Abstract
The intraocular administration of the ciliary neurotrophic factor (CNTF) has been found to attenuate the photoreceptor degeneration and preserve retinal functions in the animal research models of the inherited or induced retinal disease. Studies with the aim of CNTF transfer to the posterior segment inside the eye have been directed to determine the best method for its administration. An ideal delivery method would overcome the eye drug elimination mechanisms or barriers and provide the sustained release of the CNTF into retina in the safest fashion with the minimum harm to the quality of life. This review focuses on the present state of CNTF delivery to retina, also provides an overview of available technologies and their challenges.
Introduction
Neurotrophic factors (NTFs) are peptides or small protein molecules, with the potential for supporting the survival, proliferation and differentiation of neurons [Citation1]. NTFs were grouped in three known families including the neurotrophin, glial cell line-derived neurotrophic factor(GDNF) and the ciliary neurotrophic factor (CNTF) [Citation2]. The CNTF protein family members are Interlukein-6(IL-6), Leukaemia inhibitory factor (LIF) and CNTF [Citation3].
CNTF has high expression levels in central nervous system including brain, spinal cord and parasympathetic ganglion behind the eye (ciliary ganglia) [Citation4]. The study of CNTF-deficient mice showed that lack of CNTF impaired the ability for recovering the sciatic nerve crush [Citation5]. The coadministration of CNTF and brain neurotrophic factor (BDNF) showed re-myelination effect on regenerating nerve fibres [Citation6]. Additionally, the recent reports revealed that CNTF stopped the decay of axotomized peripheral motor neurons and also retrograded cell loss of neurons in the thalamic nuclei following dissection of the intracerebral neuronal circuits [Citation7–9]. Furthermore, CNTF seems efficient for delaying cellular and practical losses in neurodegenerative disease of the CNS [Citation10]. In rats having amyotrophic lateral sclerosis, CNTF notably decreased motor neuron destruction, enhanced motor operation and the survival period [Citation11]. CNTF neuroprotection and operational achievement were also described in the Huntington's disease in primate and mouse [Citation12,Citation13]. The CNTF as a neuropoietic cytokine, is expressed in the retina under stressful circumstances for instance in the experimental ocular hypertension [Citation14], optic nerve trauma and works as an injury-activated signal to protect neural tissues, as well as the retina [Citation15]. CNTF has been confirmed to cause a neuroprotective influence on photoreceptors, the cells responsible for sensing the light in the retina. The animal models of the retinal degeneration proposed that the mentioned neuroprotective effect decreases the vision loss speed due to photoreceptor death [Citation16,Citation17]. While the essential work of CNTF in grown-up animals is not entirely known, external CNTF influences on the survival and differentiation of the cells in the nervous structure, containing retinal cells [Citation18,Citation19]. The reported regenerative potential of CNTF led to designing and performing of the preclinical or clinical trials for the retinal degenerative diseases. Up to now, in different animal models, pre-clinical studies, and clinical trials several methods for CNTF delivery and its release to the posterior parts of eye have been examined.
Effective delivery of CNTF to target sites in the eye has proven to be a challenging task as a result of the barrier properties of the eye. Accordingly, the systemic delivery of CNTF showed undetectable therapeutic benefits and major side effects including blood chemistry changes, fever, and fatigue [Citation20]. The reason for these inacceptable results may possibly relate to the inadequate concentrations of CNTF accumulated in the target sites by the systemic administration due to the blood retinal barrier. In comparison, continuous and local delivery systems could optimize the pharmacokinetics of the CNTF as a therapeutic agent to the eye. Studied local delivery methods for CNTF are both direct intraocular injection of protein, viral vectored CNTF gene injections (CNTF gene therapy), encapsulated cell technology implantation and several other novel strategies including utilization of biomaterials or hydrogels, microspheres and so on. The local CNTF delivery to in particular, retina has likewise several limitations owing to the unique anatomy and physiology of eye, stationary barriers (such as cornea, sclera and retina, blood aqueous/retinal barrier) and dynamic barriers (such as conjunctival and choroidal blood flow, tear dilution and lymphatic clearance) [Citation21].
Encapsulated cell technology provides a safe attractive substitute to other strategies of CNTF delivery for the reason that the encapsulated cells can be engineered to express CNTF in a sustained localized manner [Citation20]. Current improvements in the field of ocular drug delivery promise a considerable progress in overcoming the challenges of CNTF transfer to retina. The aim of the present review is to discuss on the strategies employed for CNTF delivery into posterior segment of the eye or retina, their properties, advantages and drawbacks.
CNTF, CNTF receptor and signalling pathway
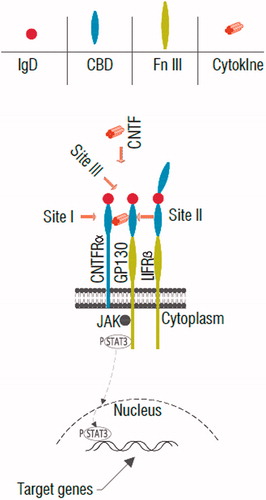
The locus for the CNTF gene is present in chromosome 11 which encodes for a 22.7 KD polypeptide [Citation22,Citation23]. CNTF is folded in a four antiparallel alpha-helices bundle, operates by a heterotrimeric receptor complex consist of CNTF receptor α (CNTFRα) and a couple of signal-transferring transmembrane subsystems including leukaemia inhibitory factor receptor β (LIFβ) and glycoprotein gp130 [Citation24] (). CNTFR α is expressed on Müller glial membranes [Citation25] on rod and cone photoreceptors [Citation26–29]. The CNTF elicited the cellular reaction through the induction of a receptor complex including the CNTF α-receptor (CNTFR) [Citation30–32]. Cellular reactions to CNTF and IL-6 type cytokines are obtained by various multipart receptor complexes that involve the membrane-spanning gp130 [Citation30–33]. CNTF first binds to the GPI-anchored CNTF receptor (CNTFRα), that is not a signal transmission factor. Regarding the limited CNTFRα expression, it is mostly accepted that CNTF adverse effects are associated with the stimulation of the IL6R-LIFRb-gp130 receptor [Citation34,Citation35]. Joining of CNTF to the membrane-bound or soluble CNTFRα produces a heterodimer of the signal-transducing β-receptors gp130 and LIF receptor (LIFR), which begins the intracellular signalling cascades [Citation36]. The research in the mechanism of the activation of the CNTF receptor, indicated that all members of the cytokines family activate intracellular signalling in the same way. CNTF activates the JAK/STAT/TyK kinase, which is joined with the beta components in an indolent state and then become activated upon the beta component dimerization [Citation37,Citation38] (). The activated CNTF receptor complex specially phosphorylates dominantly STAT3 and to a lesser extent STAT1 [Citation39,Citation40]. The MAPK and also PI3K pathways facilitate CNTF-mediated neuronal survival [Citation41]. Some studies showed the amount of the rhodopsin were preserved by cytokine signalling 3 (SOCS3), a negative feedback regulator of STAT3 activation. SOCS3 was expressed generally in the retinal cells mostly in photoreceptors [Citation42]. A recombinant modified form of CNTF, Axokine which is a truncated form (15 amino acid truncation). The Axokine has been used for the treatment of the obesity and related obstacles for instance in type-2 diabetes [Citation7].
Figure 1. Schematic diagram of CNTF receptor and its signalling pathway. First, CNTF binds to site I by cytokine-binding domain (CBD) of CNTFRα, then the complex of CNTF-CNTFRα interacts with CNTF site II-gp130 and site III-LIFRβ immunoglobulin domain (IgD) that trigger the activation of the gp130, LIFRβ and signaling chains through Jak-STAT pathway. Note: Fn III: fibronectins type-III domain.
Effect of CNTF on eye disease
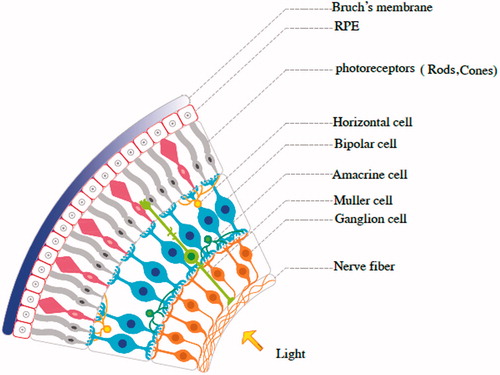
The human eye is a sense organ that responds to the light and allows vision. The eye has three layers, encircling different anatomical structures. The inner layer is the retina. The life history of retinal cells is consisted of three stages: a stage of reproduction, in which the cells frequently divide; a stage of differentiation, in which the similar population is transformed into a heterogeneous cells and a stage of regeneration, in which nothing seems to change [Citation43]. After the evolution and differentiation, the retinal cells have formed that include neurons and synapses (). The light-sensitive cells are named photoreceptors (rods and cons) (). The light-sensitive external parts of the retinal rod photoreceptor consist of many hundreds of densely packed discs. Several reports confirmed that the external parts of mature rods are frequently renewed [Citation44]. A number of neurotrophic factors, growth factors and cytokines, known as survival factors, protect neurons specially the retinal photoreceptors from cell death that caused by different forms of the retinal degeneration [Citation45,Citation46]. CNTF plays a role in rod differentiation in vivo [Citation47] and promotes the photoreceptor survival. Previous study showed that the intra-vitreal injection of the CNTF protein in rat changes in rod photoreceptors, decreases in rhodopsin expression and increases the arrestin levels [Citation48]. Muller cells are the type of glial cells that span the entire depth of the retina and these cells can express CNTF after ischaemia and protected the neurons [Citation49]. Cone photoreceptors are responsible for the colour and vision and CNTF promotes the existence and axonal regeneration of RGCs and has neuroprotective effect in RGCs against optic nerve trauma [Citation50–54]. Since CNTF is survival element for neurons and retinal cells, this neurotrophic factor is a promising candidate in treatment of inherited and orphan retinal diseases [Citation55].
