Abstract
The objective of the present study was to deliver docetaxel to cancerous cells with enhanced efficacy and safety profile, using aspartic acid linked fullerenols. This aspartic acid derivatized fullerenol conjugate linked with docetaxel was characterized by UV, FT-IR and NMR spectroscopy. Studies for particle size, PDI, zeta potential and FE-SEM were also performed. The conjugate was evaluated for release kinetics, cancer cell cytotoxicity, cellular uptake using confocal laser microscopy and also for pharmacokinetic profile. Cytotoxic studies proved that there was almost 4.3 folds decrease in IC50 with significantly enhanced cellular uptake of the nanometric conjugates. It was observed that the bioavailability was enhanced by 5.8 folds when compared to that of pure DTX. The developed nanoconstructs were erythrocyte compatible and offered decreased protein binding. The findings are encouraging and offer a novel carrier with enhanced efficacy and safety of a drug, belonging to BCS class IV.
Background
Docetaxel (DTX) is a semi-synthetic drug which belongs to a group of anti-neoplastic agents, specifically to anti-metabolites [Citation1]. When compared to crude extracts of taxanes and paclitaxel, DTX is reported to be more effective on tumor cells [Citation2]. It was first synthesized by Guenard et al. in 1980 [Citation3]. DTX is used in the management of brain tumor and as well in prostate, ovarian, breast, small cell lung, head/neck and non-small cell lung cancer, respectively. It was approved by the Food and Drug administration (FDA) in 1996 and Indian CDSCO in 2006 [Citation4]. DTX exhibits its apoptotic action by various mechanisms like stabilizing the microtubule arrays, inhibiting the spindle formation, induces IL-8 (Interleukin) gene expression, centrosomal impairment and blocking of apoptosis-blocking Bcl-2 oncoprotein. It even induces apoptosis by inhibiting certain steps of anaphase and metaphase of mitotic cell division [Citation5]. Along with various advantages, the concerns associated include low aqueous solubility, first pass metabolism (hepatic and intestinal) and poor oral bioavailability (<7%) [Citation6]. Frequently, this drug is administered via parenteral route and the product involves anionic surfactants for solubility enhancement [Citation7]. Use of these surfactants is reported to be associated with several concerns like neutropenia, anorexia, peripheral neuropathy, tissue necrosis and hypersensitivity reactions [Citation8,Citation9].
Now-a-days, fullerenes and the respective derivatives have been extensively explored for various biomedical applications [Citation10,Citation11]. When compared to other isomers of carbon (diamond and graphite), fullerenes are chemically more reactive [Citation12]. In general, C60 fullerene acts as electron deficient alkene molecule, which can easily react with electron rich moieties [Citation13]. Fullerenes easily undergo various types of reactions like pericyclic reactions, halogenations, radical reactions, formation of endohedral complexes, electrophilic and nucleophilic addition reactions [Citation11,Citation14,Citation15]. Fullerenes are generally functionalized in order to overcome the major challenge of aqueous insolubility [Citation16]. It has been observed that increasing the polar groups on fullerene molecule, reduces its hydrophobic nature, without compromising its biological activity [Citation17]. Poly hydroxylated derivatives of fullerenes, generally known as fullerenols (C60(OH)x), are highly hydrophilic in nature with capability of intracellular penetration [Citation18]. These highly polar derivatives of fullerenes have been considered to be delivered via parenteral route and ophthalmic route in rodents [Citation19]. In drug delivery, C60 fullerenes and the derivatives have been extensively employed for delivery of various anticancer drugs to the respective target sites [Citation18]. These conjugated systems with or without linkers are found to be better in drug release control, efficacy enhancement and cancer cell toxicity [Citation4]. In recent past, researchers have made various efforts to deliver anti-cancer drugs like 5-fluro uracil, cyclophosphamide, doxorubicin, paclitaxel and docetaxel by means of C60 fullerene and derivatives, however, there are no reports of delivering DTX by means of fullerenols. Though, numerous nanotechnology-based attempts other than fullerenols have been made to deliver DTX [Citation20–Citation22]. Henceforth, it was envisioned to explore the potential of fullerenols in the delivery of DTX and the influence of this conjugation on the pharmacokinetic profile of the drug.
