?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Schizonepetae Herba Carbonisata (SHC) has been used in traditional Chinese medicine (TCM) to treat haemorrhagic diseases for more than 1000 years. However, little information is available on its haemostatic components and mechanism. In this study, we developed novel water-soluble carbon dots (CDs) in aqueous extracts of SHC for the first time and a modified pyrolysis method was used to prepare the SHC using Schizonepetae Herba (SH) as the sole precursor. The SHC-CDs were characterized using transmission electron microscopy (TEM), high-resolution TEM (HRTEM), Fourier transform infrared (FT-IR), ultraviolet–visible (UV–Vis) and fluorescence spectroscopy, X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD) and high-performance liquid chromatography (HPLC). Furthermore, the CDs with a quantum yield (QY) around 2.26% exhibited no toxicity within approximately 0.84 mg/mL in the CCK-8 assay. More interestingly, tail haemorrhaging and liver haemorrhaging experiments showed that CDs-treated mice had significantly shorter bleeding time than did normal saline (NS)-treated control group. Coagulation assays suggested that SHC-CDs could stimulate the extrinsic blood coagulation system and activate the fibrinogen system. These results suggested that SHC-CDs possess a remarkable haemostatic property, which provides evidence to support the further investigation of the considerable potential and effective material basis of TCM.
Introduction
Charcoal drugs (called “tan yao” in Chinese) are specialized drugs obtained by charcoal processing of crude herbal medicine and other material including herba (such as Cirsii Japonici Herba), rhizoma (such as Rhei Radix et Rhizoma), pericarpium (such as Granati Pericarpium), pollen (such as Typhae Pollen), folium (such as Nelumbinis Folium), medulla (such as Junci Medulla) and human hair that have been used to stop bleeding for 2000 years in traditional Chinese medicine (TCM). The earliest comprehensive literature report on charcoal drugs is the Prescriptions for Fifty-two Diseases, which was written before the 3rd century B.C. and unearthed in 1973 from the Mawangdui Han Tombs (located in Changsha City, middle area of China and sealed in 168 BC by the Han dynasty). The safety profile and positive curative effect of charcoal drugs have encouraged their continued clinical application with the development of TCM. By 2015, 27 types of charcoal drugs were included in the Pharmacopoeia of the People's Republic of China (PPRC).
Schizonepetae Herba (Jingjie), the dried aerial parts of the plant Schizonepeta tenuifolia (Benth.) Briq. (family Labiatae), is a kind of medicinal herb widely used in China, Korea and Japan [Citation1]. The charcoal-processed product of Schizonepetae Herba (SH), SH Carbonisata (SHC), has been widely used to treat haemorrhagic conditions for more than 1000 years in TCM and was recorded in the Prescriptions People's Welfare Pharmacy during the Song Dynasty (960–1279 A.D. in China) at the earliest. Moreover, evidence from modern pharmacological studies and considerable clinical data have also demonstrated its significant efficacy in the treatment of numerous kinds of haemorrhage such as haematochezia, metrorrhagia, metrostaxis and postpartum haemorrhage [Citation2]. Furthermore, SHC was acknowledged in the PPRC (2015) as a haemostatic drug. Tremendous efforts have been made to elucidate the material basis of SHC and other charcoal drugs from the perspective of active small molecule compounds, but the results have been unsatisfactory. This predicament and challenge compelled us to focus on the novel substances generated after charcoal processing, which are carbon dots (CDs).
Carbon dots are a new type of biocompatible carbon-based nanomaterial that has attracted tremendous attention because of their considerable benefits such as excellent physicochemical and photochemical stability, robust chemical inertness, tunable emissions, ease of functionalization, high biocompatibility, hypotoxicity and hydrophilicity [Citation3–7]. The advantages of the unique CDs have been exploited in the intensive efforts to apply their potential usefulness in biomedical fields such as in biosensors [Citation8–10], in vitro and in vivo bioimaging [Citation11–13], drug delivery and therapy [Citation14–18], and interaction with DNA [Citation19], etc.
Moreover, as an emerging candidate carbonaceous nanomaterial, CDs do not contain any heavy metals and, thus, they are more environmentally friendly and may be much safer for biological applications than conventional materials [Citation20–22]. Currently, the intrinsic bioactivities of CDs are gradually gaining attention. Liu et al. [Citation23] described the hydrothermal carbonization of metronidazole to prepare CDs, which showed great potential selective antibacterial activity against Porphyromonas gingivalis. Shi et al. [Citation24] used candle soot as a carbon source to produce CDs and demonstrated their peroxidase-like activity while Zhao et al. [Citation25] used egg yolk to formulate CDs and investigated their haemostatic efficacy. Although the bioactivities of CDs have gained attention, the exploration of new carbon sources for the preparation the CDs with satisfactory bioactivities is still challenging. It is noteworthy that little attention has been paid to the bioactivities of CDs from traditional herbal medicines, which are currently an excellent resource worth exploring as a research area.