Figure 2. The retinal neurons are classified into three main types including: primary sensory cells (rods and cones), interneuron cells (amacrine cells, bipolar cells and horizontal cells) and output neurons: retinal ganglion cells (RGCs). The cell types are distributed in a manner that the entire retina is fully occupied with the cell types. RPE: retinal pigment epithelium.
Since various eye diseases are age-related, recent increases in life hope are bound to have a main impact on the epidemiological profile of loss vision and blindness in developed countries. In this section, we will review effect of CNTF on the common eye disorders.
Diabetic retinopathy
Diabetic retinopathy (DR) is the very frequent form of the diabetic eye condition that damages to the retina and caused by diabetes [Citation56]. DR is a particular microvascular problem about diabetes and the risk of developing DR and blindness is known to increase in working-aged persons which is the leading reason of blindness for people aged 20–64 years with diabetes. DR includes three types: diabetic maculopathy, background retinopathy and proliferative retinopathy [Citation57,Citation58]. The early stages of DR may arise without symptoms and pain, symptoms may only become obvious once the disease progresses such as pain in the eye, sudden changes in vision or blurred vision and double vision. Proliferative DR is described with the development of the additional blood vessels on the cover of the retina, these vessels may bleed that leads to the vitreous haemorrhage, consequent fibrosis and retinal separation. Diabetic macular oedema (DME), arise in all degrees of DR is described by improved vascular permeability at the retina. DME is the major reason of perceiving ability trouble in diabetic persons [Citation59]. Hyperglycaemia insistently activates PKCδ (protein kinase C delta) and p38α MAPK (P38 mitogen-activated protein kinases) that results in the cellular apoptosis in many vascular cells and is important for initiating DR [Citation60]. There are some major treatments for DR such as laser surgery, intravitreal delivery of anti-VEGF drugs and vitrectomy [Citation61]. Cell therapy for DR is also useful. The aim of the mentioned method and use of mesenchymal stem cells with promising potential in regenerative medicine [Citation62–67] is to help to replace the impaired retinal vasculature and the retinal neurons [Citation68]. CNTF as a best studied neurotrophic agent in retinal disease facilitated capillary regrowth and reduced pre-retinal neovascularization in a retinal vascular diseases like DR [Citation69,Citation70].
Glaucoma
Glaucoma is a collection of the eye conditions which leads to optic nerve damage and blindness [Citation71]. Glaucoma is described by continuous loss of RGCs and axons [Citation72]. Glaucoma diseases includes three types: most common is primary open-angle glaucoma (POAG) [Citation73] and other less common types are normal-tension glaucoma (NTG) and closed-angle glaucoma. POAG is developed slowly with no pain and is a progressive disease and all patients must be checked throughout their life [Citation74]. The closed-angle glaucoma can develop regularly or suddenly that patient suffer from severe eye pain, blurred vision and redness of the eyes. NTG develops like the open-angle kind of glaucoma, but the only difference is that NTG patients have intraocular pressures (IOP) below 21 mmHg while primary (or chronic) POAG have above this level [Citation75]. Glaucoma treatment include surgery, laser and eye drops [Citation76]. CNTF was shown to promote the RGC neuroprotection in glaucoma model. Some studies showed that CNTF has a substantial protective effect in comparison with both combined CNTF-BDNF and BDNF alone, had no statistically considerable increase in RGC axon survival in glaucoma, so using both BDNF and CNTF or with cofactors like cAMP increases neuroprotective efficiency [Citation77,Citation78]. CNTF and CNTFRα expression become upregulated in the rat retina after glaucoma suggesting that CNTF might play a role in the endogenous response of retinal neurons to injury [Citation79,Citation80].
Age-related macular degeneration (AMD)
AMD is a continuous degenerative disorder that results in loss of the central vision as an outcome of the abnormalities in macula of the retina. It does not initiate complete blindness because peripheral vision is unaffected in that disease [Citation81]. Advanced AMD is classified into two types: dry macular degeneration and wet macular degeneration. There is no medication and surgery for dry macular degeneration, but wet AMD is curable with laser coagulation therapy, drug treatment and photodynamic therapy [Citation82,Citation83]. The clinical signs of AMD consist of drusen, hyperplasia of the retinal pigment epithelium (RPE), geographic atrophy and choroidal new vessels [Citation84]. The first phase of AMD is considered with the presence of white or yellow lipid leaves in Bruch’s layer known as drusen, Then, the destruction of rod photoreceptors and RPE function happens in the high-level dry AMD patients that result in geographic atrophy [Citation85]. In dry AMD, first step of treatment is the prevention of photoreceptors and RPE cells loss. CNTF is an effective neurotrophic factor that slows down the loss of photoreceptors. The CNTF implants that used in AMD patient slowed the visual loss rate at 12 months [Citation86,Citation87].
Retinitis pigmentosa
Retinitis pigmentosa (RP) is the medical condition given to inherited and degenerative retinal diseases that leads to degeneration of the rod and cone photoreceptors and RPE that causes the severe vision impairment. RP is independent of age and can manifests the initial symptoms and occurs from the early infancy to late adulthood. RP is classified into 1: non-syndromic without any other clinical representing, 2: syndromic with other abnormalities, neurosensory disorders or other clinical findings 3: occurs with other systematic diseases. Syndromic RP can be combined with other diseases and resulting in diseases like Usher syndrome, Kearns–Sayre syndrome (also known as ragged red fibre myopathy), Bardet–Biedl syndrome, Batten disease, Bassen–Kornzweig (Abetalipoproteinemia) and Refsum disease [Citation88,Citation89]. The clinical symptoms and signs of RP (rod-cone retinitis pigmentosa) are nyctalopia, photophobia, loss of central vision, blurring of vision, photopsia, colour vision defects and visual field loss. Currently, there are no standard and certain treatment for RP patients. Some various probable treatments are currently being evaluated like the vitamin therapy, gene therapy, cell transplantation (stem cell transplantation) [Citation90]. RP as a heterogeneous-inherited disorders is appropriate candidate for the neuroprotective treatment with the techniques of gene therapy [Citation91]. Growth factors might provide effective treatment for the preservation of the photoreceptors in RP [Citation92]. Previous studies have shown that using neurotrophic elements perform as therapeutic tools for RP. In particular, CNTF is efficient in impeding retinal degeneration in the animal RP models. Recent researches showed that gene therapy with CNTF following recombinant adeno-associated virus (rAAV)-mediated gene delivery have the protective effects against retinal disease and photoreceptor loss in several retinal disease, including RP [Citation93]. These findings are promising for designing future treatments of RP.
Retinal detachment
Retinal detachment (RD) is a disease of the eye with potentially blinding condition that characterized by the accumulation of the fluid in subretina. In the RD, retina separates from the underlying pigment epithelium that causes the death of photoreceptors. The occurrence of RD is strongly associated with age, vitreoretinal degenerations and myopia. There are three types of the retinal detachment: Rhegmatogenous that this type is most common, tractional that is less common and exudative or secondary retinal detachment. RD is also more probable to occur in people who have cataract surgery and other risk factors are the retinal tears, family history and severe myopia. Some genetic or acquired factors stimulating local inflammation and photoreceptor degeneration might also be involved in the progress of the RD such as glaucoma, aids, retinoblastoma, malignant hypertension, smoking and passive smoking and DR [Citation94,Citation95]. There are some techniques for treating a detached retina, each of them depends on what types of RD have formed. These methods are cryopexy and laser photocoagulation, pneumatic retinopexy, scleral buckle surgery and vitrectomy [Citation96]. The IL-6 may serve as a photoreceptor neuroprotectant in the retinal–RPE separation [Citation97]. The CNTF showed similar functions as mentioned earlier. Some research projects showed the high quantity of IL-6 in the vitreous of RD patients, which suggests a neuroprotective effect. Therefore, IL-6 has a significant function in the protection of photoreceptors after retina separation from the pigment epithelium [Citation98].