Materials and methods
Materials
C60fullerenes were purchased from M/s Bucky USA, Houston, TX. Docetaxel (DTX) was generously supplied as a gift sample by M/s Frensius Kabi., Ltd., Gurgaon, Haryana, India. Deuterium oxide (D2O), 3-(4,5-dimethylthiazole-2-yl)-2,5-diphenyltetrazolium bromide (MTT) and dimethyl sulphoxide-d6(DMSO-d6) were procured from M/s Sigma-Aldrich, Bangalore, India. The solvents employed in the study were obtained from M/s Thermo Fisher Scientific India, Co., Ltd., Mumbai, India. The buffer ingredients, aspartic acid and tetrahydrofuran (THF) were purchased from M/s CDH Co., Ltd., New Delhi, India. HPLC-acetonitrile, HPLC-water and 1-ethyl-3–(3-dimethylaminopropyl) carbodiimide (EDC) were supplied by M/s Spectrochem Co., Ltd., Mumbai, India. Bovine serum albumin (BSA) and dialysis membrane were provided by M/s Himedia laboratories Co., Ltd., Mumbai, India. Cell lines used were maintained at UGC-Centre of Excellence in Applications of Nanomaterials, Nanoparticles and Nanocomposites, Panjab University, Chandigarh, India. Distilled water was employed throughout the studies and the chemicals/reagents were used as such without any further purification.
Synthesis of fullerenols
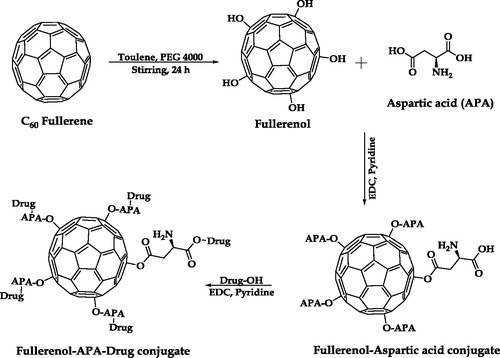
shows the synthetic scheme for the method employed to synthesize fullerenols. Phase-transfer catalyzed reaction was used for synthesizing water soluble fullerenols. In 10 ml aqueous solution of PEG 4000 (50 mg) and sodium hydroxide (20 mg), C60 fullerene solution of toluene (100 mg/20 ml) was added drop-wise with continuous stirring at room temperature. The reaction mixture was kept for stirring until the discoloration of deep violet colored toluene solution, followed by brown colored precipitation. The precipitate was collected and washed 3–4 times with methanol in order to ensure that there were no traces of unreached PEG and sodium hydroxide. Later the precipitate was dried and stored for further use [Citation23].
Extent of hydroxylation and solubility studies
The Fullerenols synthesized were characterized for the number of hydroxyl groups using acid-base titration. Fullerenol (5 mg) was dispersed in 4 ml solution of 0.5 M succinic anhydride (in pyridine) and heated at a temperature of 60 °C. After 1 h, the dispersion was titrated against standardized sodium hydroxide (0.1 N) solution in ethanol, using phenolphthalein as an indicator [Citation24]. Gravimetric method was employed to determine the solubility of fullerenols in distilled water at the ambient temperature.
Synthesis of aspartic acid derivatized fullerenols
Fullerenols (50 mg) were dispersed in 10 ml of THF. To this dispersion, aspartic acid (100 mg) and EDC (5 mg) were added with continuous stirring at room temperature (). After 24 h of stirring the reaction mixture was filtered and washed thrice with distilled water to remove the unreacted aspartic acid and to fetch the fullerenol aspartic acid conjugate, i.e. C60-OH-APA [Citation25].
Conjugation of DTX with C60-OH-APA
The synthesized C60-OH-APA (50 mg) , EDC (5 mg) and DTX (100 mg) were weighed accurately and dispersed in pyridine (8 ml). The whole dispersion was subjected to stirring for 24 h at room temperature (). Reaction was continuously monitored for unreacted DTX using thin layer chromatography. Final conjugate (C60-OH-APA-DTX) was collected and washed with THF to remove the unconjugated drug [Citation21].