In addition, there are numerous established protocols for the synthesis of luminescent CDs such as pyrolysis [Citation26,Citation27], microwave-mediated synthesis [Citation28], chemical oxidation [Citation29] and hydrothermal treatment [Citation30]. It is noteworthy that the preparation process of SHC is similar to the pyrolysis method, which is conducted under extreme environmental conditions such as high temperature. Therefore, in this study, CDs were prepared, separated and identified from SHC and were named SHC-CDs. Furthermore, we characterized the SHC-CDs using transmission electron microscopy (TEM), high-resolution TEM (HRTEM), X-ray photoelectron spectroscopy (XPS), X-ray diffraction (XRD), as well as fluorescence analysis, and ultraviolet–visible (UV–Vis), Fourier transform infrared (FT-IR) spectroscopy and high-performance liquid chromatography (HPLC). Then, we detected the effects of SHC-CDs on cell viability and assessed the anti-haemorrhagic effects as well as related haemostatic mechanisms of the SHC-CDs.
Materials and methods
Chemicals
The SH material was purchased from Beijing Qiancao Herbal Pieces Co., Ltd., and the SHC was prepared in our laboratory. Haemocoagulase (HC) for injection was purchased from Jinzhou Ahon Pharmaceutical Co., Ltd. (Liaoning, China). Dialysis membranes with a molecular weight of 1000 Da were purchased from Beijing Ruida Henghui Technology Development Co., Ltd. (Beijing, China). The cell counting kit (CCK)-8 was purchased from Dojindo Molecular Technologies, Inc. (Kumamoto, Japan). Pentobarbital sodium and other analytical grade chemical reagents were obtained from Sinopharm Chemical Reagents Beijing (Beijing, China). All the experiments were performed using deionized water (DW).
Animals
This study was performed in accordance with the Guide for the Care and Use of Laboratory Animals and was approved by the Committee of Ethics of Animal Experimentation of the Beijing University of TCM. Male Kunming mice (weighing 29.1 ± 1.0 and 35.0 ± 2.0 g) were purchased from the Laboratory Animal Center, Si Beifu with a Laboratory Animal Certificate of Conformity. They were maintained under the following conditions: temperature, 24.0 ± 1.0 °C; relative humidity, 55–65%; and a 12-h light/dark cycle with ad libitum access to food and water.
Preparation of SHC-CDs
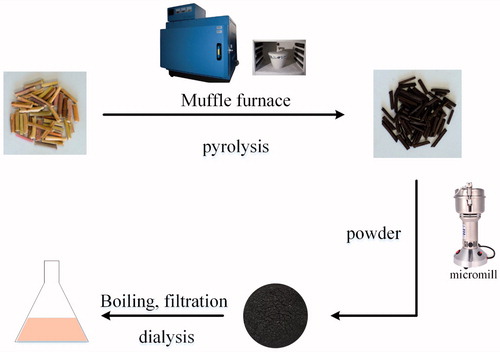
The SHC was prepared using a modified pyrolysis method using a muffle furnace (TL0612, Beijing Zhong Ke Aobo Technology Co., Ltd., Beijing, China). First, SH was placed into a crucible, which was covered with aluminium foil paper before closing the lid to form a tight seal. Then, the calcined temperature was raised to 350 °C within 1 h and maintained there for a further 1 h. Finally, the carbonized product was crushed into a powder after it had cooled to 30 °C.
All the purification procedures for the SHC-CDs were performed using DW. Briefly, 100 g of the fine powder obtained was soaked in 900 mL distilled water and boiled twice for 1 h each time until the solution turned yellowish brown. Then, the resulting solution was pre-filtered to remove the residue, concentrated, and 100 mL was dialysed using a 1000-Da dialysis membrane against DW for 72 h to purify the SHC-CDs. Finally, the solution in the dialysis membrane was collected and evaporated to obtain increasing concentrations of 0.50, 0.20 and 0.10 mg/mL (high-, medium- and low-dose, respectively) before use. The preparation process for the SHC-CDs is shown in .
Characterization of SHC-CDs
The morphology, size and microstructure of the resultant SHC-CDs were characterized using TEM (Tecnai G2 20, FEI Company, Hillsboro, OR), operating at an accelerating voltage of 200 kV. The structural details and atomic lattice fringes of the CDs were examined using HRTEM (JEN-1230, Japan Electron Optics Laboratory, Tokyo, Japan). The spectral properties of the CDs were studied using UV–Vis (CECIL, Cambridge, UK) and fluorescence spectroscopy (F-4500, Tokyo, Japan) using a standard quartz cuvette. In addition, the FTIR (Thermo Fisher, Fremont, CA) was recorded to identify the organic functional groups in the CDs within a spectral window of 400–4000 cm−1. The surface composition and elemental analysis of the CDs were recorded using XPS (ESCALAB 250Xi, Thermo Fisher Scientific, Fremont, CA) using a mono X-ray source Al Kl excitation (1486.6 eV). XRD measurement was performed on a D8 DISCOVER Plus X-ray Diffraction with Cu Ku radiation.