CNTF effect against light-induced damage
The different types of photochemical damage to the retina were reviewed elsewhere [Citation99–101]. When CNTF joins its receptors on rods and cones photoreceptors, triggers a cascade that has been associated with neuroprotection after the light-induced retinal damage. The mentioned statement was confirmed by previous studies, which showed an association between CNTF upregulation and photoreceptor resistance to damage. In a study, albino rats from Sprague–Dawley type were faced to the bright constant light (1000 lux) for one or two days just after nerve section [103]. Based on the time course of the protection against bright light, it seems that CNTF plays a major role in the protection against light-induced damage [Citation102]. In addition, studies of the CNTFRα have shown that CNTFRα is located on the outer segments of the retinal photoreceptors that CNTF/CNTFRα localization occurs on the outer segments and is upregulated in light-damage retina, suggesting that CNTF plays a major role in resistance of the photoreceptors against light-induced damage [Citation103].
CNTF delivery to retina
There are three general methods for the delivery of eye medications including topical, local and systemic. The CNTF administration with systemic and bolus injections showed limited efficacy and considerable side effects [Citation104]. Therefore, the local and sustained delivery may simultaneously reduce the adverse side effects and increase the persistence and efficiency of the CNTF.
Eye drops, containing either lubricating and tear replacing or medications included saline solutions, are commonly used as topical ocular administration route. The eye structure can be classified into anterior and posterior segments [Citation21]. The posterior segment includes the optical structures behind the eye such as vitreous humour, retina and cranial nerve II (optic nerve) (). Delivery of CNTF protein by means of eye drops to retina faces with several limitations including tear turn over, protein binding, systemic absorption, degradation by enzymes, corneal blood aqueous barrier and blood retinal barrier [Citation105]. Similar to the blood–brain barrier, the blood–retinal barrier, limits passage way from the blood current to the retina tissue. Overcoming these difficulties is necessary for long-term sustained transfer of CNTF to the retina [Citation20]. Recent research showed new interesting methods in the CNTF ocular delivery. There are three main strategies employed for the transfer of CNTF to the posterior pole of eye including the direct protein injection, viral vector–mediated gene delivery and cell-based administration (in the forms of free or encapsulated). Moreover, other novel methods for CNTF delivery have been studied recently, which are reviewed at the end of this section.
Figure 3. Schematic graphic of the routes of ocular CNTF delivery. (A) Intravitreal injection, (B) Subretinal injection, (C) encapsulated cell technology implantation.
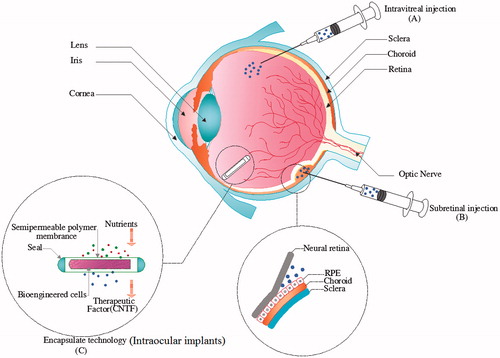
Intravitreal injection-mediated drug delivery has become a common method for injection of medications near to the retina. In the intravitreal injection procedure, needle is inserted into the vitreous, enabling highly targeted therapeutic remedy delivery to the eye posterior pole (). Using the mentioned method minimizes systemic toxicity of the eye-targeting drugs and increases the accumulation of drugs at target sites. The other delivery approach is subretinal injections of the vectors or engineered stem cells (). This technique permits for the targeting of several retinal cells such as photoreceptors or RPEs, because it locates the engineered stem cells or gene therapy viral vectors in the surrounding area of these cells [Citation106]. The intravitreal injections place the fluid containing vectors or cells inside the vitreous cavity, which should transfer through the retina to reach to the targeted cells. Since possibly not all viral particles could migrate, the efficiency of gene transduction into RPE or photoreceptors is low. Therefore, in particular, engineered stem cells which replace the injured RPE or photoreceptor cells should be delivered via subretinal injections [Citation107].
Direct injection of CNTF protein
The receptor for CNTF protein, CNTFRα, is expressed in the retina which was consistently detected in rods and cones photoreceptors [Citation27] and involves in CNTF signalling. The studies exhibited several forms of CNTF administration by the intravitreal delivery including CNTF in the form of the protein and its gene construct in the viral vectors, which expresses CNTF protein after its entrance to the retinal cells.
As it was mentioned previously, CNTF protein could be injected to the retina by intravitreal injection [Citation108–110], but this is not suitable for its sustained transportation because of dose dependency of CNTF, that impairs vision at high dose in several weeks after injection, whereas in low dose provides protection against the light damage [Citation111]. Mathew et al., showed that intravitreal injection of the CNTF improved ganglion cell survival in non-arteritic anterior ischaemic optic neuropathy (NAION) of rodent model [Citation112]. In adult hamster CNTF or BDNF, and also in a similar way, hepatocyte growth factor (HGF) showed sustained long-term survival of ganglion cell and desired sensitivity to stimulations [Citation113]. Marangoni and colleagues studied the effect of CNTF on retinal function at Achromatopsia model, that is, the CNGB3−/− mouse. They found that CNTF protein caused a transient improve of the cone-mediated function [Citation114]. The CNTF showed a role in the migration of mouse corneal epithelial stem cells via upregulation of matrix metalloproteinases and accelerated the repair of the mouse corneal epithelial wound [Citation115]. The research on the effect of CNTF on stem/progenitor cells is interesting. In a study, CNTF delivery into mouse diabetic corneal epithelial promoted wound healing and activation of corneal epithelial stem/progenitor cells along with triggering regeneration process in corneal nerve fibres [Citation116]. Although there are many studies supporting promising effects of CNTF, a human clinical trial reported no measurable improve for cone function [Citation117].
Intravitral injections are currently, the conventional clinical approach for the disease of posterior pole. However, the repeated invasive injections of CNTF could be problematical by the elevation of the intraocular pressure, endophthalmitis, retinal detachment and retinal vascular occlusion [Citation118]. As well, the effect of CNTF in the injection of protein form would be transient due to the short half-life of it. Hence, some other strategies for prolonged transportation of CNTF into posterior parts of the eye have been offered.
Viral vector-mediated CNTF gene delivery
Recent evidence implies that RGC death and visual field loss could be prevented by the continuous supplementation of CNTF. The administration of CNTF via its gene inserts in the viral vectors showed better performance compared to the intravitreous injections of the CNTF recombinant protein [Citation119,Citation120]. Additionally, since injections repetition causes injuries in the eye, the prolonged release of CNTF could be achieved through employing viral vectors that provide CNTF-gene inside the cells at posterior parts of the eye. The primary goals for the production of gene transfer vectors have been to couple the natural ability of a virus to enter the gene into a cell along with eliminating virus pathogenesis. The natural viruses have been engineered for the removal of wild type virulence genes. The current vectors show both efficiency and safety for gene delivery into the retina and human clinical trials are at phase 1 [Citation121].
Three types of the viral vectors have been used for CNTF gene transfer into the retinal cells (summarized in ), including lentiviral vectors, adenoviral vectors and adeno-associated vectors (AAV) [Citation122]. Lentiviral vectors (LeV) could just propagate in proliferating cells. Recent attention have increased for LeV vectors mediated CNTF gene delivery to CNS due to the large transgene capacity of LeVs; however, the retinal transduction ability of them is controversial in the literature [Citation121]. A study showed that quick and continued transferring of CNTF applying LeV-mediated gene transfer LV-CNTF (lentiviral-CNTF) to the retina is a useful and efficient cure for saving the axotomized RGCs for long time [Citation122].
Table 1. The characteristics of viral vectors used for CNTF gene delivery to eye in animal studies.
Previous studies revealed that the administration of CNTF can extend the survival of photoreceptor cells that have phototoxic injury or a mutant gene. Adenovirus-vectored CNTF has also been useful for retinal protection, but its impact has been temporary [Citation126] besides adenoviral vectors raises non-specific antivector cellular immunity [Citation121].
The AAV vectors are used more than others since they can proliferate in any proliferating/non-proliferating cells, elicit no immune responses and perform long-lasting transgene expression in various retinal cells [Citation131]. As well, AAV-vectored minigenes offer important hope for long-term survival. The rds gene provides instructions for producing a protein, playing a key role in the normal vision. In a rat model carrying negative point mutation for rds gene, the AAV vectors have been employed to provide long term, CNTF-based rescue of rat photoreceptors [Citation126]. Earlier research revealed that after intraorbital optic nerve (ON) transection 85–90% of grown-up mouse RGCs die in 2 weeks. The survival and re-formation of these neurons can be improved by intraocular injections of AAV-2 vectors encoding recombinant CNTF [Citation127].
In other recent study, AAV-mediated transfer of the CNTF and rhodopsin A-short hairpin RNA (RhoA-shRNA) showed the synergistic effects regarding promoting of the survival and axon regeneration of RGCs after the optic nerve crush. Silencing of RhoA and CNTF in AAV vectors increased the regenerative potential and resulted in the successful therapeutic outcomes after neurotrauma in the rat model [Citation128].
Antirecoverin autoantibodies are secreted into the serum of the patients having cancer-associated retinopathy syndrome, which induce photoreceptor apoptosis. The AAV-mediated delivery of the human CNTF gene into eye (via subretinal injection) resulted in the efficient transduction of photoreceptors. Therefore, it prevented the antirecoverin autoantibodies induced apoptosis through suppression of Caspase3 as an apoptotic protein and activation of STAT3 [Citation125].