Drug loading studies
From the above step the washings of THF were collected and evaporated using rotary evaporator (R- 215, M/s Buchi, Flawil, Switzerland). HPLC acetonitrile was used to dissolve the residue and the concentration of unreacted DTX was determined using high performance liquid chromatography (LC-2010 C HT, M/s Schimadzu Co., Ltd., Chiyoda-ku, Tokyo, Japan) with the following conditions: flow rate of 1.0 ml/min; run time of 30 min; injection volume of 20 µl; mobile phase as acetonitrile: water 50:50 v/v; Merck HPLC Column, Oyster BDS C18 (25 × 4.6 mm, 5 mm); detection wavelength of 231 nm; detector used was PDA (SPD-M20A) and column temperature was maintained at 30 °C [Citation26].
Characterization studies
UV-Visible spectroscopy
To infer the drug conjugation, UV-Visible spectra of pure DTX, naïve fullerenes and C60-OH-APA-DTX in chloroform were observed in the range of 200 to 800 nm [Citation4].
Fourier transform infra-red spectroscopy (FT-IR)
FT-IR data were recorded by scanning a punched tablet (potassium bromide and sample, in the mass ratio of 98:2) at a wave number of 4000–400 cm−1, using FT-IR spectrometer (Spectrum two, M/s Perkin Elmer Co., Waltham, MA).
Nuclear magnetic resonance spectroscopy (NMR)
All the samples were prepared as saturated solutions in various solvents like deuterated water and DMSO-d6. Spectra were recorded on ASCEND 500WB NMR Spectrometer, M/s Bruker Bio Spin Corporation., IN.
Powdered X-ray diffraction (XRD)
XRD is one of the characterization techniques, generally used to determine the materialistic attributes of the powder sample. XRD studies were performed at Department of Physics, Central University of Rajasthan, India on the XRD instrument (EMPYREAN, PANalytical, Germany). In this study, the powder sample was mounted on the sample holder and scanned at a wavelength of 1.54 A°, using copper Kα, as the X-ray source. X-rays were irradiated at angles ranging from 5 to 60° [Citation21].
Elemental analysis
Percentage of various elements like C, Hand N were measured using a CHN analyzer (Thermo Scientific FLASH 2000 Elemental Analyzer, CHNS-O analyzer, Thermo Fisher Scientific, Italy). Sample (1 mg) was weighed accurately in a cup shaped tin foil and made as a ball. This ball was dropped into the analyzer for the estimation of % of elements present in respective samples.
Field emission scanning electron microscopy (FE-SEM)
FE-SEM installed at Advance Materials Research Centre, Malaviya National Institute of Technology (MNIT), Jaipur, India was used for the morphological characterization. Sample was subjected to gold coating, using carbon tape and observed under suitable magnification.
Micromeritics and zeta potential
Particle size analysis and poly dispersity index (PDI) of developed nanocarriers were determined using Malvern Zetasizer (M/s Malvern, Worcestershire, UK) installed at Panjab University, Chandigarh, India. Zeta potential was determined employing the same equipment. For both particle size and zeta potential studies, the solid samples were dispersed in phosphate buffered saline (PBS) of pH 7.4 (1 mg/ml). Final reported results were average of three consecutive readings [Citation27].
Evaluation studies
Release kinetic studies
Both pure DTX (1 mg of DTX dispersed in 1 ml in PBS of pH 5.6 and 7.4) and C60-OH-APA-DTX dispersions (equivalent to 1 mg/ml of DTX in PBS of pH 5.6 and 7.4) were prepared and packed in dialysis bags, separately. These packed bags were dipped separately in beakers filled with 30 ml PBS of pH 5.6 and 7.4. Each set-up was maintained at 37 ± 0.5 °C and the sink was continuously stirred at 50 rpm. Sampling was done at regular time intervals, which was quantified using RP-HPLC, as already disclosed above. Obtained data was fitted into various release kinetic models like zero-order, first-order, Higuchi equation and Korsmeyer-Peppas equation and suitable inferences were drawn [Citation28].
