2 protein [Citation226]; introduction of p21 as well as p27 tumour suppressor proteins [Citation227]; induces demethylation and apoptosis [Citation228]; down-regulation of Notch-1 [Citation229] and others [Citation230,Citation231].
Nanocarriers approved by FDA and in clinical trials currently
Scientists are trying to explore new drug candidates to overcome drug resistance offered by conventional pharmaceutics. Nanotechnology based products are being actively used against breast cancer which provide an effective and efficient tool and aid in the reduction of mortality rate caused by this devastating disease. Recently, there are number of nanotechnology based products that have been approved by FDA to be applied for breast cancer and some are in clinical trials too be rigorously tested either can be applicable for breast cancer or not ().
Table 2. Nanotechnology based FDA approved products against breast cancer.
Table 3. Nanotechnology based products in clinical trials against breast cancer.
Table 4. Nanotechnology based inorganic nanocarriers used in research against breast cancer.
Fate of metallic NPs in clinical treatment of breast cancer
Traditional treatment to cure cancer are toxic for normal cells which results in severe side effects in patient’s even damages their immune system due to non-targeted drug delivery. On the other way round, nanotechnology offers targeted drug delivery by limiting side effects linked with conventional techniques. Various products are in clinical trials which have been proved to be most effective than other medications because of smaller particle size. Lot of FDA approved organic nanocarriers are in practice for breast cancer while inorganic nanocarriers are being actively explored for anticancer activity. Various metallic based NPs are in progress which show superior anticancer potential in breast cancer (). Abraxane is among the most obvious product of nanotechnology towards the treatment of breast cancer along with many more. In case of inorganic nanocarriers gold, iron, platinum, arsenic trioxide etc. show enhanced anticancer activity and are in rigorous clinical phase to evaluate their superior effects in contrast to other drugs. Some of them are CYT-6091, Oxaliplatin, Trisenox® etc. are FDA approved inorganic nanocarriers. Obvious advantages offered by nanotechnology over conventional anticancer agents attribute due to the ability of NPs to cross the fine capillaries, easiest penetration within cells and tissue to reach at the targeted place, sustained drug release and reduced toxicity. In spite of these characteristics nanotechnological products are still not be able to replace conventional techniques used against breast cancer and are used with adjuvant conventional treatments.
Conclusion
Uncontrolled cell division results in a group of pathologies known as cancer. Among various types of cancer; breast cancer (BC) with various phenotypes has become problematic for humanity since a long ago due to association of high mortality rate. Many conventional techniques have been available to cure cancer like surgery, hormonal therapy, radiation and chemical treatment. All such treatments are quite painful and associate with severe side effects. For example, in surgery in some cases, whole of the breast of the patient is removed that bring serious social issues for the female patient. Besides this, conventional therapies also face major limitations since form the detection to the treatment of BC because of non-specific targeting and drug resistance. These limitations demand an urgent need to develop a novel and targeted method that can deliberately overcome human sufferings associated with BC. Recently nanotechnological based products offer many advantages to be employed as a powerful tool against BC. Various organic and inorganic based nanocarriers are in practice and some are in clinical phases with outstanding results against BC due to their specific targeting. From above all it is concluded that nanotechnological based nanocarriers like liposomes, micelles, gold, iron, platinum etc. will be specifically delivered by taking advantage of active and passive drug targeting and useful to cure BC. By this way, we can omit the need of conventional therapies although plenty of research work is needed to translate potentials of nanotechnology in medicines to bring a breakthrough in cancer technology. However, issues linked with nanotechnology are like problematic control in liquid and dry forms, limited drug loading, burst release and toxicity. So, in order to translate the potential advantages offered by nanotechnology, more research is needed to rigorously analyze the toxicity associated with NPs and systemic studies to evaluate the mechanism of action etc. It establishes new grounds for enlists to bring these NPs to demolish the roots of breast cancer and overcome the sufferings.
Disclosure Statement
No potential conflict of interest was reported by the authors.
References
- Bhandare N, Narayana A. Applications of nanotechnology in cancer: a literature review of imaging and treatment. J Nuclear Med Rad Ther. 2014;5:195.
- Parhi P, Mohanty C, Sahoo SK. Nanotechnology-based combinational drug delivery: an emerging approach for cancer therapy. Drug Dis. Today. 2012;17:1044–1052.
- Jemal A, Bray F, Center MM, et al. Global cancer statistics. Can J Clinic. 2011;61:69–90.
- Misra R, Acharya S, Sahoo SK. Cancer nanotechnology: application of nanotechnology in cancer therapy. Drug Dis Today. 2010;15:842–850.
- Hamaoka T, Madewell JE, Podoloff DA, et al. Bone imaging in metastatic breast cancer. J Clin Oncol. 2004;22:2942–2953.
- Weigelt B, Reis-Filho JS. Histological and molecular types of breast cancer: is there a unifying taxonomy? Nat Rev Clinical Oncol. 2009;6:718–730.
- Lacey JV, Devesa SS, Brinton LA. Recent trends in breast cancer incidence and mortality. Environ Mol Mutag. 2002;39:82–88.
- Plodinec M, Loparic M, Monnier CA. et al. The nanomechanical signature of breast cancer. Nature Nanotechnol. 2012;7:757–765.
- Giordano SH, Cohen DS, Buzdar AU, et al. Breast carcinoma in men. Cancer. 2004;101(1):51–57.
- Vance GH, Barry TS, Bloom KJ, et al. Genetic heterogeneity in HER2 testing in breast cancer: panel summary and guidelines. Arc Pathol Lab Med. 2009;133(4):611–612.
- Tanaka T, Decuzzi P, Cristofanilli M, et al. Nanotechnology for breast cancer therapy. Biomedical Microdev. 2009;11(1):49–63.
- Carlson RW, Allred DC, Anderson BO, et al. Invasive breast cancer. J Nat Compreh Cancer Net. 2011;9(2):136–222.
- Benson JR, Jatoi I, Keisch M, et al. Early breast cancer. Lancet. 2009;373(9673):1463–1479.
- El Saghir NS, Eniu A, Carlson RW, et al. Locally advanced breast cancer. Cancer. 2008;113(S8):2315–2324.
- Baselga J, Campone M, Piccart M, et al. Everolimus in postmenopausal hormone-receptor–positive advanced breast cancer. New Eng J Med. 2012;366(6):520–529.
- Engel RH, Kaklamani VG. HER2-positive breast cancer. Drugs. 2007;67(9):1329–1341.