Other study showed that the administration of rAAV2-CNTF with doxorubicin via subretinal coinjection into the rat eyes having inherited retinal degeneration resulted in an increase in thickness of the photoreceptor layer and higher cellular density at the outer nuclear layer which emphasizes on a noticeable protection effect by the high expression levels of CNTF. This simple delivery method improved rAAV2-based gene delivery without presenting any cytotoxicity. This method could be therapeutically used in the gene therapy of retinal degenerative diseases [Citation129].
In the study by Hellstrom et al., the rAAV-mediated gene delivery just after, neurotrauma caused most successful effects when performed with the temporary postinjury trophic support, so is a viable strategy for the treatment of the patients having acute CNS injury [Citation120].
Administration of rAAV2-SOCS3-GFP demonstrated that SOCS3 overexpression has negative impact on regeneration of mature CNS neurons. Viral vectors mediated CNTF delivery to the retina is more effective than the injections of the bolus recombinant CNTF due to the observations that the gene therapy has a less effect on neuron-intrinsic SOCS (suppressor of cytokine signalling) repressor pathways [Citation119].
Although the recombinant AAV vectors have various benefits, one possible difficulty remains the pause in the onset of the transgene expression, usually needing up to six weeks for peak degrees of protein, which could restrict their use in acute retinal condition.
Cell-based delivery of CNTF protein
Accurate control of the CNTF dose has not be reached for the clinical application, which uses the viral vectors. The difficulties of using the viral vectors for CNTF delivery are promoter type, which determine the expression level, target cell categories and percentage of transduced cells. Additional issues exist including the tuning of CNTF production according to the disease site and the timely termination of treatment. As a result, using other cell-based technologies are necessary in some cases [Citation132]. While CNTF, other proteins and polypeptides may offer therapeutic performance in treatment of the destructive retinal conditions, localized therapy of the retinal diseases is complexed by the blood–retinal barrier (BRB) and makes the transportation of CNTF and other curative proteins to the retina a notable difficulty [Citation133]. This is a special problem in the chronic conditions where repeated treatments may be required, fortunately, cell based approaches for delivering CNTF showed promising potential to overcome aforementioned difficulties.
Cell-based delivery of CNTF into eye is designed in two forms including either transplantation of the biomaterial-encapsulated cells carrying genetic modifications for over expression of CNTF or direct transfer of cells engineered to express CNTF.
The eye is an easily accessible and immunoprivileged organ that provides a good target for the application of encapsulated cell technology (ECT). The current two ECT devices are NT-501 and NT-503. NT-503 delivers a soluble form of the antivascular endothelial growth factor receptor (VEGF-R). NT-501 stands a cylinder-shaped device that has one millimetre diameter and six millimetre length. This device is composed of a semipermeable polyethersulfone peripheral membrane, a core of polyethylene terephthalate, as well as 2 × 105 cells secreting-CNTF.
ECT, particularly the NT-501 implant, was developed to facilitate sustained transportation of the therapeutic elements right into the vitreous cavity [Citation133]. ECT implants consist of living cells encapsulated in a semipermeable polymer layer and matrices. The collected retinal pigment epithelium cells (ARPE-19) from human origin are genetically engineered to create CNTF to target a specific illness or situation [Citation133,Citation134]. Once operationally implanted into the eye, the semipermeable polymer layer has two main operations: it permits the outward crossing of the curative product while guarding the collected cells from exclusion by the patient's immune system and also allows easy access to oxygen and nutrients [Citation135]. The device is implanted throughout a small pars plana incision and anchored to the sclera by a small titanium line loop that facilitates anchorage in sclera and its easy retrieving [Citation136] (). The active intravitreal portion of the cylindrical NT-501 dimensions is established outside the visual axis [Citation134,Citation135]. ECT gives the possibility for measured, constant, long-term transportation of medication, covering a wide range of different proteins like CNTF and other compounds right into the retina, detouring the BRB. Besides, the implants can be recovered, bringing an added degree of protection [Citation136]. Thus, ECT has encouraging operations to important kinds of ocular conditions such as retinal degeneration, ocular inflammation and angiogenesis [Citation135]. A number of pre-clinical and clinical studies reported the remarkable potential of ECT technology in terms of treating retinal diseases [Citation137,Citation138] ().
There are some clear advantages to ECT. First, it offers the possibility for any gene encoding a curative protein to be engineered into a cell and accordingly has a wide range of utilization. Also, the curative protein is newly synthesized and delivered in situ; thus, a comparatively little volume of the protein is needed to carry out a curative impact [Citation133,Citation134]. Steady, endogenous secretion of the protein guarantees that the availability of the protein at the destination site is not only continuous but also long time [Citation134,Citation135]. Moreover, the output of an ECT insert can be managed to deliver the optimal dose for therapy. Eventually, therapy by ECT can be terminated if essential by simply recovering of the implant. Hence, ECT is a potentially useful tool for long-term transfer of proteins and polypeptides to the retina [Citation133,Citation136,Citation139]. NT-501 has been examined in four clinical trials, three of them were for RP in phase 1 for six months, late-stage RP for one year in phase 2 and for two years in phase 2 of early-stage RP, and finally, one study at phase 2 was directed for GA. Presently, NT-501 is under phase-2 trials performing in patients with glaucoma and macular telangiectasia [Citation136].
As previously mentioned, RP illustrates a group of neurodegenerative retinal conditions that induce the death of photoreceptor cells and begin growing vision damage and blindness. More than 39 genetic loci and genes are involved in monogenic forms of RP [Citation140]. With the exclusion of vitamin A nutritional supplementation, not any therapies have been confirmed to be useful for the series of these diseases. Intervention investigations have shown the feasibility of utilizing neurotrophic elements as curative agents for RP, particularly CNTF is active in delaying retinal destruction in leastwise 13 animal RP subjects stage I. Study of NT-501 illustrated the safety and possible advantage of ECT-based transportation of CNTF [Citation141–143]. AMD, the first reason of the blindness in humans age 55 or greater, is a heterogeneous clinical entity in that retinal destruction happens mostly in the macula and drives to deterioration of the central visual awareness [Citation144]. There is no therapy accessible for vision damage connected to the high-level dry AMD or GA. The previous study analysed the CNTF transported via an intraocular ECT insert for the therapy of GA. Consequently, the application of the inserted NT-501 implant may be useful in human with atrophic macular destruction. CNTF transferred by the ECT insert seems to slow the progress of vision loss in GA [Citation145]. In type 2 of macular telangiectasia, the intraocular delivery of CNTF by the ECT implant was reported to be safe and well stand in human eyes [Citation146].
There were no reports of adverse side effects of NT-501 or its implantation events. Regarding immunologic responses, there are no antibodies against NT-501 encapsulated cells or CNTF. As well, there is no detectable amounts of CNTF in serum. Thus, the NT501 is a well-tolerated route for sustained CNTF delivery [Citation137].
The efficacy of the NT-501 for geographic atrophy (GA) and RP were reported to be moderate [Citation145]. Also, in CNTF groups at late stage, there were not any detectable improvements, and even in high doses early-stage RP showed deterioration of the visual field sensitivity in comparison with non-treated patients during one year [Citation142,Citation143].
Since early findings from phase-1 trial for glucoma showed promising results by enhancing the survival of RGCs [Citation147]. A phase–2 trial which is estimated to be finalized in 2020 is at present underway with the aim of recruit 60 patients.
ECT-based methods employ NTC-200 cells that are originated from human retinal pigment epithelium cells (RPEs). Cell-based methods, other than ECT for CNTF delivery, have been studied utilizing for example neural stem cells. Because the main drawback of ECT is its large size in mouse models, the neural stem cells (NSCs) based CNTF delivery may work more effective in pre-clinical studies [Citation123]. In this way, intraocular-grafted NSCs were differentiated into astrocytes that stably express CNTF. In a period of 4 months or more, considerably attenuated the loss of the axotomized RGCs in the mice models of glaucoma [Citation148].
In a study by Jankowiak et al. sustained intraocular delivery of CNTF using NSCs reduced photoreceptor loss in the neuronal ceroid lipofuscinosis (nclf) mice model [Citation123]. The aforementioned cell-based approaches are similar to ECT since the modified cells, which are transferred into eye, are utilized. A polycistronic lentiviral vector was employed for establishing a cell-based intraocular sustained CNTF delivery system to the dystrophic mice retina. The transduction of the polycystronic lentiviral vector carrying a secretable variant of CNTF, genetically modified adherent cultivated murine neural stem cells (NSCs). The intravitreal grafting of the engineered cells into the mice models of retinitis pigmentosa was performed. The cells were differentiated into astrocytes and neurons. Interestingly, the administration of CNTF-secreting NSCs prevented photoreceptor degeneration in mutant mice. Intravitreal transplantations of CNTF secreting NSCs may represent a proper method for the pre-clinical studies with the aim of evaluating the therapeutic effects of cell-mediated intra-ocular CNTF delivery in mice models of photoreceptor degeneration [Citation130]. The direct intravitreal transplantation of engineered cells has been performed using cell types other than NSCs carrying genetic modifications for the overexpression of CNTF. A recent cell-combined administration of CNTF report is interesting, in which a combination of CNTF and olfactory ensheathing cells (OECs) were administered into vitreous body of rat after optic nerve injury. Which showed the additive positive effects rather than CNTF or OECs monotherapy in regeneration of the optic nerve injury [Citation34].