Protein binding studies
Pure DTX (1 mg) and C60-OH-APA-DTX (equivalent to 1 mg of DTX) were dispersed in 1 ml of 2% w/v BSA solution in PBS of pH 7.4. Both the dispersions were packed in dialysis bags and suspended individually in 30 ml of PBS of pH 7.4. Till six hours, for every one hour, 1 ml of sample was collected and analyzed using HPLC for the amount of unbound drug [Citation29].
Blood compatibility studies
This protocol was approved duly by Institutional Ethics Committee, Central University of Rajasthan, Bandar Sindri, Kishangarh, Ajmer, Rajasthan, India. From a healthy human volunteer, 5 ml of blood sample was collected into EDTA-coated vial, under strict medical supervision. To harvest erythrocytes, collected blood sample was centrifuged for 5 min at the speed of 2000 rpm. After centrifugation, these erythrocytes were decanted, washed three times with normal saline and re-suspended in 10 ml of normal saline. To 1 ml of RBC dispersion, both plain DTX (1 mg) and C60-OH-APA-DTX (equivalent to 1 mg of DTX) were added. To serve as negative and positive controls, erythrocytes were dispersed in distilled water and normal saline, respectively. All the tubes were subjected to incubation for 1 h at 37 °C in dark. Subsequent to that, the samples were centrifuged for 5 min for the transparent supernatant. Collected supernatant was analyzed spectrophotometrically at a wavelength of 415 nm [Citation30]. The calculation for percent hemolysis were performed, as already disclosed elsewhere [Citation31].
Cell viability studies
For this study, MDA MB-231 cells were used. The cells were cultured using Dulbecco’s modified Eagle’s medium/Ham’s F-12 containing 5% fetal bovine serum supplemented with 1% 1-glutamine and 1% penicillin/streptomycin in presence of 5% CO2. Test samples in various concentrations (plain DTX and C60-OH-APA-DTX) were added to these culture plates and incubated for 24 h at 37 °C. MTT solution (10 µl) was added into every well of the cultured plate and mixed properly. Purple colored formazan crystals were harvested, after 4 h of incubation. Extracted crystals were dissolved in DMSO (200 µl) and scanned at λmax of 570 nm for the optical density. The optical density was correlated with % cell viability and IC50 values were calculated [Citation28].
Cellular uptake studies
Coumarin-6 tagged C60-OH-APA-DTX was added into culture of MDA-MB-231 cells and incubated for 2 h at 37 °C. After incubation, the cells were washed to remove unpenetrated dye and the nuclei was further stained with DAPI (300 nM). After washing of DAPI, the cells were observed under confocal laser microscope (Nikon C2 Plus, with NIS Elements Version 4.3 Software, M/s NIKON Instruments Inc., Melville, NY), installed at UGC-Centre of Excellence in Applications of Nanomaterials, Nanoparticles & Nanocomposites, Panjab University, Chandigarh, India. For excitation and emission of coumarin-6, wave length of 488 and 500–550 nm were used, whereas the respective wavelengths for DAPI were 405 and 417–477 nm, respectively. All the microphotographs were captured at 60X magnification.
Pharmacokinetic studies
All the animal protocols were duly approved by Institutional Animal Ethics Committee, Panjab University, Chandigarh, India. Unisex Wistar rats, 4–6 weeks old, with average mass of 225 g were employed. All the animals were divided into two groups with four animals each. Samples (plain DTX and C60-OH-APA-DTX) were dispersed in 0.3 ml of “water for injection”. Both the samples were aseptically administered through tail vein of rat at the dose of 5 mg/kg. At pre-determined time points, 0.3 ml of blood sample was collected through retro-orbital plexus. Plasma was separated and mixed with 1 drop of 0.1 N HCl to hydrolyze the ester linkage of DTX. The samples were analyzed by RP-HPLC and the data was analyzed as per 1 CBM IV push model [Citation32].