Quantum yield (QY) of SHC-CDs
The fluorescence QY of the SHC-CDs was measured using quinine sulphate (% QY was 54 in 0.1 M sulphuric acid [H2SO4] solution) as a standard sample and calculated using the following equation [Citation31].
where Q represents the QY, I is the integrated area under the emission spectrum, A is the absorbance at 355 nm wavelength and η is the refractive index of the solvent. The subscripts “CDs” and “R” refer to SHC-CDs and standard, respectively. To minimize the reabsorption effect, the AR and ACDs were maintained below 0.05.
Cell viability assay
We assessed the effects of SHC-CDs on the viability of RAW 264.7 cells using a CCK-8 assay [Citation32]. The cells were cultured in Dulbecco’s modified Eagle’s medium (DMEM) supplemented with 20% foetal bovine serum in a humidified 5% CO2 atmosphere at 37 °C. Briefly, cells were seeded in 96-well plates at a density of 1 × 105/well, with 100 μL medium per well. After incubation for 24 h, the original medium in each well was discarded, and 100 μL various concentrations of SHC-CDs were added to the designated wells. The controls were treated with medium only. The cells were cultured for 24 h before adding 10 μL/well CCK-8 solution for an additional 4 h incubation. A microplate reader (Biotek, Winooski, VT) was used to detect the optical density (OD) of each well at a wavelength of 450 nm. The cell viability was calculated using the following formula:
Ae, Ab and Ac represent the A450 of the experimental, blank and control groups, respectively. The experiments were performed in triplicate, independently.
Fingerprint analysis of SH and SHC-CDs using high-performance liquid chromatography
To evaluate the component change of the SHC-CDs, a comparative analysis of the SH and SHC-CDs was performed using an Agilent series 1260 HPLC instrument (Agilent, Waldbronn, Germany) equipped with a column compartment, an autosampler, degasser, quaternary pump and diode-array detector. The detection condition of the SHC-CDs was consistent with that of SH. An aqueous solution of the SHC-CDs and methanol extracts of SH were initially prepared. For the HPLC analysis, a 10-µL aliquot was injected into the Reliasil-C18 column (250 mm × 4.6 mm, Orochem, Naperville, IL) and eluted at 35 °C. The mobile phase consisted of water (solvent A) and methanol (solvent B), which were both filtered through 0.22 µm cellulose acetate membrane filters (Jin Teng, Tianjin, China) prior to use. The gradient program started with 80% solvent A, followed by a linear decrease to 20% solvent A for 10 min, which was maintained for another 30 min. The flow rate was 1.0 mL/min, and the detection wavelength was 254 nm [Citation33].
Haemostasis studies of SHC-CDs
The mouse tail amputation and liver scratch models were established according to previous methods [Citation34,Citation35] with modifications. Briefly, Kunming mice (weighing 29.1 ± 1.0 g) were divided into the following groups and treated subcutaneously as indicated: control (normal saline [NS]), positive (HC, 0.67 ku/kg); and high-, medium- and low-dose SHC-CDs (8.33, 3.33 and 1.67 mg/kg, respectively). For the experiment, the animals were anaesthetized with 4% pentobarbital sodium via intraperitoneal injections (50 mg/kg). In the tail amputation model, the mouse tails were transected using a sterile scalpel at the point where the tail diameter was approximately 1.08–1.12 mm (approximately 10 mm from the tip). Then, each tail was immediately placed on a filter paper.
In the liver scratch model, the liver injury was established by scratching the left lateral lobe with a 2-mL syringe, causing it to bleed and then the incision was overlaid with a filter paper. The bleeding was monitored at an interval of 30 s until haemostasis was achieved. The haemostatic endpoint was defined as the maintenance of haemostasis for 30 min [Citation36], and the time was recorded until the haemorrhage ceased. All the mice were euthanized via cervical dislocation under anaesthesia at the end of each experiment.
Measurement of coagulation parameters [Citation37,Citation38]
A total of 30 Kunming mice (weighing 35.0 ± 2.0 g) were randomly divided into the following five groups (n = 6/group) and subcutaneously treated as indicated: control (NS); HC (0.67 ku/kg); and high-, medium- and low-dose SHC-CDs (8.33, 3.33 and 1.67 mg/kg, respectively). The SHC-CDs doses were determined as the equivalent amount of the SHC-CDs solution based on the analysis results of the haemostasis studies. Two hours after the subcutaneous injections, blood samples were withdrawn from the mice by removing the eyeballs and then placed into plastic tubes with 3.2% sodium citrate (citrate/blood: 1/9, v/v) for at least 10 min before the analysis. Then, the samples were centrifuged at 750×g for 10 min to obtain the supernatant. The prothrombin (PT), activated partial thromboplastin time (APTT), thrombin time (TT) and fibrinogen (FIB) content were measured using an automatic coagulation analyser.
Statistical analysis
The statistical analysis was performed using the statistical package for the social sciences (SPSS, version 13.0, Chicago, IL). The non-normally distributed data were expressed as the median (quartile range). The within group differences were assessed using a non-parametric test while the Wilcoxon rank test was used to compare two groups.
The normally distributed data and homogeneous variances were expressed as the mean ± standard deviation (SD). Multiple comparisons were performed using a one-way analysis of variance (ANOVA) followed by the least significant difference (LSD) test. A p value <.05 was considered statistically significant for the analyses.