In an interesting study, directed by Huang et. al., a combination of the liposome technology and cell-mediated CNTF transfer into subretinal space of rat right eye was performed. The fetal lung fibroblasts from human origin were engineered to express CNTF at high levels using transfection with liposome. The transplanted fibrobasts (having high expression levels of CNTF) were successful to rescue photoreceptor degeneration of the dystrophic retina in rat [Citation149].
CNTF delivery by means of liposome, nanospheres and hydrogels
Liposomes are artificial membrane-bound vesicles that mimic biological membranes and used as delivering tools for entering the foreign materials into cells [Citation150]. The study by Wang and colleagues, which delivered CNTF gene by liposomes, could enter to the retinal cells. The expression of therapeutic CNTF gene in retinal cells rescued the photoreceptors [Citation151]. The viral vector method was combined with liposome technology to target retina in another research mentioned earlier [Citation149]. The liposome-mediated gene delivery to cells is transient, that is, continues no more than 4 weeks. Hence, repeated administrations are needed, which is considered a draw back for this method.
The hydrogels having biocompatibility [Citation152] are useful for growth factors delivery into diseased tissues, which triggers the start of regeneration. The hydrogels, especially loaded with neurotrophins (NTs), were employed for the treatment of spinal cord injuries.
The release kinetics of the NTs could be controlled via changes in the density of network cross linking. The network cross-linking density influences diffusion and release of NTs from gels ranging from weeks to months [Citation153].
Hydrogels are a group of delivery vehicles that release the therapeutic proteins to trigger regeneration of the damaged tissues [Citation154]. In the study by Burdick et al., CNTF loaded in a degradable hydrogel, acrylated PLA-b-PEG-b-PLA and released in an explanted retina. Subsequently, stimulated outgrowth of a significant more neurites than controls. Besides no toxicity of the PLA-b-PEG-b-PLA hydrogel degradation monomers were detected. Even, the researchers suggested development of microsphere/hydrogel composites intended for codelivery of the multiple neurotrophins having certain release kinetics [Citation155]. Nkansah and colleagues used poly (DL-lactic-co-glycolic acid) (PLGA) nano- and microspheres for the optimized encapsulation/release of CNTF. The nanospheres containing CNTF showed sustained delivery over two weeks. On the other hand, microspheres delivered in a longer period for 70 days. The bioactivity of CNTF after encapsulation in nanospheres was assessed by treating NSCs with released CNTF in comparison with those treated with free CNTF. Significantly, nanospheres released CNTF-treated NSCs exhibited a similar differentiation results as compared to those treated with free same concentration of CNTF; therefore, they suggested that in addition to ideal release, bioactivity of protein did not reduce through encapsulation [Citation104]. In the future, nanotechnology-based CNTF delivery developments would be remarkable regarding their desired performance in retinal delivery [Citation156].
CNTF delivery by cell penetrating peptides (CPP)
Cell-penetrating peptides (CPPs) are a group of short-length peptides having the ability to transfer cargos into cells [Citation157]. The transactivator of the transcription (TAT) protein is a natural CPP found in human immunodeficiency virus. A group of scientists described a CNTF molecule combined with TAT that retains neurotrophic activity after subcutaneous injection without any cytokine side effects [Citation158].
Conclusions
The CNTF showed encouraging outcomes for treating retinal degeneration/disease in recent animal and pre-clinical studies. However, due to specific anatomy, physiology and particular barriers of the eye, there are several limitations in terms of transfer or delivery, and the dose/duration of CNTF administrations. The different delivering strategies have been employed ranging from direct protein injections to more complex viral vectors mediated gene therapy, cell-based transfer and implantation of encapsulated cells. The goal was to determine a safe (with minimum side effects), effectively bio-available and sustained method to release CNTF to retina. Viral vectors, polymer-based ECT or other cell-based delivery methods could facilitate timely releasing of CNTF. Although primary safety of these clinical methods was adequate, therapeutic efficacy of the delivered CNTF is yet far from addressing the expected outcomes. Future studies should be designed for the proper delivery of CNTF to retina with the ultimate purpose of bringing CNTF therapy to routine practice.
Disclosure statement
The authors declared no conflict of interest.
Additional information
Funding
References
- Nejati-Koshki K, Mortazavi Y, Pilehvar-Soltanahmadi Y, et al. An update on application of nanotechnology and stem cells in spinal cord injury regeneration. Biomed Pharmacother. 2017;90:85–92.
- Henderson CE. Role of neurotrophic factors in neuronal development. Curr Opin Neurobiol. 1996;6:64–70.
- Sato C. Chapter five – Releasing mechanism of neurotrophic factors via polysialic acid. In: L. Gerald, editor. Vitamins and hormones. Cambridge (MA): Academic Press; 2017. p. 89–112.
- Pasquin S, Sharma M, Gauchat JF. Ciliary neurotrophic factor (CNTF): New facets of an old molecule for treating neurodegenerative and metabolic syndrome pathologies. Cytokine Growth Factor Rev. 2015;26:507–515.
- Yao M, Moir MS, Wang MZ, et al. Peripheral nerve regeneration in CNTF knockout mice. Laryngoscope. 1999;109:1263–1268.
- Lang EM, Schlegel N, Reiners K, et al. Single-dose application of CNTF and BDNF improves remyelination of regenerating nerve fibers after C7 ventral root avulsion and replantation. J Neurotrauma. 2008;25:384–400.
- Preti A. Axokine (Regeneron). IDrugs. 2003;6:696–701.
- Vigneswara V, Akpan N, Berry M, et al. Combined suppression of CASP2 and CASP6 protects retinal ganglion cells from apoptosis and promotes axon regeneration through CNTF-mediated JAK/STAT signalling. Brain. 2014;137:1656–1675.
- Thoenen H. Ciliary neurotrophic factor prevents the degeneration of motor neurons after axotomy. Nature. 1990;345:31.
- Linker RA, Mäurer M, Gaupp S, et al. CNTF is a major protective factor in demyelinating CNS disease: a neurotrophic cytokine as modulator in neuroinflammation. Nat Med. 2002;8:620–624.
- Aebischer P, Schluep M, Déglon N, et al. Intrathecal delivery of CNTF using encapsulated genetically modifiedxenogeneic cells in amyotrophic lateral sclerosis patients. Nat Med. 1996;2:696–699.
- Mittoux V, Joseph JM, Conde F, et al. Restoration of cognitive and motor functions by ciliary neurotrophic factor in a primate model of Huntington's disease. Hum Gene Ther. 2000;11:1177–1188.
- Emerich DF, et al. Implants of encapsulated human CNTF-producing fibroblasts prevent behavioral deficits and striatal degeneration in a rodent model of Huntington’s disease. J Neurosci. 1996;16:5168–5181.
- Yu S, Tanabe T, Yoshimura N. A rat model of glaucoma induced by episcleral vein ligation. Exp Eye Res. 2006;83:758–770.
- Adler R. Ciliary neurotrophic factor as an injury factor. Curr Opin Neurobiol. 1993;3:785–789.
- LaVail MM, Yasumura D, Matthes MT, et al. Protection of mouse photoreceptors by survival factors in retinal degenerations. Invest Ophthalmol Vis Sci. 1998;39:592–602.
- Liang FQ, Dejneka NS, Cohen DR, et al. AAV-mediated delivery of ciliary neurotrophic factor prolongs photoreceptor survival in the rhodopsin knockout mouse. Mol Ther. 2001;3:241.
- Dutt K, Cao Y, Ezeonu I. Ciliary neurotrophic factor: a survival and differentiation inducer in human retinal progenitors. In Vitro Cell Dev Biol Anim. 2010;46:635–646.
- Emsley J, Hagg T. Endogenous and exogenous ciliary neurotrophic factor enhances forebrain neurogenesis in adult mice. Exp Neurol. 2003;183:298–310.
- Sieving PA, Caruso RC, Tao W, et al. Ciliary neurotrophic factor (CNTF) for human retinal degeneration: Phase I trial of CNTF delivered by encapsulated cell intraocular implants. Proc Natl Acad Sci USA. 2006;103:3896–3901.
- Gaudana R, Ananthula HK, Parenky A, et al. Ocular drug delivery. AAPS J. 2010;12:348–360.
- Lam A, Fuller F, Miller J, et al. Sequence and structural organization of the human gene encoding ciliary neurotrophic factor. Gene. 1991;102:271–276.
- Lin LF, Mismer D, Lile JD, et al. Purification, cloning, and expression of ciliary neurotrophic factor (CNTF). Science. 1989;246:1023–1025.
- Panayotatos N, Radziejewska E, Acheson A, et al. Localization of functional receptor epitopes on the structure of ciliary neurotrophic factor indicates a conserved, function-related epitope topography among helical cytokines. J Biol Chem. 1995;270:14007–14014.