- Ray-Coquard I, Cropet C, Van Glabbeke M, et al. Lymphopenia as a prognostic factor for overall survival in advanced carcinomas, sarcomas, and lymphomas. Cancer Res. 2009;69(13):5383–5391.
- Takkouche B, Regueira-Méndez C, Etminan M. Breast cancer and use of nonsteroidal anti-inflammatory drugs: a meta-analysis. J Nat Can Inst. 2008;100(20):1439–1447.
- Brockhausen I. Mucin‐type O‐glycans in human colon and breast cancer: glycodynamics and functions. EMBO Reports. 2006;7(6):599–604.
- Goshen R, Chu W, Elit L, et al. Is uterine papillary serous adenocarcinoma a manifestation of the hereditary breast–ovarian cancer syndrome? Gynecol Oncol. 2000;79(3):477–481.
- Ferrari M. Cancer nanotechnology: opportunities and challenges. Nat Rev Cancer. 2005;5(3):161–171.
- Shapira A, Livney YD, Broxterman HJ, et al. Nanomedicine for targeted cancer therapy: towards the overcoming of drug resistance. Drug Resist. Updat. 2011;14(3):150–163.
- Grobmyer SR, Morse DL, Fletcher B, et al. The promise of nanotechnology for solving clinical problems in breast cancer. J Surg Oncol. 2011;103(4):317–325.
- Zamboni WC, Torchilin V, Patri AK, et al. Best practices in cancer nanotechnology: perspective from NCI nanotechnology alliance. Clin Cancer Res. 2012;18(12):3229–3241.
- Loebinger MR, Eddaoudi A, Davies D, et al. Mesenchymal stem cell delivery of TRAIL can eliminate metastatic cancer. Cancer Res. 2009;69(10):4134–4142.
- Chariou PL, Lee KL, Wen AM, et al. Detection and imaging of aggressive cancer cells using an epidermal growth factor receptor (EGFR)-targeted filamentous plant virus-based nanoparticle. Bioconjug Chem. 2015;26(2):262–269.
- Nie S, Xing Y, Kim GJ, et al. Nanotechnology applications in cancer. Annu Rev Biomed Eng. 2007;9:257–288.
- Kwon IK, Lee SC, Han B, et al. Analysis on the current status of targeted drug delivery to tumors. J Control Release. 2012;164(2):108–114.
- Yamashita F, Hashida M. Pharmacokinetic considerations for targeted drug delivery. Adv Drug Deliv Rev. 2013;65(1):139–147.
- Mura S, Nicolas J, Couvreur P. Stimuli-responsive nanocarriers for drug delivery. Nat Mater. 2013;12(11):991–1003.
- Azagury A, Khoury L, Enden G, et al. Ultrasound mediated transdermal drug delivery. Adv Drug Deliv Rev. 2014;72:127–143.
- Koo OM, Rubinstein I, Onyuksel H. Role of nanotechnology in targeted drug delivery and imaging: a concise review. Nanomed. 2005;1(3):193–212.
- Nguyen KT. Targeted nanoparticles for cancer therapy: promises and challenges. J Nanomed Nanotechnol. 2011;2(5):1000103e.
- Dwivedi M, Kemp EH, Laddha NC, et al. Regulatory T cells in vitiligo: Implications for pathogenesis and therapeutics. Autoimmun Rev. 2015;14(1):49–56.
- Malhotra M, Tomaro-Duchesneau C, Prakash S. Synthesis of TAT peptide-tagged PEGylated chitosan nanoparticles for siRNA delivery targeting neurodegenerative diseases. Biomaterials. 2013;34(4):1270–1280.
- Kasinski AL, Kelnar K, Stahlhut C, et al. A combinatorial microRNA therapeutics approach to suppressing non-small cell lung cancer. Oncogene. 2015;34(27):3547–3555.
- Mo R, Jiang T, Gu Z. Enhanced anticancer efficacy by ATP‐mediated liposomal drug delivery. Angew Chem Int Ed. 2014;53(23):5815–5820.
- Hrubý M, Filippov SK, Štěpánek P. Smart polymers in drug delivery systems on crossroads: Which way deserves following? Eur Polym J. 2015;65:82–97.
- Loo C, Lowery A, Halas N, et al. Immunotargeted nanoshells for integrated cancer imaging and therapy. Nano Lett. 2005;5(4):709–711.
- Sinha R, Kim GJ, Nie S, et al. Nanotechnology in cancer therapeutics: bioconjugated nanoparticles for drug delivery. Mol Cancer Ther. 2006;5(8):1909–1917.
- Lal S, Clare SE, Halas NJ. Nanoshell-enabled photothermal cancer therapy: impending clinical impact. Acc Chem Res. 200841(12):1842–1851.
- Portney NG, Ozkan M. Nano-oncology: drug delivery, imaging, and sensing. Anal Bioanal Chem. 2006;384(3):620–630.
- Salzano G, Zappavigna S, Luce A, et al. Transferrin-Targeted Nanoparticles Containing Zoledronic Acid as a Potential Tool to Inhibit Glioblastoma Growth. J Biomed Nanotechnol. 2016;12(4):811–830.
- Avci CB, Kurt CC, Tepedelen BE, et al. Zoledronic acid induces apoptosis via stimulating the expressions of ERN1, TLR2, and IRF5 genes in glioma cells. Tumor Biol. 2016;37(5):6673–6679.
- Kopecka J, Porto S, Lusa S, et al. Zoledronic acid-encapsulating self-assembling nanoparticles and doxorubicin: a combinatorial approach to overcome simultaneously chemoresistance and immunoresistance in breast tumors. Oncotarget. 2016;7(15):20753.
- Murphy SF, Varghese RT, Lamouille S, et al. Connexin 43 inhibition sensitizes chemoresistant glioblastoma cells to temozolomide. Cancer Res. 2016;76(1):139–149.
- Sultana S, Khan MR, Kumar M, et al. Nanoparticles-mediated drug delivery approaches for cancer targeting: a review. J Drug Target. 2013;21(2):107–125.
- Early Breast Cancer Trialists’ Collaborative Group. Tamoxifen for early breast cancer: an overview of the randomised trials. Lancet. 1998;351(9114):1451–1467.
- Burstein HJ, Temin S, Anderson H, et al. Adjuvant endocrine therapy for women with hormone receptor–positive breast cancer: American Society of Clinical Oncology clinical practice guideline focused update. J Clin Oncol. 2014;32(21):2255–2269.