Results
Characterization of SHC-CDs
The SHC-CDs extracted from the SHC solution were characterized in the present study. As shown in , the TEM image of the SHC-CDs revealed that the CDs were nearly spherical and well separated from each other. The size distribution of the CDs was in the range of 0.8–4.0 nm (). Furthermore, the HRTEM showed that the CDs had a lattice spacing of 0.393 nm (). In addition, the powder XRD pattern as shown in exhibited a distinct diffraction peaks (2θ = 22.8), which is attributed to amorphous carbon composed in a considerably random fashion [Citation39].
Figure 2. (A) Transmission electron microscopy (TEM) images of Schizonepetae Herba Carbonisata-carbon dots (SHC-CDs) displaying ultra-small particles. Inset: histogram depicting particle size distribution. (B) High-resolution TEM (HRTEM) image of SHC-CDs. Inset: HRTEM image of atomic lattice fringes. (C) XRD pattern, (D) ultraviolet--visible (UV--vis), (E) fluorescence and (F) FTIR spectra.
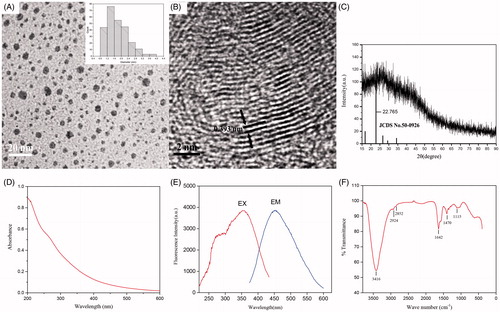
The emission spectrum of SHC-CDs showed the strongest emission at approximately 454 nm with the strongest excitation at 355 nm (). The QY of the SHC-CDs was determined to be 2.26% using quinine sulphate as a reference QY of CDs in aqueous solution and calculated at an excitation wavelength of 355 nm. The UV–Vis absorption spectrum, which was also analysed to determine the optical properties of the SHC-CDs, revealed that the aqueous SHC-CDs solution exhibited a broad absorption spectrum without any obvious peak ().
To gain a better insight into the organic functional groups on the surface of the SHC-CDs, we further analysed the CDs using FTIR, and the purified SHC-CDs spectra () showed characteristic peaks at 3416, 2924, 2852, 1642, 1407 and 1115 cm−1. The presence of O–H groups was indicated by the peak at 3416 cm−1. The weak absorption signals at 2924 and 2852 cm−1 were assigned as –C–H stretching, which may have occurred because of the association of methyl or methylene groups with the aliphatic hydrocarbons present in the SHC-CDs. Moreover, the absorption peak at 1642 cm−1 in the SHC-CDs was the characteristic absorption of the C=O group. In addition, the peak at 1407 cm−1 in the SHC-CDs spectrum was related to the –C–N– band, and the weak C–O stretching band occurred at ∼1115 cm−1 (alkoxy) [Citation40,Citation41].
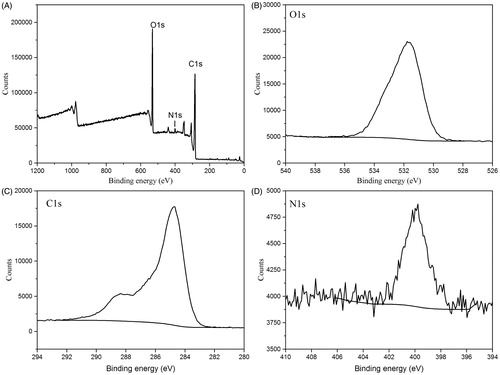
The surface composition and elemental analysis of the prepared SHC-CDs were characterized using an XPS technique. As shown in , the synthesized CDs were mainly composed of the elements C 1s, O 1s at relative percentage compositions of 66.42%, 26.79%, respectively. In addition, elements N, Mg, Ca also exist in CDs. The limited Mg, Ca elements may come from the trace minerals in SH. The elements C, O and N may correspond to C–C, C–OH, C=O, C–N and C–O–C. Moreover, no heavy metal elements (Fe and Al) were detected. This result was in accordance with the surface composition of SHC-CDs shown in the FTIR analysis.
Cellular toxicity
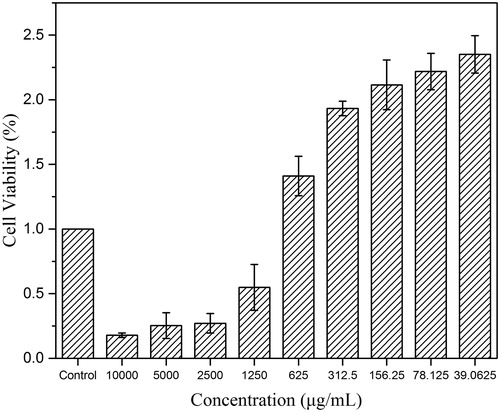
The toxicity of CDs has always been of considerable concern in their biological application. shows the cell viabilities of RAW 264.7 cells treated with SHC-CDs in the concentration range from 39.06 to 10,000 µg/mL for 24 h. The SHC-CDs promoted RAW 264.7 cell growth at CDs concentrations within approximately 840 µg/mL. The maximum viability of the RAW 264.7 cells was 235% at a CDs concentration of 39.06 μg/mL. However, their viability decreased with increasing CDs concentration from 840 to 10,000 µg/mL, which indicated that the cytotoxicity of SHC-CDs was negligible at up to 840 µg/mL.