Statistical analysis
Data obtained from various studies were subjected to statistical analysis to draw an inference. One-way ANOVA was employed for all the comparisons, as the same is most prescribed for such data sets [Citation33]. Unless mentioned, the level of significance studied was p < .05. All the studies were repeated thrice, unless mentioned and the data is represented in the form of mean ± SE.
Results
Extent of hydroxylation and solubility studies
From titrimetry, it was calculated that there were 0.02158 milli moles of -OH groups present per mg of the fullerenols. Stoichiometric calculations revealed the molecular mass of fullerenols as 1111 g/mol, which fetched the molecular formula of fullerenol as C60(OH)23. The gravimetric method revealed the average aqueous solubility of fullerenol as 8.5 ± 0.3 mg/ml (7.65 mM).
Spectroscopic characterization
From UV-Visible spectra for naïve fullerenes, plain DTX and C60-OH-APA-DTX, it was observed that both pure DTX and C60-OH-APA-DTX showed sharp peaks at the wavelength of 232 nm peculiar to DTX. In the spectrum of naïve C60, no such peak was visible [Citation8].
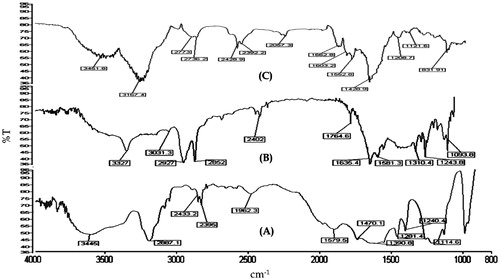
portray the FT-IR spectra of fullerenol, C60-OH-APA and C60-OH-APA-DTX, respectively. From , it can be concluded that fullerenes were successfully hydroxylated. Peaks at 1593 cm−1 showed the -C = C- groups of fullerenes. Peaks at 1281.4 , 1240 and 1114.6 cm−1, respectively were ascribed to -C-O groups of fullerenols whereas, the broad peak at 3445 cm−1 was attributed to presence of -O-H groups due to hydroxylation. From FT-IR spectrum of , the conjugation of aspartic acid on to fullerenols was also confirmed, as the peaks related to stretching’s of -N-H, -O-H, -C = O and -C-O were conspicuous at 3327, 1764.6, 1243.8 and 1093.8 cm−1, respectively. In , peak observed at 3451.8 cm−1 showed the presence of -O-H and -N-H groups of DTX and aspartic acid, respectively. Peaks at 1662.8, 1208.71 and 1121.6 cm−1represented the presence of -C = O and -C-O groups of ester bond formed between -COOH group of aspartic acid and -OH group of DTX [Citation4].
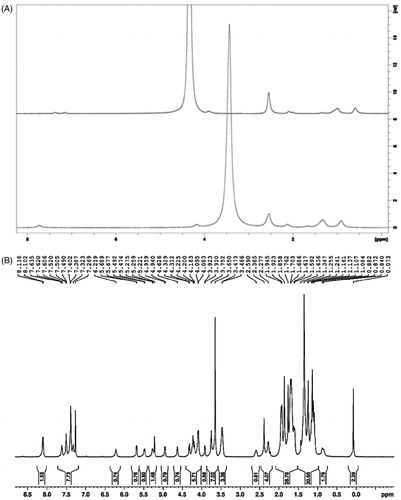
1H NMR spectra in shows the successful hydroxylation and conjugation of DTX with C60-OH-APA. In , the sharp peak at δ of 3.4 represents the protons of -OH in fullerenol. This peak disappeared from the graph, after treating with D2O due to exchange of -OH protons with deuterium. In , aromatic region of DTX was observed in the region of δ of 7.269 to 8.116. Peaks in the region of δ of 5.221 to 6.219 show the -CO-NH of both aspartic acid and DTX. Peaks at δ of 3.466 to 4.959 show the ester linkage between DTX and C60-OH-APA.