- Yuzhakova DV, Shirmanova MV, Sergeeva TF, et al. Immunotherapy of Cancer. Med Technol Med. 2016;8(1):173–181.
- Wang AZ, Langer R, Farokhzad OC. Nanoparticle delivery of cancer drugs. Annu Rev Med. 2012;63:185–198.
- Cuenca AG, Jiang H, Hochwald SN, et al. Emerging implications of nanotechnology on cancer diagnostics and therapeutics. Cancer. 2006;107(3):459–466.
- Sahu SK, Mallick SK, Santra S, et al. In vitro evaluation of folic acid modified carboxymethyl chitosan nanoparticles loaded with doxorubicin for targeted delivery. J Mater Sci Mater Med. 2010;21(5):1587–1597.
- Conde J, Doria G, Baptista P. Noble metal nanoparticles applications in cancer. J Drug Deliv. 2011;2012(2012):1–12.
- Jannesari M, Varshosaz J, Morshed M, et al. Composite poly (vinyl alcohol)/poly (vinyl acetate) electrospun nanofibrous mats as a novel wound dressing matrix for controlled release of drugs. Int J Nanomed. 2011; (6):993–1003.
- Wang J, Chen BA, Cheng J, et al. Apoptotic mechanism of human leukemia K562/A02 cells induced by magnetic iron oxide nanoparticles co-loaded with daunorubicin and 5-bromotetrandrin. Int J Nanomedicine. 2011;6:1027–1034.
- New RRC. Liposomes: a practical approach. Oxford: Oxford University Press; 1990.
- Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat Rev Drug Discov. 2005;4(2):145–160.
- Bawarski WE, Chidlowsky E, Bharali DJ, et al. Emerging nanopharmaceuticals. Nanomed. 2008;4(4):273–282.
- Bangham AD, Standish MM, Watkins JC. Diffusion of univalent ions across the lamellae of swollen phospholipids. J Mol Biol. 1965;13(1):238–IN27.
- Bangham AD. Liposomes: the Babraham connection. Chem Phys Lipids. 1993;64(1):275–285.
- Hofheinz RD, Gnad-Vogt SU, Beyer U, et al. Liposomal encapsulated anti-cancer drugs. Anticancer Drugs. 2005;16(7):691–707.
- Gabizon A, Shmeeda H, Barenholz Y. Pharmacokinetics of pegylated liposomal doxorubicin. Clin Pharmacokinet. 2003;42(5):419–436.
- Zhang JA, Anyarambhatla G, Ma L, et al. Development and characterization of a novel Cremophor® EL free liposome-based paclitaxel (LEP-ETU) formulation. Eur J Pharm Biopharm. 2005;59(1):177–187.
- Krieger ML, Eckstein N, Schneider V, et al. Overcoming cisplatin resistance of ovarian cancer cells by targeted liposomes in vitro. Int J Pharm. 2010;389(1):10–17.
- Zamboni WC, Gervais AC, Egorin MJ, et al. Systemic and tumor disposition of platinum after administration of cisplatin or STEALTH liposomal-cisplatin formulations (SPI-077 and SPI-077 B103) in a preclinical tumor model of melanoma. Cancer Chemother Pharmacol. 2004;53(4):329–336.
- Auguste DT, Furman K, Wong A, et al. Triggered release of siRNA from poly (ethylene glycol)-protected, pH-dependent liposomes. J Control Release. 2008;130(3):266–274.
- Schroeder A, Kost J, Barenholz Y. Ultrasound, liposomes, and drug delivery: principles for using ultrasound to control the release of drugs from liposomes. Chem Phys Lipids. 2009;162(1): 1–16.
- Pashkovskaya A, Kotova E, Zorlu Y, et al. Light-triggered liposomal release: membrane permeabilization by photodynamic action. Langmuir. 2009;26(8):5726–5733.
- Pradhan P, Giri J, Rieken F, et al. Targeted temperature sensitive magnetic liposomes for thermo-chemotherapy. J Control Release. 2010;142(1):108–121.
- Ong W, Yang Y, Cruciano AC, et al. Redox-triggered contents release from liposomes. J Am Chem Soc. 2008;130(44):14739–14744.
- Torchilin V. Multifunctional and stimuli-sensitive pharmaceutical nanocarriers. J Pharm Biopharm. 2009;71(3):431–444.
- Kumari A, Yadav SK, Yadav SC. Biodegradable polymeric nanoparticles based drug delivery systems. Colloids Surf B Biointerfaces. 2010;75(1):1–18.
- Wang X, Wang Y, Chen ZG, et al. Advances of cancer therapy by nanotechnology. Cancer Res Treat. 2009;41(1):1–11.
- Vauthier C, Bouchemal K. Methods for the preparation and manufacture of polymeric nanoparticles. Pharm Res. 2009;26(5):1025–1058.
- Mora-Huertas CE, Fessi H, Elaissari A. Polymer-based nanocapsules for drug delivery. Int J Pharm. 2010;385(1):113–142.
- Langer R, Folkman J. Polymers for the sustained release of proteins and other macromolecules. Nature. 1976;28:797–800.
- Couvreur P, Kante B, Roland M, et al. Adsorption of antineoplastic drugs to polyalkylcyanoacrylate nanoparticles and their release in calf serum. J Pharm Sci. 1979;68(12):1521–1524.
- Gref R, Minamitake Y, Peracchia MT, et al. Biodegradable long-circulating polymeric nanospheres. Science. 1994;263(5153):1600–1603.
- Gref R, Lück M, Quellec P, et al. ‘Stealth’corona-core nanoparticles surface modified by polyethylene glycol (PEG): influences of the corona (PEG chain length and surface density) and of the core composition on phagocytic uptake and plasma protein adsorption. Colloids Surf B Biointerfaces. 2000;18(3):301–313.
- Tanford C. The hydrophobic effect: formation of micelles and biological membranes. 2nd ed. Malabar (Fla)7 Kreiger Publishing Company; 1991.
- Zhang L, Gu FX, Chan JM, et al. Nanoparticles in medicine: therapeutic applications and developments. Clin Pharmacol Ther. 2008;83(5):761–769.
- Farokhzad OC, Jon S, Khademhosseini A, et al. Nanoparticle-aptamer bioconjugates a new approach for targeting prostate cancer cells. Cancer Res. 2004;64(21):7668–7672.
- Gu F, Zhang L, Teply BA, et al. Precise engineering of targeted nanoparticles by using self-assembled biointegrated block copolymers. Proc Natl Acad Sci. 2008;105(7):2586–2591.