HPLC profile of SH and SHC-CDs
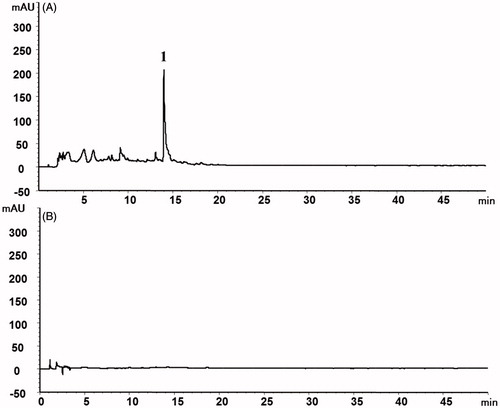
The HPLC results of this study showed that SH the extract was present based on observations such as the pulegone (peak 1). In contrast, no active small molecule compounds of the SH were detected in the prepared SHC-CDs, as shown in .
Haemostasis studies of SHC-CDs
To explore the haemostatic effect of SHC-CDs, the mouse tail amputation and liver scratch models were used. A significant decrease in the bleeding time was observed after SHC-CDs treatment in both injury models (). The tail bleeding time of the groups treated with the SHC-CDs at 8.33, 3.33 and 1.67 mg/kg decreased to 0.75 ± 1.65, 0.80 ± 2.78 and 0.43 ± 2.74 min, respectively compared with that in the control group [13.94 ± 3.61 min, p < .05]. Furthermore, these results were not significantly different from those of the HC group [0.00 ± 2.18 min].
Figure 6. Study of time to haemostasis in Kunming mouse: (A) Tail amputation (n = 6) and (B) liver scratch (n = 8) models treated with normal saline (NS), haemocoagulase (HC) and different concentrations of Schizonepetae Herba Carbonisata-carbon dots (SHC-CDs). **p < .01 and *p < .05 compared with control group.
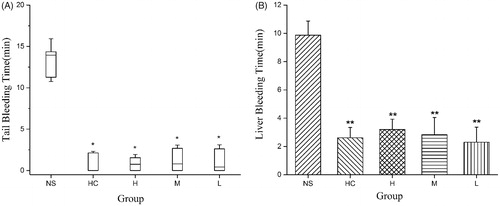
In the liver scratch model, the SHC-CDs (8.33, 3.33 and 1.67 mg/mL) and HC treatment significantly decreased (p < .01) the liver bleeding time (2.62 ± 0.73, 3.20 ± 0.72, 2.83 ± 1.21 and 2.30 ± 1.06 min, respectively) compared with that of the control group (9.88 ± 0.99 min). No significant difference was observed between the SHC-CDs and HC groups.
Measurements of coagulation parameters
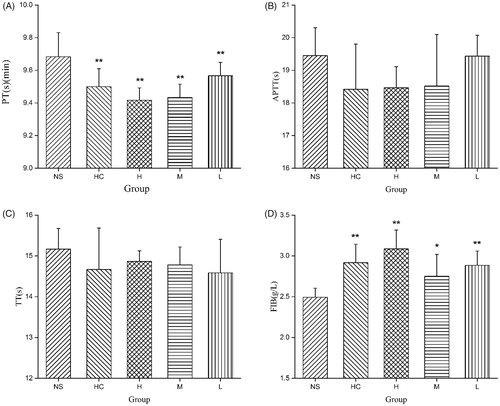
To determine whether the SHC-CDs had coagulant properties, relevant parameters (PT, APTT, TT and FIB) were evaluated using mouse plasma. The PT was decreased significantly by treatment with SHC-CDs at high- (9.41 s), medium- (9.43 s) and low doses (9.51 s) and HC compared with the NS treatment (control group, 9.68 s, p < .01). Moreover, the SHC-CDs (8.33 and 1.67 mg/kg) and HC groups showed a significant increase (p < .01) in the FIB (2.92, 3.09 and 2.88 g/L, respectively) compared with that of the control group (2.49 g/L). Furthermore, the SHC-CDs (3.33 mg/kg, 2.75 g/L) had a significant (p < .05) effect on the FIB but not the APTT and TT ().
Figure 7. (A) Prothrombin time (PT), (B) activated partial thromboplastin time (APTT), (C) thrombin (TT) and (D) fibrinogen (FIB) analysis of mice treated with normal saline (NS), haemocoagulase (HC), and different concentration of Schizonepetae Herba Carbonisata-carbon dots (SHC-CDs). **p < .01 and *p < .05 compared with control group (n = 6).