Powdered X-ray diffraction (XRD)
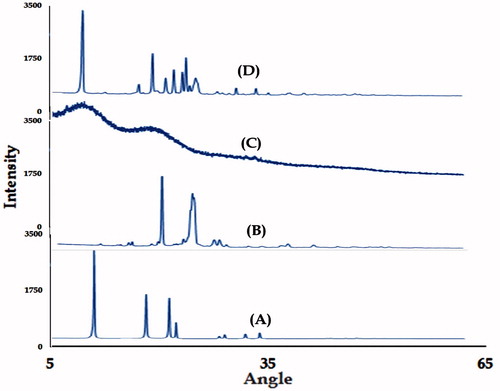
XRD patterns of naïve fullerenes, C60-OH, plain DTX and C60-OH-APA-DTX conjugate were portrayed in . In , diffraction peaks observed at 10.76, 17.72, 20.74 and 21.71°, respectively indicated the plane reflections of naive fullerenes. Crystal lattice constant for fullerene was found to be 14.18 Ǻ. In , peaks at 7.8, 17.43, 19.31, 20.24 and 22.06°, respectively showed the existence of plane reflections from the synthesized conjugate. Crystal lattice constant of synthesized conjugate was found to be 8.15 Ǻ. It was proved that pure DTX existed in amorphous form, whereas change in lattice structure was observed after conjugation.
Elemental analysis
The results showed that the fullerenes contained 101.4%, indicating pure carbon, devoid of any oxidation and other impurities. The percentages of C, H and O for the deduced formula of fullerenols were in the closed agreement with the experimental results of elemental analysis. The results from quantification of the unreacted reactants at various reaction stages and elemental analysis for the final conjugate C60-OH-APA-DTX indicated that only 22% of the hydroxyl groups were conjugated with APA and 30% of the APA reacted with the DTX molecules. The final conjugate was able to load five APA molecules and two DTX molecules.
Drug loading studies
Through RP-HPLC method, amount of drug conjugation was found to be 95.93 ± 0.39%, whereas the drug loading was observed to be 48.80 ± 0.17%.
Field emission scanning electron microscopy
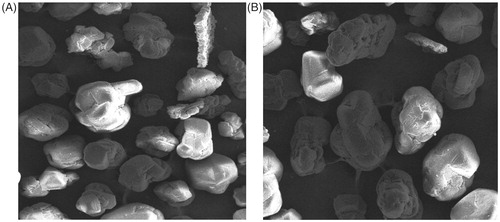
FE-SEM images of naïve fullerenes and C60-OH-APA-DTX conjugate is depicted in . Though the FE-SEM images offered near to spherical structures for both the samples of fullerenols and the designed conjugate, however, the pictorial views were not of the individual molecules.
Micromeritics & zeta potential
shows particle size, zeta potential and PDI values of C60-OH-APA-DTX conjugate along with various intermediates. Pattern of size was observed as C60-fullerenes > fullerenols > C60-OH-APA > C60-H-APA-DTX conjugate. From , it was also clear that all the results were acceptable as the PDI values were <0.3. In general, the surface charge of electron rich fullerene/derivatives remained on negative side ranging from −2.71 to −7.99 mV.
Table 1. Results of particle size, poly dispersity index (PDI) and zeta potential studies.
Release kinetic studies
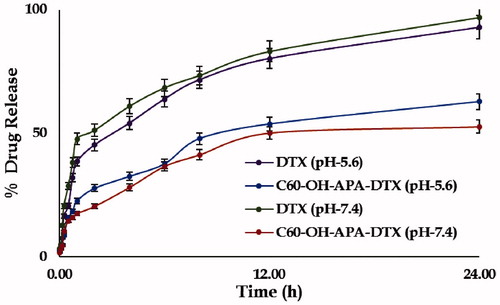
Percent drug release of DTX and C60-OH-APA-DTX conjugate at pH 5.6 and 7.4 is shown in . At pH 5.6, DTX and C60-OH-APA-DTX conjugate were able to show respective drug release of 92.91 and 62.79%, whereas at pH 7.4, it was found to be 96.87 and 52.68%, respectively. At pH 5.6, average release flux values of drug from pure DTX and C60-OH-APA-DTX were calculated to be 5.57 ± 0.12 and 3.73 ± 0.07 µg.h−1 . cm−2, respectively. The same flux values were respectively observed as 5.79 ± 0.02 and 3.15 ± 0.13 µg.h−1 . cm−2 at pH 7.4. It was calculated that there was significant difference in release profile of DTX from pure DTX and C60-OH-APA-DTX conjugate in both pH 5.6 and 7.4 at p < .05. After release model fitting, it was observed that both the drug as well the conjugate offered the Higuchian drug release profile. This kind of release behavior is very common for the systems where there is neither matrix swelling nor matrix dissolution, as in the case with fullerenols. However, Higuchian drug release profile of plain drug can be ascribed to its BCS class IV nature [Citation34].