- Aliabadi HM, Shahin M, Brocks DR, et al. Disposition of drugs in block copolymer micelle delivery systems. Clin Pharmacokinet. 2008;47(10):619–634.
- Oerlemans C, Bult W, Bos M, et al. Polymeric micelles in anticancer therapy: targeting, imaging and triggered release. Pharm Res. 2010;27(12):2569–2589.
- Mainardes RM, Silva LP. Drug delivery systems: past, present, and future. Curr Drug Targets. 2004;5(5):449–455.
- Quintana A, Raczka E, Piehler L, et al. Design and function of a dendrimer-based therapeutic nanodevice targeted to tumor cells through the folate receptor. Pharm Res. 2002;19:1310–6.
- Ballauff M, Likos CN. Dendrimers in solution: insight from theory and simulation. Angew Chem Int Ed. 2004;43(23):2998–3020.
- Boas U, Heegaard PM. Dendrimers in drug research. Chem Soc Rev. 2004;33(1):43–63.
- Lee CC, MacKay JA, Fréchet JM, et al. Designing dendrimers for biological applications. Nat Biotechnol. 2005;23(12):1517–1526.
- Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nat Biotechnol. 2007;2(12):751–760.
- Menjoge AR, Kannan RM, Tomalia DA. Dendrimer-based drug and imaging conjugates: design considerations for nanomedical applications. Drug Discov Today. 2010;15(5):171–185.
- Stasko NA, Johnson CB, Schoenfisch MH, et al. Cytotoxicity of polypropylenimine dendrimer conjugates on cultured endothelial cells. Biomacromolecules. 2007;8(12):3853–3859.
- Ke W, Zhao Y, Huang R, et al. Enhanced oral bioavailability of doxorubicin in a dendrimer drug delivery system. J Pharm Sci. 2008;97(6):2208–2216.
- Chauhan AS, Jain NK, Diwan PV, et al. Solubility enhancement of indomethacin with poly (amidoamine) dendrimers and targeting to inflammatory regions of arthritic rats. J Drug Target. 2004;12(9-10):575–583.
- Tripathi PK, Khopade AJ, Nagaich S, et al. Dendrimer grafts for delivery of 5-fluorouracil. Pharmazie. 2002;57(4):261–264.
- Hussain M, Shchepinov M, Sohail M, et al. A novel anionic dendrimer for improved cellular delivery of antisense oligonucleotides. J Control Release. 2004;99(1):139–155.
- Agarwal A, Asthana A, Gupta U, et al. Tumour and dendrimers: a review on drug delivery aspects. J Pharm Pharmacol. 2008;60(6):671–688.
- Boswell CA, Eck PK, Regino CA, et al. Synthesis, characterization, and biological evaluation of integrin αvβ3-targeted PAMAM dendrimers. Mol Pharm. 2008;5(4):527–539.
- Kratz F. Albumin as a drug carrier: design of prodrugs, drug conjugates and nanoparticles. J Control Release. 2008;132(3):171–183.
- Tiruppathi C, Song W, Bergenfeldt M, et al. Gp60 activation mediates albumin transcytosis in endothelial cells by tyrosine kinase-dependent pathway. J Biol Chem. 1997;272(41):25968–25975.
- Arruebo M, Galán M, Navascués N, et al. Development of magnetic nanostructured silica-based materials as potential vectors for drug-delivery applications. Chem Mater. 2006;18(7):1911–1919.
- Frechet JMJ. Functional polymers: from plastic electronics to polymer-assisted therapeutics. Prog Polym Sci. 2005;30(8):844–857.
- Daniel MC, Astruc D. Gold nanoparticles: assembly, supramolecular chemistry, quantum-size-related properties, and applications toward biology, catalysis, and nanotechnology. Chem Rev. 2004;104(1):293–346.
- Murphy CJ, Sau TK, Gole AM, et al. Anisotropic metal nanoparticles: synthesis, assembly, and optical applications. J Phys Chem B. 2005;109(29):13857–13870.
- Bardhan R, Lal S, Joshi A, et al. Theranostic nanoshells: from probe design to imaging and treatment of cancer. Acc Chem Res. 2011;44(10):936–946.
- Skrabalak SE, Chen J, Sun Y, et al. Gold nanocages: synthesis, properties, and applications. Acc Chem Res. 2008;41(12):1587–1595.
- Link S, El-Sayed MA. Optical properties and ultrafast dynamics of metallic nanocrystals. Annu Rev Phys Chem. 2003;54(1): 331–366.
- Kelly KL, Coronado E, Zhao LL, et al. The optical properties of metal nanoparticles: the influence of size, shape, and dielectric environment. J Phys Chem B. 2003;107(3):668–677.
- Levy Y, Onuchic JN. Mechanisms of protein assembly: lessons from minimalist models. Acc Chem Res. 2006;39(2):135–142.
- Yuan H, Fales AM, Vo-Dinh T. TAT peptide-functionalized gold nanostars: enhanced intracellular delivery and efficient NIR photothermal therapy using ultralow irradiance. J Am Chem Soc. 2012;134(28):11358–11361.
- Ali MR, Panikkanvalappil SR, El-Sayed MA. Enhancing the efficiency of gold nanoparticles treatment of cancer by increasing their rate of endocytosis and cell accumulation using rifampicin. J Am Chem Soc. 2014;136(12):4464–4467.
- Jain S, Hirst DG, O’Sullivan JM. Gold nanoparticles as novel agents for cancer therapy. Br J Radiol. 2012;85 (1010):101–113.
- Sam M, Hwang JH, Chanfreau G, et al. Hydroxyl radical is the active species in photochemical DNA strand scission by bis (peroxo) vanadium (V) phenanthroline. Inorg Chem. 2004;43(26):8447–8455.
- Hirsch L, Stafford RJ, Bankson JA, et al. Nanoshell-mediated near-infrared thermal therapy of tumors under magnetic resonance guidance. Proc Natl Acad Sci. 2003;100(23):13549–13554.
- Libutti SK, Paciotti GF, Byrnes AA, et al. Phase I and pharmacokinetic studies of CYT-6091, a novel PEGylated colloidal gold-rhTNF nanomedicine. Clin Cancer Res. 2010;16(24):6139–6149.
- Kauer-Dorner D, Pötter R, Resch A, et al. Partial breast irradiation for locally recurrent breast cancer within a second breast conserving treatment: alternative to mastectomy? Results from a prospective trial. Radiother Oncol. 2012;102(1):96–101.