Discussion
CDs are zero-dimensional carbon nanostructure materials with particle sizes <10 nm [Citation42,Citation43], which typically display size- and excitation wavelength-dependent photoluminescence behaviour [Citation44]. As an emerging novel nanomaterial, a variety of synthesis methods has been developed. However, there is still lacking of ideal choice, because most of these approaches have some disadvantages such as requiring a large quantity of strong CDs acids and complex process [Citation45]. From the environmental conservation point of view, the study of new carbon sources for facile, economical, simple and green synthesis is highly desired, especially for CDs with bioactivities. Pyrolysis, which is described as the direct thermal decomposition of a biomass in the absence of oxygen [Citation46], has numerous merits such as a simple experimental setup, easy control of the reaction, facile operation and environmental friendliness [Citation47]. In this study, a modified pyrolysis method was used to prepared SHC-CDs, which was conducive to the further investigation of the bioactivities.
SHC, called Jingjietan in China, is used in the treatment of various kinds of haemorrhage and has exhibited apparent efficacious clinical effects for thousands of years. However, the mechanism of haemostatic action of SHC remains unclear. With the advancement of CDs, various plant materials have been widely used as precursors of CDs, such as Trapa bispinosa peel [Citation41], sweet potatoes [Citation48] and orange waste peels [Citation49]. These reports are the motivation for our exploration of SHC-CDs prepared from SHC.
In this work, the novel SHC-CDs material isolated from the SHC aqueous extract with a considerable QY of 2.26% was identified using TEM, HRTEM, XPS, XRD and FTIR, as well as UV–Vis and fluorescence spectroscopy and HPLC. We evaluated the component variation and excluded the possible interference of active small molecule compounds of the SHC dialysis solution by comparing the chemical components of the SH and SHC-CDs solution using an HPLC method. In the SH, components such as pulegone were detected. In contrast, the HPLC fingerprint of the SHC dialysis solution revealed no small molecule components existed after charcoal processing and dialysis. Moreover, it is worth noting that applying CDs in biological field should take their safe concentration into account. From the perspective of the precursor of CDs, SH is a kind of green, economical and eco-friendly traditional Chinese herbal source, which accords with Green Chem nature [Citation50]. Moreover, the prepared CDs without heavy metal are more environmental friendly, and could be much safer for biological use, which was consistent with the result of the CCK-8 assay.
More importantly, the novel SHC-CDs possessed charming haemostatic performance, which could be demonstrated in the tail amputation and liver scratch mouse models. The results demonstrated that HC and the SHC-CDs (8.33, 3.33 and 1.67 mg/kg) treatments were highly effective in controlling the haemorrhage compared to NS treatment (control group) in both bleeding models. Moreover, the bleeding time was not significantly different between the HC and SHC-CDs groups, indicating, for the first time, that SHC-CDs are a potentially useful haemostatic medicine.
To explore the mechanism underlying the haemostatic activity of the SHC-CDs (8.33, 3.33 and 1.67 mg/mL), coagulation parameters were measured in treated Kunming mice. The PT and APTT values are correlated with the overall efficiency of the extrinsic clotting pathway and the intrinsic coagulation phase, respectively. The common coagulation pathway and the activities promoting the transformation of FIB into fibrin in the plasma are related to the TT and FIB levels [Citation51]. In our study, the PT decreased, and the FIB content increased in the SHC-CDs groups, whereas the APTT and TT values showed no significant difference compared to those of the control group. It is worth noting that the APTT of the high and medium SHC dose-treated showed a decreasing tendency. The observation above indicates that the SHC-CDs had beneficial effects on the extrinsic coagulation pathway and activating FIB system and may be relevant in the intrinsic coagulation phase. This study was a preliminary evaluation of the haemostatic activity of the SHC-CDs, and further investigations are needed to elucidate the underlying mechanisms of these effects.
Conclusions
In the present study, the SHC aqueous extract was first demonstrated to produce CDs using a modified pyrolysis method. The obtained SHC-CDs with a QY of 2.26% were characterized using TEM, HRTEM, XPS, XRD, FTIR and HPLC, as well as UV–Vis and fluorescence spectroscopy. The cell viability assay demonstrated that the SHC-CDs were safe at concentrations <840 µg/mL. Further pharmacodynamic experiments in mice revealed that the SHC-CDs remarkably inhibited haemorrhage in the mouse tail amputation and liver scratch models. Furthermore, these effects may be associated with extrinsic coagulation activity and the activation of the FIB system according to the results of the evaluation of the mouse coagulation parameters. To the best of our knowledge, this is the first study to investigate the haemostatic effects of SHC-CDs, and it has provided salient information for facilitating the development of potential haemostatic medications in the future. Moreover, we also provided a novel strategy for exploring the material basis of TCM formulations used as anti-haemorrhagic agents.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Shan MQ, Qian Y, Yu S, et al. Anti-inflammatory effect of volatile oil from Schizonepeta tenuifolia on carrageenin-induced pleurisy in rats and its application to study of appropriate harvesting time coupled with multi-attribute comprehensive index method. J Ethnopharmacol. 2016;194:580–586.
- Ye DJ. Components of essential oils from different parts and the charcoal of Schizonepeta tenuifolia. Zhong Yao Tong Bao. 1985;10:19–21.