Protein binding studies
From protein binding studies, it was observed that protein binding was 65 ± 0.3% and 25 ± 0.13%, in case of pure DTX and C60-OH-APA-DTX, respectively after 6 h (p < .05).
Blood compatibility studies
From the results, it was clear that there was substantial decrease in % hemolysis by around 1.8 times after conjugation of DTX with C60-OH-APA. Even fullerenols were able to offer reduced hemolysis in comparison to that of plain DTX.
Cell viability studies
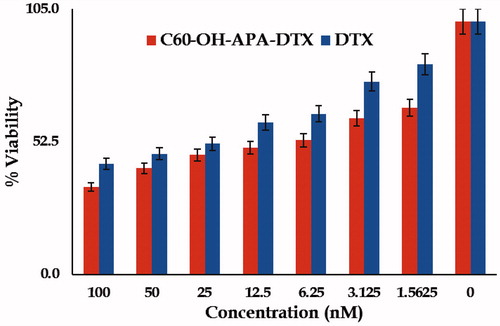
showcases the % cell viability of MDA-MB-231 cells incubated with pure DTX and C60-OH-APA-DTX conjugate at various concentrations in the form of bar diagram. It was observed that naïve fullerenes and fullerenols were non-toxic to MDA-MB-231 cells, even at the concentration of >100 nM. IC50 values for the DTX and C60-OH-APA-DTX conjugate observed values were 37.5 and 12.5 nM, respectively.
Cellular uptake studies
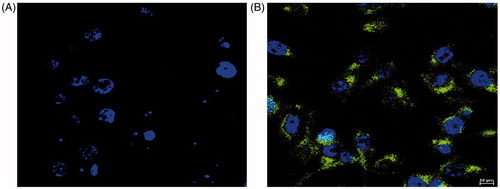
Confocal laser scanning microphotographs of control and C60-OH-APA-DTX conjugated have been showcased in . From the it was clear that the dye (coumarin-6) alone was not able to penetrate into MDA-MB-231 cells in consonance with the published literature [Citation35]. shows both green and blue spots of coumarin-6 tagged conjugate along with DAPI stained nucleus, simultaneously. From this image, it was clear that the conjugate was able to penetrate into cytoplasm as well as nucleoplasm of the cell.
Pharmacokinetic studies
From the calculated plasma drug concentrations, it was concluded that, rodents group injected with C60-OH-APA-DTX conjugate shows more amount of drug in the central compartment when compared to that of group treated with pure DTX.
By fitting the obtained data in one compartmental I.V push model various pharmacokinetic parameters were calculated as represented in . It was observed that there was almost 5.8 times increase in the bioavailability of drug by conjugation. Half-life of drug was also increased by approximately six times by conjugation when compared to pure drug. Decreased clearance and increased volume of distribution shows better delivery of DTX with enhanced efficacy and safety.
Table 2. Various pharmacokinetic parameters obtained from in-vivo pharmacokinetic studies.
Discussion
The first synthetic step resulted in a fullerenol molecule with a stoichiometric formula of C60(OH)23. The findings are in consonance with the reports from previous results, however, the slight variation in number of -OH groups can be ascribed to the type of PEG employed [Citation23]. The solubility studies offered the solubility of the order of 7.65 mM, which is in close agreement with the previously published solubility of fullerenols in the range of 1 to 5 mM [Citation18].