- Lowery AR, Gobin AM, Day ES, et al. Immunonanoshells for targeted photothermal ablation of tumor cells. Int J Nanomedicine. 2006;1(2):149–154.
- Kim ST, Chompoosor A, Yeh YC, et al. Dendronized gold nanoparticles for siRNA delivery. Small. 2012;8(21):3253–3256.
- Joshi P, Chakraborti S, Ramirez-Vick JE, et al. The anticancer activity of chloroquine-gold nanoparticles against MCF-7 breast cancer cells. Colloids Surf B Biointerfaces. 2012;95:195–200.
- Kuo TR, Hovhannisyan VA, Chao YC, et al. Multiple release kinetics of targeted drug from gold nanorod embedded polyelectrolyte conjugates induced by near-infrared laser irradiation. J Am Chem Soc. 2010;132(40):14163–14171.
- Decuzzi P, Godin B, Tanaka T, et al. Size and shape effects in the biodistribution of intravascularly injected particles. J Control Release. 2010;141(3):320–327.
- El-Sayed IH, Huang X, El-Sayed MA. Selective laser photo-thermal therapy of epithelial carcinoma using anti-EGFR antibody conjugated gold nanoparticles. Cancer Lett. 2006;239(1):129–135.
- Wu P, Gao Y, Zhang H, et al. Aptamer-guided silver–gold bimetallic nanostructures with highly active surface-enhanced raman scattering for specific detection and near-infrared photothermal therapy of human breast cancer cells. Anal Chem. 2012;84(18):7692–7699.
- Yang X, Liu X, Liu Z, et al. Near‐Infrared Light‐Triggered, Targeted Drug Delivery to Cancer Cells by Aptamer Gated Nanovehicles. Adv Mater. 2012;24(21):2890–2895.
- Shi P, Qu K, Wang J, et al. pH-responsive NIR enhanced drug release from gold nanocages possesses high potency against cancer cells. Chem Commun. 2012;48(61):7640–7642.
- Ma Y, Liang X, Tong S, et al. Gold Nanoshell Nanomicelles for Potential Magnetic Resonance Imaging, Light‐Triggered Drug Release, and Photothermal Therapy. Adv Funct Mater. 2013;23(7):815–822.
- Gao Y, Li Y, Wang Y, et al. Controlled synthesis of multilayered gold nanoshells for enhanced photothermal therapy and SERS detection. Small. 2015;11(1):77–83.
- Lin J, Wang S, Huang P, et al. Photosensitizer-loaded gold vesicles with strong plasmonic coupling effect for imaging-guided photothermal/photodynamic therapy. ACS Nano. 2013;7(6):5320–5329.
- Chattopadhyay N, Cai Z, Pignol JP, et al. Design and characterization of HER-2-targeted gold nanoparticles for enhanced X-radiation treatment of locally advanced breast cancer. Mol Pharm. 2010;7(6):2194–2206.
- Koyama T, Shimura M, Minemoto Y, et al. Evaluation of selective tumor detection by clinical magnetic resonance imaging using antibody-conjugated superparamagnetic iron oxide. J Control Release. 2012;159(3):413–418.
- Pai AB, Garba AO. Ferumoxytol: a silver lining in the treatment of anemia of chronic kidney disease or another dark cloud? J Blood Med. 2012;3:77–85.
- Schütt W, Grüttner C, Häfeli U, et al. Applications of magnetic targeting in diagnosis and therapy—possibilities and limitations: a mini-review. Hybridoma. 1997;16(1):109–117.
- Gupta AK, Gupta M. Synthesis and surface engineering of iron oxide nanoparticles for biomedical applications. Biomaterials. 2005;26(18):3995–4021.
- Dilnawaz F, Singh A, Mohanty C, et al. Dual drug loaded superparamagnetic iron oxide nanoparticles for targeted cancer therapy. Biomaterials. 2010;31(13):3694–3706.
- Jurgons R, Seliger C, Hilpert A, et al. Drug loaded magnetic nanoparticles for cancer therapy. J Phys Condens Matter. 2006;18(38):S2893–S2902.
- Hu FX, Neoh KG, Kang ET. Synthesis and in vitro anti-cancer evaluation of tamoxifen-loaded magnetite/PLLA composite nanoparticles. Biomaterials. 2006;27(33):5725–5733.
- Zhang Y, Li L, Tang F, et al. Controlled drug delivery system based on magnetic hollow spheres/polyelectrolyte multilayer core–shell structure. J Nanosci Nanotechnol. 2006;6(9–10):3210–3214.
- Ma Y, Manolache S, Denes F, et al. Plasma synthesis of carbon-iron magnetic nanoparticles and immobilization of doxorubicin for targeted drug delivery. J Mater Eng Perform. 2006;15(3):376–382.
- Zhang R, Wang X, Wu C, et al. Synergistic enhancement effect of magnetic nanoparticles on anticancer drug accumulation in cancer cells. Nanotechnology. 2006;17(14):3622.
- Yang J, Lee H, Hyung W, et al. Magnetic PECA nanoparticles as drug carriers for targeted delivery: synthesis and release characteristics. J Microencapsul. 2006;23(2):203–212.
- Zhang JQ, Zhang ZR, Yang H, et al. Lyophilized paclitaxel magnetoliposomes as a potential drug delivery system for breast carcinoma via parenteral administration: in vitro and in vivo studies. Pharm Res. 2005;22(4):573–583.
- Tadahikokubo TS, Shimose S, Nitta Y, et al. Targeted systemic chemotherapy using magnetic liposomes with incorporated adriamycin for osteosarcoma in hamsters. Int J Oncol. 2001;18:121–125.
- Jain TK, Morales MA, Sahoo SK, et al. Iron oxide nanoparticles for sustained delivery of anticancer agents. Mol Pharm. 2005;2(3):194–205.
- Gazeau F, Lévy M, Wilhelm C. Optimizing magnetic nanoparticle design for nanothermotherapy. Nanomed. 2008;3:831–844.
- Sanson C, Diou O, Thevenot J, et al. Doxorubicin loaded magnetic polymersomes: theranostic nanocarriers for MR imaging and magneto-chemotherapy. ACS Nano. 2011;5(2):1122–1140
- Kim D, Lee N, Park M, et al. Synthesis of uniform ferrimagnetic magnetite nanocubes. J Am Chem Soc. 2008;131(2):454–455.