- Abdelhamid HN, Talib A, Wu HF. One pot synthesis of gold - carbon dots nanocomposite and its application for cytosensing of metals for cancer cells. Talanta. 2017;166:357–363.
- Gowthaman NS, Sinduja B, Karthikeyan R, et al. Fabrication of nitrogen-doped carbon dots for screening the purine metabolic disorder in human fluids. Biosens Bioelectron. 2017;94:30–38.
- Campos BB, Oliva MM, Contreras-Caceres R, et al. Carbon dots on based folic acid coated with PAMAM dendrimer as platform for Pt(IV) detection. J Colloid Interface Sci. 2016;465:165–173.
- Qu S, Wang X, Lu Q, et al. A biocompatible fluorescent ink based on water-soluble luminescent carbon nanodots. Angew Chem Int Ed. 2012;51:12215–12218.
- Wu Y, Wei P, Pengpumkiat S, et al. Development of a carbon dot (C-Dot)-linked immunosorbent assay for the detection of human alpha-fetoprotein. Anal Chem. 2015;87:8510–8516.
- Zhou L, Lin Y, Huang Z, et al. Carbon nanodots as fluorescence probes for rapid, sensitive, and label-free detection of Hg2+ and biothiols in complex matrices. Chem Commun (Cambridge, England). 2012;48:1147–1149.
- Qu K, Wang J, Ren J, et al. Carbon dots prepared by hydrothermal treatment of dopamine as an effective fluorescent sensing platform for the label-free detection of iron(III) ions and dopamine. Chem Eur J. 2013;19:7243–7249.
- Lu W, Qin X, Liu S, et al. Economical, green synthesis of fluorescent carbon nanoparticles and their use as probes for sensitive and selective detection of mercury(II) ions. Anal Chem. 2012;84:5351–5357.
- Han C, Wang R, Wang K, et al. Highly fluorescent carbon dots as selective and sensitive “on-off-on” probes for iron(III) ion and apoferritin detection and imaging in living cells. Biosens Bioelectron. 2016;83:229–236.
- Wei W, Xu C, Wu L, et al. Non-enzymatic-browning-reaction: a versatile route for production of nitrogen-doped carbon dots with tunable multicolor luminescent display. Sci Rep. 2014;4:3564.
- Sahu S, Behera B, Maiti TK, et al. Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun. 2012;48:8835–8837.
- Kim J, Park J, Kim H, et al. Transfection and intracellular trafficking properties of carbon dot-gold nanoparticle molecular assembly conjugated with PEI-pDNA. Biomaterials. 2013;34:7168–7180.
- Wang L, Wang X, Bhirde A, et al. Carbon-dot-based two-photon visible nanocarriers for safe and highly efficient delivery of siRNA and DNA. Adv Healthcare Mater. 2014;3:1203–1209.
- Tang J, Kong B, Wu H, et al. Carbon nanodots featuring efficient FRET for real-time monitoring of drug delivery and two-photon imaging. Adv Mater. 2013;25:6569–6574.
- Fowley C, Nomikou N, McHale AP, et al. Extending the tissue penetration capability of conventional photosensitisers: a carbon quantum dot-protoporphyrin IX conjugate for use in two-photon excited photodynamic therapy. Chem Commun. 2013;49:8934–8936.
- Ye Q, Yan F, Kong D, et al. Constructing a fluorescent probe for specific detection of catechol based on 4-carboxyphenylboronic acid-functionalized carbon dots. Sens Actuators B Chem. 2017;250:712--720.
- Heller DA, Jeng ES, Yeung TK, et al. Optical detection of DNA conformational polymorphism on single-walled carbon nanotubes. Nano Lett. 2006;311:508–511.
- Tao H, Yang K, Ma Z, et al. In vivo NIR fluorescence imaging, biodistribution, and toxicology of photoluminescent carbon dots produced from carbon nanotubes and graphite. Small. 2012;8:281–290.
- Abbasi E, Kafshdooz T, Bakhtiary M, et al. Biomedical and biological applications of quantum dots. Artif Cells Nanomed Biotechnol. 2016;44:885–891.
- Bajwa N, Mehra NK, Jain K, et al. Pharmaceutical and biomedical applications of quantum dots. Artif Cells Nanomed Biotechnol. 2016;44:758.
- Liu J, Lu S, Tang Q, et al. One-step hydrothermal synthesis of photoluminescent carbon nanodots with selective antibacterial activity against Porphyromonas gingivalis. Nanoscale. 2017;9:7135–7142.
- Shi W, Wang Q, Long Y, et al. Carbon nanodots as peroxidase mimetics and their applications to glucose detection. Chem Commun. 2011;47:6695–6697.
- Yan Z, Yue Z, Liu X, et al. Novel carbon quantum dots from egg yolk oil and their haemostatic effects. Sci Rep. 2017;7:4452.
- Jia X, Li J, Wang E. One-pot green synthesis of optically pH-sensitive carbon dots with upconversion luminescence. Nanoscale. 2012;4:5572–5575.