- Wahlin KJ, Campochiaro PA, Zack DJ, Adler R. Neurotrophic factors cause activation of intracellular signaling pathways in Muller cells and other cells of the inner retina, but not photoreceptors. Invest Ophthalmol Vis Sci. 2000;41:927–936.
- Fuhrmann S, Kirsch M, Heller S, et al. Differential regulation of ciliary neurotrophic factor receptor – expression in all major neuronal cell classes during development of the chick retina. J Comp Neurol. 1998;400:244–254.
- Beltran W, Rohrer H, Aguirre GD. Immunolocalization of ciliary neurotrophic factor receptor α (CNTFRα) in mammalian photoreceptor cells. Molecul Vision. 2005;11:232.
- Valter K, Bisti S, Stone J. Location of CNTFRα on outer segments: evidence of the site of action of CNTF in rat retina. Brain Res. 2003;985:169–175.
- Seydewitz V, Rothermel A´e, Fuhrmann S, et al. Expression of CNTF receptor-α in chick violet-sensitive cones with unique morphologic properties. Invest Ophthalmol Vis Sci. 2004;45:655–661.
- Davis S, Aldrich TH, Valenzuela DM, et al. The receptor for ciliary neurotrophic factor. Science. 1991;253:59–63.
- Schuster B, Kovaleva M, Sun Y, et al. Signaling of human ciliary neurotrophic factor (CNTF) revisited the interleukin-6 receptor can serve as an α-receptor for CNTF. J Biol Chem. 2003; 278:9528–9535.
- Ichihara M, Hara T, Kim H, et al. Oncostatin M and leukemia inhibitory factor do not use the same functional receptor in mice. Blood. 1997;90:165–173.
- Wagener E-M, Aurich M, Aparicio-Siegmund S, et al. The amino acid exchange R28E in ciliary neurotrophic factor (CNTF) abrogates interleukin-6 receptor-dependent but retains CNTF receptor-dependent signaling via glycoprotein 130 (gp130)/leukemia inhibitory factor receptor (LIFR). J Biol Chem. 2014;289:18442–18450.
- Gearing DP, Ziegler SF, Comeau MR, et al. Proliferative responses and binding properties of hematopoietic cells transfected with low-affinity receptors for leukemia inhibitory factor, oncostatin M, and ciliary neurotrophic factor. Proc Natl Acad Sci. 1994;91:1119–1123.
- De Serio A, Graziani R, Laufer R, et al. In vitro binding of ciliary neurotrophic factor to its receptors: evidence for the formation of an IL-6-type hexameric complex. J Molecul Biol. 1995;254:795–800.
- Mullberg J, Geib T, Jostock T, et al. IL-6 receptor independent stimulation of human gp130 by viral IL-6. J Immunol. 2000;164:4672–4677.
- Stahl N, Yancopoulos GD. The tripartite CNTF receptor complex: activation and signaling involves components shared with other cytokines. J Neurobiol. 1994;25:1454–1466.
- Bonni A, Frank D, Schindler C, et al. Characterization of a pathway for ciliary neurotrophic factor signaling to the nucleus. Science. 1993;262:1575-1575.
- Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–4090.
- Ji JZ, Elyaman W, Yip HK, et al. CNTF promotes survival of retinal ganglion cells after induction of ocular hypertension in rats: the possible involvement of STAT3 pathway. Eur J Neurosci. 2004;19:265–272.
- Askvig JM, Watt JA. The MAPK and PI3K pathways mediate CNTF-induced neuronal survival and process outgrowth in hypothalamic organotypic cultures. J Cell Commun Signal. 2015;9:217–231.
- Ozawa Y, Nakao K, Kurihara T, et al. Roles of STAT3/SOCS3 pathway in regulating the visual function and ubiquitin-proteasome-dependent degradation of rhodopsin during retinal inflammation. J Biol Chem. 2008;283:24561–24570.
- Young RW. The ninth Frederick H. Verhoeff lecture. The life history of retinal cells. Trans Am Ophthal Soc. 1983;81:193.
- Young RW, Bok D. Participation of the retinal pigment epithelium in the rod outer segment renewal process. J Cell Biol. 1969;42:392–403.
- Xue W, Cojocaru RI, Dudley VJ, et al. Ciliary neurotrophic factor induces genes associated with inflammation and gliosis in the retina: a gene profiling study of flow-sorted, Müller cells. PLoS One. 2011;6:e20326.
- Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, et al. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol Neurobiol. 2008;38:253–269.
- Ezzeddine ZD, Yang X, DeChiara T, et al. Postmitotic cells fated to become rod photoreceptors can be respecified by CNTF treatment of the retina. Development. 1997;124:1055–1067.
- Wen R, Song Y, Kjellstrom S, et al. Regulation of rod phototransduction machinery by ciliary neurotrophic factor. J Neurosci. 2006;26:13523–13530.
- Ju WK, Lee MY, Hofmann HD, et al. Expression of CNTF in Müller cells of the rat retina after pressure‐induced ischemia. Neuroreport. 1999;10:419–422.
- Weise J, Isenmann S, Klöcker N, et al. Adenovirus-mediated expression of ciliary neurotrophic factor (CNTF) rescues axotomized rat retinal ganglion cells but does not support axonal regeneration in vivo. Neurobiol Dis. 2000;7:212–223.
- Li Y, Tao W, Luo L, et al. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PLoS One. 2010;5:e9495.
- Jo S, Wang E, Benowitz L. Ciliary neurotrophic factor is an axogenesis factor for retinal ganglion cells. Neurosci. 1999;89:579–591.
- Leibinger M, Muller A, Andreadaki A, et al. Neuroprotective and axon growth-promoting effects following inflammatory stimulation on mature retinal ganglion cells in mice depend on ciliary neurotrophic factor and leukemia inhibitory factor. J Neurosci. 2009;29:14334–14341.
- van Adel BA, Arnold JM, Phipps J, et al. Ciliary neurotrophic factor protects retinal ganglion cells from axotomy‐induced apoptosis via modulation of retinal glia in vivo. J Neurobiol. 2005;63:215–234.
- Weleber RG. Inherited and orphan retinal diseases: phenotypes, genotypes, and probable treatment groups. Retina. 2005;25:S4–S7.
- Baharivand N, Zarghami N, Panahi F, et al. Relationship between vitreous and serum vascular endothelial growth factor levels, control of diabetes and microalbuminuria in proliferative diabetic retinopathy. Clin Ophthalmol. 2012;6:185.
- Mohamed Q, Gillies MC, Wong TY. Management of diabetic retinopathy: a systematic review. JAMA. 2007;298:902–916.
- Williams R, Airey M, Baxter H, et al. Epidemiology of diabetic retinopathy and macular oedema: a systematic review. Eye (Lond). 2004;18:963–983.
- Wilkinson CP, Ferris FL, Klein RE, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110:1677–1682.
- Geraldes P, Hiraoka-Yamamoto J, Matsumoto M, et al. Activation of PKC-δ and SHP-1 by hyperglycemia causes vascular cell apoptosis and diabetic retinopathy. Nat Med. 2009;15:1298–1306.
- Kollias AN, Ulbig MW. Diabetic retinopathy: early diagnosis and effective treatment. Dtsch Arztebl Int. 2010;107:75–83.
- Alizadeh E, Akbarzadeh A, Eslaminejad MB, et al. Up regulation of liver‐enriched transcription factors HNF4a and HNF6 and liver‐specific microRNA (miR‐122) by inhibition of Let‐7b in mesenchymal stem cells. Chem Biol Drug Des. 2015;85:268–279.
- Alizadeh E, Zarghami N, Eslaminejad MB, et al. The effect of dimethyl sulfoxide on hepatic differentiation of mesenchymal stem cells. Artif Cells Nanomed Biotechnol. 2016;44:157–164.
- Alizadeh E, Eslaminejad M, Reza B, Akbarzadeh A, et al. Upregulation of MiR‐122 via trichostatin a treatments in hepatocyte‐like cells derived from mesenchymal stem cells. Chem Biol Drug Des. 2016;87:296–305.
- Hoseinzadeh S, Atashi A, Soleimani M, et al. MiR-221-inhibited adipose tissue-derived mesenchymal stem cells bioengineered in a nano-hydroxy apatite scaffold. In Vitro Cell Dev Biol-Animal. 2016;52:479–487.
- Saei Arezoumand K, Alizadeh E, Pilehvar-Soltanahmadi Y, et al. An overview on different strategies for the stemness maintenance of MSCs. Artif Cells Nano Med Biotechnol. 2017;45:1255–1271.
- Shotorbani BB, Alizadeh E, Salehi R, Barzegar A. Adhesion of mesenchymal stem cells to biomimetic polymers: a review. Mater SciEng: C. 2016;71:1192–1200.
- Park SS. Cell therapy applications for retinal vascular diseases: diabetic retinopathy and retinal vein occlusion. Invest Ophthalmol Vis Sci. 2016;57(5):ORSFj1-ORSFj10. doi: 10.1167/iovs.15-17594
- Bucher F, Walz JM, Bühler A, et al. CNTF attenuates vasoproliferative changes through upregulation of SOCS3 in a mouse-model of oxygen-induced retinopathy CNTF attenuates vasoproliferative changes in OIR. Invest Ophthalmol Vis Sci. 2016;57:4017–4026.