- Lee N, Kim H, Choi SH, et al. Magnetosome-like ferrimagnetic iron oxide nanocubes for highly sensitive MRI of single cells and transplanted pancreatic islets. Proc Natl Acad Sci. 2011;108(7):2662–2667.
- Lee JH, Huh YM, Jun YW, et al. Artificially engineered magnetic nanoparticles for ultra-sensitive molecular imaging. Nat Med. 2007;13(1):95–99.
- Zhao C, Rehman FU, Yang Y, et al. Bio-imaging and Photodynamic Therapy with Tetra Sulphonatophenyl Porphyrin (TSPP)-TiO2 Nanowhiskers: New Approaches in Rheumatoid Arthritis Theranostics. Sci Rep. 2015;5:1–11.
- Setyawati MI, Tay CY, Leong DT. Mechanistic investigation of the biological effects of SiO2, TiO2, and zno nanoparticles on intestinal cells. Small. 2015;11(28):3458–3468.
- Jin C, Tang Y, Yang FG, et al. Cellular toxicity of TiO2 nanoparticles in anatase and rutile crystal phase. Biol Trace Elem Res. 2011;141(1–3):3–15.
- Carvalho C, Santos RX, Cardoso S, et al. Doxorubicin: the good, the bad and the ugly effect. Curr Med Chem. 2009;16(25):3267–3285.
- Pages BJ, Ang DL, Wright EP, et al. Metal complex interactions with DNA. Dalton Trans. 2015;44(8):3505–3526.
- Ali A, Bhattacharya S. DNA binders in clinical trials and chemotherapy. Bioorg Med Chem. 2014;22(16):4506–4521.
- Glasner H, Tshuva EY. C 1-Symmetrical Titanium (IV) Complexes of Salan Ligands with Differently Substituted Aromatic Rings: Enhanced Cytotoxic Activity. Inorg Chem. 2014;53(6):3170–3176.
- Chen Y, Wan Y, Wang Y, et al. Anticancer efficacy enhancement and attenuation of side effects of doxorubicin with titanium dioxide nanoparticles. Int J Nanomedicine. 2011;6:2321–6.
- Hall MD, Mellor HR, Callaghan R, et al. Basis for design and development of platinum (IV) anticancer complexes. J Med Chem. 2007;50(15):3403–3411.
- Schiesser S, Hackner B, Vrabel M, et al. Synthesis and DNA‐Damaging Properties of Cisplatin‐N‐Mustard Conjugates. European J Org Chem. 2015; (12):2654–2660.
- Gao J, Liang G, Zhang B, et al. FePt@ CoS2 yolk-shell nanocrystals as a potent agent to kill HeLa cells. J Am Chem Soc. 2007;129(5):1428–1433.
- Wang X, Liu F, Andavan GT, et al. Carbon nanotube–DNA nanoarchitectures and electronic functionality. Small. 2006;2(11):1356–1365.
- Xu C, Yuan Z, Kohler N, et al. FePt nanoparticles as an Fe reservoir for controlled Fe release and tumor inhibition. J Am Chem Soc. 2009;131(42):15346–15351.
- Xu C, Wang B, Sun S. Dumbbell-like Au − Fe3O4 nanoparticles for target-specific platin delivery. J Am Chem Soc. 2009;131(12):4216–4217.
- Galluzzi L, Senovilla L, Vitale I, et al. Molecular mechanisms of cisplatin resistance. Oncogene. 2012;31(15):1869–1883.
- Akshintala S, Marcus L, Warren KE, et al. Phase 1 trial and pharmacokinetic study of the oral platinum analog satraplatin in children and young adults with refractory solid tumors including brain tumors. Pediatr Blood Cancer. 2015;62(4):603–610.
- Weeks ME. The discovery of the elements. XVI. The rare earth elements. J Chem Educ. 1932;9(10):1751.
- Jakupec MA, Unfried P, Keppler BK. Pharmacological properties of cerium compunds. Rev Physiol Biochem Pharmacol. 2005;153:101–111.
- Hirst SM, Karakoti A, Singh S, et al. Bio‐distribution and in vivo antioxidant effects of cerium oxide nanoparticles in mice. Environ Toxicol. 2013;28(2):107–118.
- Xu C, Qu, X. Cerium oxide nanoparticle: a remarkably versatile rare earth nanomaterial for biological applications. NPG Asia Mater. 2014;6(3):e90.
- Walkey C, Das S, Seal S, et al. Catalytic properties and biomedical applications of cerium oxide nanoparticles. Environ Sci Nano. 2015;2(1):33–53.
- Colon J, Hsieh N, Ferguson A, et al. Cerium oxide nanoparticles protect gastrointestinal epithelium from radiation-induced damage by reduction of reactive oxygen species and upregulation of superoxide dismutase 2. Nanomedicine. 2010;6(5):698–705.
- Xue Y, Luan Q, Yang D, et al. Direct evidence for hydroxyl radical scavenging activity of cerium oxide nanoparticles. J Phys Chem C. 2011;115(11):4433–4438.
- Celardo I, De Nicola M, Mandoli C, et al. Ce3+ ions determine redox-dependent anti-apoptotic effect of cerium oxide nanoparticles. ACS Nano. 2011;5(6):4537–4549.
- Gao Y, Chen K, Ma JL, et al. Cerium oxide nanoparticles in cancer. Onco Targets Ther. 2014;7:835–840.
- Ouyang Z, Mainali MK, Sinha N, et al. Potential of using cerium oxide nanoparticles for protecting healthy tissue during accelerated partial breast irradiation (APBI). Phys Med. 2016;32(4):631–635.
- Khanna PK, More PV, Jawalkar JP, Bharate BG. Effect of reducing agent on the synthesis of nickel nanoparticles. Mater Lett. 2009;63(16):1384–1386.
- Hossain MZ, Kleve MG. Nickel nanowires induced and reactive oxygen species mediated apoptosis in human pancreatic adenocarcinoma cells. Int J Nanomedicine. 2011;6:1475–1485.
- Ahamed M, Alhadlaq HA. Nickel nanoparticle-induced dose-dependent cyto-genotoxicity in human breast carcinoma MCF-7 cells. Onco Targets Ther. 2014;7:269–280.
- Guo D, Wu C, Hu H, et al. Study on the enhanced cellular uptake effect of daunorubicin on leukemia cells mediated via functionalized nickel nanoparticles. Biomed Mater. 2009;4(2):025013.
- Chen M, Zhang Y, Huang B, et al. Evaluation of the antitumor activity by Ni nanoparticles with verbascoside. J Nanomater. 2013;2013:623497.