- Bourlinos AB, Anglos SA, Zboril D, et al. Pyrolytic formation and photoluminescence properties of a new layered carbonaceous material with graphite oxide-mimicking characteristics. Carbon. 2009;47:519–526.
- Lin Z, Xue W, Chen H, et al. Peroxynitrous-acid-induced chemiluminescence of fluorescent carbon dots for nitrite sensing. Anal Chem. 2011;83:8245–8251.
- Qiao ZA, Wang Y, Gao Y, et al. Commercially activated carbon as the source for producing multicolor photoluminescent carbon dots by chemical oxidation. Chem Commun. 2010;46:8812–8814.
- Yang ZC, Wang M, Yong AM, et al. Intrinsically fluorescent carbon dots with tunable emission derived from hydrothermal treatment of glucose in the presence of monopotassium phosphate. Chem Commun. 2011;47:11615–11617.
- Yang L, Jiang W, Qiu L, et al. One pot synthesis of highly luminescent polyethylene glycol anchored carbon dots functionalized with a nuclear localization signal peptide for cell nucleus imaging. Nanoscale. 2015;7:6104–6113.
- Jia P, Yu L, Tao C, et al. Chitosan oligosaccharides protect nucleus pulposus cells from hydrogen peroxide-induced apoptosis in a rat experimental model. Biomed Pharmacother. 2017;93:807–815.
- Ko BS. Quantitative analysis of Pulegone in Schizonepeta tenuifolia Briq. Angew Chem. 2006;42:5321–5324.
- Wu J, Lemarie CA, Barralet J, et al. Amphiphilic peptide-loaded nanofibrous calcium phosphate microspheres promote hemostasis in vivo. Acta Biomater. 2013;9:9194–9200.
- Liu Y, Jennings NL, Dart AM, et al. Standardizing a simpler, more sensitive and accurate tail bleeding assay in mice. World J Exp Med. 2012;2:30–36.
- Zhu Y, Qiu Y, Liao L. Evaluation of hemostatic effects of carbonized hair-loaded poly(l-lactic) acid nanofabrics. J Nanosci Nanotechnol. 2015;15:4193–4199.
- Xu G, Cheng L, Zhang Q, et al. In situ thiolated alginate hydrogel: instant formation and its application in hemostasis. J Biomater Appl. 2016;31:721–729.
- Dang X, Miao JJ, Chen AQ, et al. The antithrombotic effect of RSNK in blood-stasis model rats. J Ethnopharmacol. 2015;173:266–272.
- Wei J, Zhang X, Sheng Y, et al. Simple one-step synthesis of water-soluble fluorescent carbon dots from waste paper. New J Chem. 2014;38:906–909.
- Zhang JH, Niu A, Li J, et al. In vivo characterization of hair and skin derived carbon quantum dots with high quantum yield as long-term bioprobes in zebrafish. Sci Rep. 2016;6:37860.
- Mewada A, Pandey S, Shinde S, et al. Green synthesis of biocompatible carbon dots using aqueous extract of Trapa bispinosa peel. Mater Sci Eng C Mater Biol Appl. 2013;33:2914–2917.
- Li Y, Zhao Y, Cheng H, et al. Nitrogen-doped graphene quantum dots with oxygen-rich functional groups. J Am Chem Soc. 2012;134:15–18.
- Shen J, Zhu Y, Yang X, et al. Graphene quantum dots: emergent nanolights for bioimaging, sensors, catalysis and photovoltaic devices. Chem Commun (Cambridge, England). 2012;48:3686–3699.
- Jiang Z, Nolan A, Walton JG, et al. Photoluminescent carbon dots from 1,4-addition polymers. Chem Eur J. 2014;20:10926–10931.
- Gedda G, Lee CY, Lin YC, et al. Green synthesis of carbon dots from prawn shells for highly selective and sensitive detection of copper ions. Sens Actuators B Chem. 2016;224:396–403.
- Uslu A, Faaij APC, Bergman PCA. Pre-treatment technologies, and their effect on international bioenergy supply chain logistics. Techno-economic evaluation of torrefaction, fast pyrolysis and pelletisation. Energy. 2008;33:1206–1223.
- Shi L, Li Y, Li X, et al. Controllable synthesis of green and blue fluorescent carbon nanodots for pH and Cu(2+) sensing in living cells. Biosens Bioelectron. 2016;77:598–602.
- Lu W, Qin X, Asiri AM, et al. Green synthesis of carbon nanodots as an effective fluorescent probe for sensitive and selective detection of mercury(II) ions. J Nanopart Res. 2013;15:1344.
- Prasannan A, Imae T. One-pot synthesis of fluorescent carbon dots from orange waste peels. Ind Eng Chem Res. 2015;52:15673–15678.
- Shen J, Shang S, Chen X, et al. Facile synthesis of fluorescence carbon dots from sweet potato for Fe3+ sensing and cell imaging. Mater Sci Eng C Mater Biol Appl. 2017;76:856–864.
- Xin N, Li YJ, Li Y, et al. Dragon's Blood extract has antithrombotic properties, affecting platelet aggregation functions and anticoagulation activities. J Ethnopharmacol. 2011;135:510–514.