- Ola MS, Nawaz MI, Siddiquei MM, et al. Recent advances in understanding the biochemical and molecular mechanism of diabetic retinopathy. J Diabetes Complications. 2012;26:56–64.
- Sah AK, Suresh PK. Medical management of glaucoma: focus on ophthalmologic drug delivery systems of timolol maleate. Artif Cells Nanomed Biotechnol. 2017;45:448–459.
- Chan KK-W, Ho JCH, Leung DYL, et al. Ocular perfusion pressure and glaucoma: a review. Hong Kong J Ophthalmol. 2016;20:19–28.
- Motlagh BF. Medical therapy versus trabeculectomy in patients with open-angle glaucoma. Arquivos Brasileiros De Oftalmologia. 2016;79:233–237.
- Brusini P, Johnson CA. Staging functional damage in glaucoma: review of different classification methods. Survey Ophthalmol. 2007;52:156–179.
- Shields MB. Normal-tension glaucoma: is it different from primary open-angle glaucoma? Curr Opin Ophthalmol. 2008;19:85–88.
- Djafari F, Lesk MR, Giguère CÉ, et al. Impact of a brief educational intervention on glaucoma persistence: a randomized controlled clinical trial. Ophthalmic Epidemiol. 2015;22:380–386.
- Pease ME, Zack DJ, Berlinicke C, et al. Effect of CNTF on retinal ganglion cell survival in experimental glaucoma. Invest Ophthalmol Vis Sci. 2009;50:2194–2200.
- Kimura A, Namekata K, Guo X, et al. Neuroprotection, growth factors and BDNF-TrkB signalling in retinal degeneration. IJMS. 2016;17:1584.
- Almasieh M, Wilson AM, Morquette B, et al. The molecular basis of retinal ganglion cell death in glaucoma. Prog Retin Eye Res. 2012;31:152–181.
- Wu Q, Zhang M, Song BW, et al. Expression of ciliary neurotrophic factor after induction of ocular hypertension in the retina of rats. Chin Med J 2007;120:1825–1829.
- Mehta S. Age-related macular degeneration. Prim Care. 2015;42:377–391.
- Gheorghe A, Mahdi L, Musat O. Age-related macular degeneration. Rom J Ophthalmol. 2015;59:74–77.
- Emerson MV, Lauer AK. Emerging therapies for the treatment of neovascular age-related macular degeneration and diabetic macular edema. BioDrugs 2007;21:245–257.
- Zarbin MA. Current concepts in the pathogenesis of age-related macular degeneration. Arch Ophthalmol. 2004;122:598–614.
- Wei CX, Sun A, Yu Y, et al. Challenges in the development of therapy for dry age-related macular degeneration. In: Retinal degenerative diseases. Springer; 2016. p. 103–109.
- Sheth VS, et al. Ciliary neurotrophic factor in the treatment of advanced nonexudative age-related macular degeneration. Lower Gwynedd Township, PA: PentaVision LLC.
- Michalska-Małecka K, Kabiesz A, Nowak M, et al. Age related macular degeneration–challenge for future: Pathogenesis and new perspectives for the treatment. Eur Geriatr Med. 2015;6:69–75.
- Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809.
- Jones BW, Pfeiffer RL, Ferrell WD, et al. Retinal remodeling in human retinitis pigmentosa. Exp Eye Res. 2016;150:149–165.
- Shintani K, Shechtman DL, Gurwood AS. Review and update: current treatment trends for patients with retinitis pigmentosa. J Am Optom Assoc. 2009;80:384–401.
- Sanftner L. HMGee, Abel H, Hauswirth WW, et al. Glial cell line derived neurotrophic factor delays photoreceptor degeneration in a transgenic rat model of retinitis pigmentosa. Mol Ther. 2001;4:622.
- Lipinski DM, Singh MS, MacLaren RE. Assessment of cone survival in response to CNTF, GDNF, and VEGF165b in a novel ex vivo model of end-stage retinitis pigmentosa. Invest Ophthalmol Vis Sci. 2011; 52: 7340–7346.
- Lipinski DM, Barnard AR, Singh MS, et al. CNTF gene therapy confers lifelong neuroprotection in a mouse model of human retinitis pigmentosa. Mol Ther. 2015;23:1308–1319.
- Delyfer M-N, Raffelsberger W, Mercier D, et al. Transcriptomic analysis of human retinal detachment reveals both inflammatory response and photoreceptor death. PLoS One. 2011;6:e28791.
- Ho VY, Wehmeier JM, Shah GK. Wide-field infrared imaging: a descriptive review of characteristics of retinoschisis, retinal detachment, and schisis detachments. Retina (Philadelphia, Pa.). 2016;36(8):1439–1445.
- Chaudhary R, Dretzke J, Scott R, et al. Clinical and surgical risk factors in the development of proliferative vitreoretinopathy following retinal detachment surgery: a systematic review protocol. Syst Rev. 2016;5:107–112.
- Chong DY, Boehlke CS, Zheng QD, et al. Interleukin-6 as a photoreceptor neuroprotectant in an experimental model of retinal detachment. Invest Ophthalmol Vis Sci. 2008;49:3193–3200.
- Asensio-Sánchez V, Collazos J, Cantón M. Interleukin-6 concentrations in the vitreous body of patients with retinal detachment. Arch Soc Esp Oftalmol. 2015;90:527–530.
- van Norren D. Photochemical damage of the retina. In 8. Symposium Licht und Gesundheit; 2014.
- Wu J, Seregard S, Algvere PV. Photochemical damage of the retina. Surv Ophthalmol. 2006;51:461–481.
- Kremers JJ, van Norren D. Two classes of photochemical damage of the retina. Lasers Light Ophthalmol. 1988;2:41–52.
- Valter K, Bisti S, Gargini C, et al. Time course of neurotrophic factor upregulation and retinal protection against light-induced damage after optic nerve section. Invest Ophthalmol Vis Sci. 2005;46:1748–1754.
- LaVail MM, Unoki K, Yasumura D, et al. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc Natl Acad Sci. 1992;89:11249–11253.
- Nkansah MK, Tzeng SY, Holdt AM, et al. Poly(lactic-co-glycolic acid) nanospheres and microspheres for short- and long-term delivery of bioactive ciliary neurotrophic factor. Biotechnol Bioeng. 2008;100:1010–1019.
- Kuno N, Fujii S. Recent advances in ocular drug delivery systems. Polymers. 2011;3(1): 193–221.
- Wert KJ, Skeie JM, Davis RJ, et al. Subretinal injection of gene therapy vectors and stem cells in the perinatal mouse eye. J Vis Exp: 2012;67:4286.
- Wang NK, Tosi J, Kasanuki JM, et al. Transplantation of reprogrammed embryonic stem cells improves visual function in a mouse model for retinitis pigmentosa. Transplantation. 2010;89:911.
- Cayouette M, et al. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293.
- Chong NH, Alexander RA, Waters L, et al. Repeated injections of a ciliary neurotrophic factor analogue leading to long-term photoreceptor survival in hereditary retinal degeneration. Invest Ophthalmol Vis Sci. 1999;40:1298–1305.
- Beltran WA, Wen R, Acland GM, et al. Intravitreal injection of ciliary neurotrophic factor (CNTF) causes peripheral remodeling and does not prevent photoreceptor loss in canine RPGR mutant retina. Exp Eye Res. 2007;84:753–771.
- McGill TJ, Prusky GT, Douglas RM, et al. Intraocular CNTF reduces vision in normal rats in a dose-dependent manner. Invest Ophthalmol Vis Sci. 2007;48:5756–5766.
- Mathews MK, Guo Y, Langenberg P, et al. Ciliary neurotrophic factor (CNTF)-mediated ganglion cell survival in a rodent model of non-arteritic anterior ischaemic optic neuropathy (NAION). Br J Ophthalmol. 2015;99:133–137.
- Wong WK, Cheung AWS, Yu SW, et al. Hepatocyte growth factor promotes long-term survival and axonal regeneration of retinal ganglion cells after optic nerve injury: comparison with CNTF and BDNF. CNS Neurosci Ther. 2014;20:916–929.
- Marangoni D, Vijayasarathy C, Bush RA, et al. Intravitreal Ciliary Neurotrophic Factor Transiently Improves Cone-Mediated Function in a CNGB3-/- Mouse Model of Achromatopsia. Invest Ophthalmol Vis Sci. 2015;56:6810–6822.
- Chen J, Chen P, Backman LJ, et al. Ciliary neurotrophic factor promotes the migration of corneal epithelial stem/progenitor cells by up-regulation of MMPs through the phosphorylation of Akt. Sci Rep. 2016;6:25870.
- Boye SE, Boye SL, Lewin AS, et al. A comprehensive review of retinal gene therapy. Mol Ther. 2013;21:509–519.