- Umaralikhan L, Jaffar MJM. Antibacterial and anticancer properties of NiO nanoparticles by co-precipitation method. J Adv Appl Sci Res. 2016;1(4):24–35.
- Kelleher SL, McCormick NH, Velasquez V, et al. Zinc in specialized secretory tissues: roles in the pancreas, prostate, and mammary gland. Advances in Nutrition: Int Rev J. 2011;2(2):101–111.
- Hanley C, Layne J, Punnoose A, et al. Preferential killing of cancer cells and activated human T cells using ZnO nanoparticles. Nanotechnology. 2008;19(29):295103.
- Hanley C, Thurber A, Hanna C, et al. The influences of cell type and ZnO nanoparticle size on immune cell cytotoxicity and cytokine induction. Nanoscale Res Lett. 2009;4(12):1409–1420.
- Akhtar MJ, Ahamed M, Kumar S, et al. Zinc oxide nanoparticles selectively induce apoptosis in human cancer cells through reactive oxygen species. Int J Nanomedicine. 2012;7:845–857.
- Madani F, Lindberg S, Langel Ü, et al. Mechanisms of cellular uptake of cell-penetrating peptides. J Biophys. 2011;2011
- Papo N, Shahar M, Eisenbach L, et al. A novel lytic peptide composed of DL-amino acids selectively kills cancer cells in culture and in mice. J Biol Chem. 2003;278(23):21018–21023.
- Asati A, Santra S, Kaittanis C, et al. Surface-charge-dependent cell localization and cytotoxicity of cerium oxide nanoparticles. ACS Nano. 2010;4(9):5321–5331.
- Tripathy N, Ahmad R, Ko HA, et al. Enhanced anticancer potency using an acid-responsive ZnO-incorporated liposomal drug-delivery system. Nanoscale. 2015;7(9):4088–4096.
- Xia T, Kovochich M, Brant J, et al. Comparison of the abilities of ambient and manufactured nanoparticles to induce cellular toxicity according to an oxidative stress paradigm. Nano Lett. 2006;6(8):1794–1807.
- Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35(9):505–513.
- Taccola L, Raffa V, Riggio C, et al. Zinc oxide nanoparticles as selective killers of proliferating cells. Int J Nanomed. 2011;6:1129–1140.
- Wingett D, Louka P, Anders CB, et al. A role of ZnO nanoparticle electrostatic properties in cancer cell cytotoxicity. Nanotechnol Sci Appl. 2016;9:29–45.
- Wang K, Xu JJ, Chen HY. A novel glucose biosensor based on the nanoscaled cobalt phthalocyanine–glucose oxidase biocomposite. Biosens Bioelectron. 2005;20(7):1388–1396.
- Papis E, Rossi F, Raspanti M, et al. Engineered cobalt oxide nanoparticles readily enter cells. Toxicol Lett. 2009;189(3):253–259.
- Chattopadhyay S, Dash SK, Ghosh T, et al. Surface modification of cobalt oxide nanoparticles using phosphonomethyl iminodiacetic acid followed by folic acid: a biocompatible vehicle for targeted anticancer drug delivery. Cancer Nanotechnol. 2013;4(4–5):103–116.
- Bouchard LS, Anwar MS, Liu GL, et al. Picomolar sensitivity MRI and photoacoustic imaging of cobalt nanoparticles. Proc Natl Acad Sci. 2009;106(11):4085–4089.
- Sadjadi MS, Pourahmad A, Sohrabnezhad S, et al. Formation of NiS and CoS semiconductor nanoparticles inside mordenite-type zeolite. Mater Lett. 2007;61(14):2923–2926.
- Ansari SM, Bhor RD, Pai KR, et al. Size and Chemistry Controlled Cobalt-Ferrite Nanoparticles and Their Anti-Proliferative Effect against the MCF-7 Breast Cancer Cells. ACS Biomater Sci Eng. 2016;2(12):2139–2152.
- Levine B, Klionsky DJ. Development by self-digestion: molecular mechanisms and biological functions of autophagy. Dev Cell. 2004;6(4):463–477.
- Wang HY, Hua XW, Wu FG, et al. Synthesis of ultrastable copper sulfide nanoclusters via trapping the reaction intermediate: potential anticancer and antibacterial applications. ACS Appl Mater Interfaces. 2015;7(13):7082–092.
- Lee T, El-Said WA, Min J, et al. Multifunctional DNA-based biomemory device consisting of ssDNA/Cu heterolayers. Biosens Bioelectron. 2011;26(5):2304–2310.
- Meghana S, Kabra P, Chakraborty S, et al. Understanding the pathway of antibacterial activity of copper oxide nanoparticles. RSC Adv. 2015;5(16):12293–12299.
- Fahmy B, Cormier SA. Copper oxide nanoparticles induce oxidative stress and cytotoxicity in airway epithelial cells. Toxicol In Vitro. 2009;23(7):1365–1371.
- Wang Y, Zi XY, Su J, et al. Cuprous oxide nanoparticles selectively induce apoptosis of tumor cells. Int J Nanomedicine. 2012;7:2641–2652.
- Laha D, Pramanik A, Maity J, et al. Interplay between autophagy and apoptosis mediated by copper oxide nanoparticles in human breast cancer cells MCF7. Biochim Biophys Acta. 2014;1840(1):1–9.
- Simstein R, Burow M, Parker A, et al. Apoptosis, chemoresistance, and breast cancer: insights from the MCF-7 cell model system. Exp Biol Med. 2003;228(9):995–1003.
- Fu LL, Yang Y, Xu HL, et al. Identification of novel caspase/autophagy‐related gene switch to cell fate decisions in breast cancers. Cell Prolif. 2013;46(1):67–75.
- Hanagata N, Zhuang F, Connolly S, et al. Molecular responses of human lung epithelial cells to the toxicity of copper oxide nanoparticles inferred from whole genome expression analysis. ACS Nano. 2011;5(12):9326–9338.
- Laurent A, Nicco C, Chéreau C, et al. Controlling tumor growth by modulating endogenous production of reactive oxygen species. Cancer Res. 2005;65(3):948–956.
- Foldbjerg R, Dang DA, Autrup H. Cytotoxicity and genotoxicity of silver nanoparticles in the human lung cancer cell line, A549. Arch Toxicol. 2011;85(7):743–750.
- Gurunathan S, Han JW, Eppakayala V, et al. Cytotoxicity of biologically synthesized silver nanoparticles in MDA-MB-231 human breast cancer cells. Biomed Res Int. 2013; 2013:1–10
- Franco-Molina MA, Mendoza-Gamboa E, Sierra-Rivera CA, et al. Antitumor activity of colloidal silver on MCF-7 human breast cancer cells. J Exp Clin Cancer Res. 2010;29(1):148.
- Sanpui P, Chattopadhyay A, Ghosh SS. Induction of apoptosis in cancer cells at low silver nanoparticle concentrations using chitosan nanocarrier. ACS Appl Mater Interfaces. 2011;3(2):218–228
- Hsin YH, Chen CF, Huang S, et al. The apoptotic effect of nanosilver is mediated by a ROS-and JNK-dependent mechanism involving the mitochondrial pathway in NIH3T3 cells. Toxicol Lett. 2008;179(3):130–139.
- Foldbjerg R, Olesen P, Hougaard M, et al. PVP-coated silver nanoparticles and silver ions induce reactive oxygen species, apoptosis and necrosis in THP-1 monocytes. Toxicol Lett. 2009;190(2):156–162.
- Ahmad P, Sarwat M, Sharma S. Reactive oxygen species, antioxidants and signaling in plants. J Plant Biol. 2008;51(3):167–173.
- Sriram MI, Kanth SBM, Kalishwaralal K, et al. Antitumor activity of silver nanoparticles in Dalton’s lymphoma ascites tumor model. Int J Nanomedicine. 2010;5(1):753–762.
- Vernhet L, Allain N, Le Vée M, et al. Blockage of multidrug resistance-associated proteins potentiates the inhibitory effects of arsenic trioxide on CYP1A1 induction by polycyclic aromatic hydrocarbons. J Pharmacol Exp Ther. 2003;304(1):145–155.
- Emadi A, Gore SD. Arsenic trioxide—an old drug rediscovered. Blood Rev. 2010;24(4):191–199.
- Lengfelder E, Hofmann WK, Nowak D. Impact of arsenic trioxide in the treatment of acute promyelocytic leukemia. Leukemia. 2012;26(3):433–442.
- Chen GC, Guan LS, Hu WL, et al. Functional repression of estrogen receptor a by arsenic trioxide in human breast cancer cells. Anticancer Res. 2001;22(2A):633–638.
- Chow SK, Chan JY, Fung KP. Inhibition of cell proliferation and the action mechanisms of arsenic trioxide (As2O3) on human breast cancer cells. J Cell Biochem. 2004;93(1):173–187.
- Wang X, Gao P, Long M, et al. Essential role of cell cycle regulatory genes p21 and p27 expression in inhibition of breast cancer cells by arsenic trioxide. Med Oncol. 2011;28(4):1225–1254.
- Davison K, Mann KK, Miller WH. Arsenic trioxide: mechanisms of action. Semin Hematol. 2002;39(2):3–7.
- Xia J, Li Y, Yang Q, et al. Arsenic trioxide inhibits cell growth and induces apoptosis through inactivation of notch signaling pathway in breast cancer. Int J Mol Sci. 2012;13(8):9627–9641.
- Du J, Zhou N, Liu H, et al. Arsenic induces functional re-expression of estrogen receptor α by demethylation of DNA in estrogen receptor-negative human breast cancer. PLoS One. 2012;7(4):e35957.
- Wang Y, Zhang Y, Yang L, et al. Arsenic trioxide induces the apoptosis of human breast cancer MCF-7 cells through activation of caspase-3 and inhibition of HERG channels. Exp Ther Med. 2011;2(3):481–486.
- https://clinicaltrials.gov/ct2/show/record/NCT01190982 (Acessesd on 19-12, 2016)
- https://clinicaltrials.gov/show/NCT01151384 (Acessesd on 19-12, 2016)
- Stathopoulos GP, Boulikas T. Lipoplatin formulation review article. J Drug Deliv. 2011;2012:1–10.
- Saif MW. MM-398 achieves primary endpoint of overall survival in phase III study in patients with gemcitabine refractory metastatic pancreatic cancer. JOP. 2014;15(3):278–279.
- Awada A, Bondarenko IN, Bonneterre J, et al. A randomized controlled phase II trial of a novel composition of paclitaxel embedded into neutral and cationic lipids targeting tumor endothelial cells in advanced triple-negative breast cancer (TNBC). Ann Oncol. 2014;25(4):824–831.
- Cattaneo AG, Gornati R, Sabbioni E, et al. Nanotechnology and human health: risks and benefits. J Appl Toxicol. 2010;30(8):730–744.
- Zagar TM, Vujaskovic Z, Formenti S, et al. Two phase I dose-escalation/pharmacokinetics studies of low temperature liposomal doxorubicin (LTLD) and mild local hyperthermia in heavily pretreated patients with local regionally recurrent breast cancer. Int J Hyperthermia. 2014;30(5):285–294.
- https://clinicaltrials.gov/ct2/show/record/NCT02213744 (Accessed on -12, 2016)
- Immordino ML, Dosio F, Cattel L. Stealth liposomes: review of the basic science, rationale, and clinical applications, existing and potential. Int J Nanomed. 2006;1(3):297–315.
- https://clinicaltrials.gov/ct2/show/record/NCT00876486(Accessed on 19-12, 2016)
- Booser DJ, Perez-Soler R, Cossum P, et al. Phase I study of liposomal annamycin. Cancer Chemother Pharmacol. 2000;46(5):427–432.
- Couvreur P, Vauthier C. Nanotechnology: intelligent design to treat complex disease. Pharm Res. 2006;23(7):1417–1450.
- Vivek R, Thangam R, Nipunbabu V, et al. Oxaliplatinchitosan nanoparticles induced intrinsic apoptotic signaling pathway: A “smart” drug delivery system to breast cancer cell therapy. Int J Biol Macromol. 2014;65:289–297.
- https://clinicaltrials.gov/ct2/show/record/NCT01644890 (Accessed on 19-12, 2016)
- Liu F, Park JY, Zhang Y, et al. Targeted cancer therapy with novel high drug‐loading nanocrystals. J Pharm Sci. 2010;99(8):3542–3551.
- Kono K, Mimura K, Kiessling R. Immunogenic tumor cell death induced by chemoradiotherapy: molecular mechanisms and a clinical translation. Cell Death Dis. 2013;4(6):e688.
- https://clinicaltrials.gov/ct2/show/record/NCT00075413 (Accessed on 19-12, 2016)