- Zein WM, Jeffrey BG, Wiley HE, et al. CNGB3-achromatopsia clinical trial with CNTF: diminished rod pathway responses with no evidence of improvement in cone function. Invest Ophthalmol Vis Sci. 2014;55: 6301–6308.
- Schwartz SG, Scott IU, Flynn HW, et al. Drug delivery techniques for treating age-related macular degeneration. Expert Opin Drug Deliv. 2014;11:61–68.
- Hellström M, Muhling J, Ehlert EM, et al. Negative impact of rAAV2 mediated expression of SOCS3 on the regeneration of adult retinal ganglion cell axons. Mol Cell Neurosci. 2011;46:507–515.
- Hellström M, Pollett MA, Harvey AR. Post-injury delivery of rAAV2-CNTF combined with short-term pharmacotherapy is neuroprotective and promotes extensive axonal regeneration after optic nerve trauma. J Neurotrauma. 2011;28:2475–2483.
- Greenberg KP, et al. Gene delivery to the retina using lentiviral vectors. In: Retinal degenerative diseases. Basel, Switzerland: Springer; 2006. p. 255–266.
- van Adel BA, Kostic C, Déglon N, et al. Delivery of ciliary neurotrophic factor via lentiviral-mediated transfer protects axotomized retinal ganglion cells for an extended period of time. Hum Gene Ther. 2003;14:103–115.
- Jankowiak W, Kruszewski K, Flachsbarth K, et al. Sustained neural stem cell-based intraocular delivery of CNTF attenuates photoreceptor loss in the nclf mouse model of neuronal ceroid lipofuscinosis. PLoS One. 2015;10:e0127204.
- Schlichtenbrede F, MacNeil A, Bainbridge JW, et al. Intraocular gene delivery of ciliary neurotrophic factor results in significant loss of retinal function in normal mice and in the Prph2Rd2/Rd2 model of retinal degeneration. Gene therapy, 2003;10(6):523–527.
- Adamus G, Sugden B, Shiraga S, et al. Anti-apoptotic effects of CNTF gene transfer on photoreceptor degeneration in experimental antibody-induced retinopathy. J Autoimmun. 2003;21:121–129.
- Bok D, Yasumura D, Matthes MT, et al. Effects of adeno-associated virus-vectored ciliary neurotrophic factor on retinal structure and function in mice with a P216L rds/peripherin mutation. Exp Eye Res. 2002;74:719–735.
- Leaver SG, Cui Q, Plant GW, et al. AAV-mediated expression of CNTF promotes long-term survival and regeneration of adult rat retinal ganglion cells. Gene Ther. 2006;13:1328–1341.
- Cen LP, Liang JJ, Chen JH, et al. AAV-mediated transfer of RhoA shRNA and CNTF promotes retinal ganglion cell survival and axon regeneration. Neuroscience. 2017;343:472–482.
- Zhang S, Wu J, Wu X, et al. Enhancement of rAAV2-mediated transgene expression in retina cells in vitro and in vivo by coadministration of low-dose chemotherapeutic drugs. Invest Ophthalmol Vis Sci. 2012;53:2675–2684.
- Jung G, Sun J, Petrowitz B, et al. Genetically modified neural stem cells for a local and sustained delivery of neuroprotective factors to the dystrophic mouse retina. Stem cells. Transl Med. 2013;2:1001–1010.
- Buch P, Bainbridge J, Ali R. AAV-mediated gene therapy for retinal disorders: from mouse to man. Gene Ther. 2008;15: 849–857.
- Wen R, Tao W, Li Y, et al. CNTF and retina. Prog Retin Eye Res. 2012;31:136–151.
- Emerich DF, Thanos CG. NT-501: an ophthalmic implant of polymer-encapsulated ciliary neurotrophic factor-producing cells. Curr Opin Mol Ther. 2008;10:506–515.
- Tao W, R. Wen Application of encapsulated cell technology for retinal degenerative diseases. In: Retinal degenerations. New York: Humana Press; 2007. p. 401–413.
- Tao W. Application of encapsulated cell technology for retinal degenerative diseases. Expert Opin Biol Ther. 2006;6:717–726.
- Kauper K, McGovern C, Sherman S, et al. Two-year intraocular delivery of ciliary neurotrophic factor by encapsulated cell technology implants in patients with chronic retinal degenerative diseases intraocular delivery of CNTF via ECT. Invest Ophthalmol Vis Sci. 2012;53:7484–7491.
- Wong F, Tsang K, Lo A. Delivery of therapeutics to posterior eye segment: cell-encapsulating systems. Neural Regen Res. 2017;12:576–577.
- Wong FSY, Lo ACY. Current development in encapsulated cell therapy for degenerative retinopathies. CTE. 2016;5:30–38.
- Lee SS, Robinson MR. Novel drug delivery systems for retinal diseases. A review. Ophthalmic Res. 2009;41:124–135.
- Hims M, S, Daiger, C. Inglehearn. Retinitis pigmentosa: genes, proteins and prospects. In: Genetics in ophthalmology. Basel, Switzerland: Karger Publishers; 2004. p. 109–125.
- Thanos CG, Bell WJ, O'Rourke P, et al. Sustained secretion of ciliary neurotrophic factor to the vitreous, using the encapsulated cell therapy-based NT-501 intraocular device. Tissue Eng. 2004;10:1617–1622.
- Birch DG, Bennett LD, Duncan JL, et al. Long-term follow-up of patients with retinitis pigmentosa receiving intraocular ciliary neurotrophic factor implants. Am J Ophthalmol. 2016;170:10–14.
- Birch DG, Weleber RG, Duncan JL, et al. Randomized trial of ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for retinitis pigmentosa. Am J Ophthalmol. 2013;156:283–292.
- Bressler NM. Age-related macular degeneration is the leading cause of blindness. JAMA. 2004;291:1900–1901.
- Zhang K, Hopkins JJ, Heier JS, et al. Ciliary neurotrophic factor delivered by encapsulated cell intraocular implants for treatment of geographic atrophy in age-related macular degeneration. Proc Natl Acad Sci. 2011;108:6241–6245.
- Chew EY, Clemons TE, Peto T, et al. Ciliary neurotrophic factor for macular telangiectasia type 2: results from a phase 1 safety trial. Am J Ophthalmol. 2015;159:659–666.e1.
- Neurotech, NT-501 & CNTF. 2016.
- Flachsbarth K, Kruszewski K, Jung G, et al. Neural stem cell-based intraocular administration of ciliary neurotrophic factor attenuates the loss of axotomized ganglion cells in adult mice. Invest Ophthalmol Vis Sci. 2014;55:7029–7039.
- Huang Q, Xu P, Xia X, et al. Subretinal transplantation of human fetal lung fibroblasts expressed ciliary neurotrophic factor gene prevent photoreceptor degeneration in RCS rats. Zhonghua Yan Ke Za Zhi. 2006;42:127–130.
- Rezaei-Sadabady R, Eidi A, Zarghami N, et al. Intracellular ROS protection efficiency and free radical-scavenging activity of quercetin and quercetin-encapsulated liposomes. Artif Cells Nanomed Biotechnol. 2016;44:128–134.
- Wang F, Xia X, Hu H, et al. Liposome-mediated gene transfer into retina. Zhonghua Yan Ke Za Zhi. 2002;38:520–522.
- Bazmi zeynabad F, Salehi R, Alizadeh E, et al. pH-Controlled multiple-drug delivery by a novel antibacterial nanocomposite for combination therapy. RSC Adv. 2015;5:105678–105691.
- Asghari F, Salehi R, Agazadeh M, et al. The odontogenic differentiation of human dental pulp stem cells on hydroxyapatite-coated biodegradable nanofibrous scaffolds. Int J Polym Mater Polym Biomater. 2016;65:720–728.
- Pandit J, Sultana Y, Aqil M. Chitosan-coated PLGA nanoparticles of bevacizumab as novel drug delivery to target retina: optimization, characterization, and in vitro toxicity evaluation. Artif Cells Nanomed Biotechnol. 2017;45:1397–1407.
- Burdick JA, Ward M, Liang E, et al. Stimulation of neurite outgrowth by neurotrophins delivered from degradable hydrogels. Biomaterials. 2006;27:452–459.
- Zhu M, Wang J, Li N. A novel thermo-sensitive hydrogel-based on poly(N-isopropylacrylamide)/hyaluronic acid of ketoconazole for ophthalmic delivery. Artif Cells Nanomed Biotechnol. 2017 [Aug 21];[1–6]. doi: 10.1080/21691401.2017.1368024.
- Sun M, Zhu Z, Wang H, et al. Polyarginine and PEG-AEYLR comodified nanostructured lipid carrier: 10mol% uncleavable PEG-AEYLR showed no shielding effect to polyarginine in vitro while maintaining good tumor targeting in vivo. Artif Cells Nanomed Biotechnol. 2017 [Aug 30];[1–9]. doi: 10.1080/21691401.2017.1307211
- Vieira AS, Rezende ACS, Grigoletto J, et al. Ciliary neurotrophic factor infused intracerebroventricularly shows reduced catabolic effects when linked to the TAT protein transduction domain. J Neurochem. 2009;110:1557–1566.