Spectroscopic characterization by UV, FT-IR and NMR was performed. Presence of DTX absorption maxima in UV-Visible spectrum inferred the conjugation of DTX with fullerenols, which was further confirmed by specific stretching and bending peaks offered by various bonds/groups in FT-IR spectra [Citation8]. 1HNMR spectrum of fullerenol offered a peculiar peak corresponding to -OH protons of fullerenols, which disappeared after deuterium exchange with D2O [Citation36]. This distinct peak of -OH groups of fullerenol could be easily ruled out from that of the moisture of DMSO-d6 [Citation36]. The 1HNMR successfully demonstrated the conjugation of APA to C60-fullerenols to fetch with C60-OH-APA and its further conjugation with DTX to form the desired conjugate, i.e. C60-OH-APA-DTX [Citation36].
XRD studies revealed the amorphous nature of pure DTX due to absence of any characteristic crystalline peak. Crystal lattice constant of C60-fullerene was determined to be 14.18 Ǻ and the observed value was in consonance with reported value for these carbon-based sub-micron structures [Citation37]. The final conjugate was also observed to be crystalline in nature with a crystal lattice constant of 8.15 Ǻ. The difference in crystal lattice constant clearly vouched the difference in the crystal structure of the fullerenes and the synthesized nanoconjugate [Citation38].
The results of drug loading and conjugation efficiency proved that there was significant capacity of drug loading onto water soluble fullerenols. Such drug payload carrying capability of the nanocarriers is a desired attribute in chemotherapy so as to deliver the desired dose in a sustained manner [Citation21]. As fullerenols and its derivatives are known to possess diameter of ≈1 nm, hence, molecular depiction is not possible from FE-SEM or TEM. However, the findings of FE-SEM were in consonance with the previous reports [Citation18]. On the other hand, the pictures supported the findings from XRD, indicating an organized clustering as well as absence of any visible contaminant spots [Citation39].
From the data obtained from micromeritics, it was observed that there was decrease in the hydrodynamic diameter of the nanostructures at every synthetic step. From the particle size data, it can be concluded that decrease in particle size results in increase of solubility nature. However, the hydrodynamic diameters should not be confused with the diameter of segregated molecule, as these fullerene-based systems are established to form clusters in water and never offer molecular solutions [Citation18]. The negative surface charge of fullerene and its derivatives can be attributed to electron rich sp2_hybridized carbon network of naïve fullerenes. The negative charge has been further increased by conjugation of DTX, which is due to free -OH groups of DTX [Citation22]. However, nanoparticles with surface charges between 0 to ±10 mV are regarded as pharmacokinetically neutral and are reported to bypass mononuclear phagocytic system, resulting in prolonged circulation [Citation40].
It was observed that drug release from the conjugate was controlled and pH dependent, when compared to that of pure DTX in both the pH environments (pH 5.6 and 7.4). There was higher amount of drug release from conjugate at cancer cell pH of 5.6, which ensured the increased availability of drug to cancer cells and retarded drug release at plasma pH ensured substantial carriage to the target site with minimal transportation loss [Citation4]. The drug release from the conjugate followed Higuchi model indicating non-involvement of matrix swelling and drug dissolution, which is peculiar to inorganic drug delivery systems [Citation34]. From protein binding studies, it was observed that there was significant difference between binding of pure drug and C60-OH-APA-DTX conjugate [Citation28]. The decrease in protein binding has been directly correlated with the incresed drug availability for the target site and is generally regarded as a favorable phenomenon [Citation41]. Meanwhile, the results of hemolysis unequivocally demonstrate the hemocompatible nature of the developed system, which is an indispensable and desirable characteristic of the drug carrier intended to be administered by intravenous route [Citation42].
In MTT assay, naïve fullerenes and the fullerenols were observed to be non-toxic for the studied breast cancer cells. Similar findings in literature have been reported for fullerenes [Citation4], but for fullerenols reports on MDA-MB-231 are seldom available. However, data for fullerenols on LLC-PK1 cell viability also suggest that these carriers are not cytotoxic below the concentrations of 600 nM, the concentration employed far above the range explored in the present study [Citation20]. On the other hand, there was 4.3folds (p < .05) decrease in the IC50 value of drug against MDA-MB-231 cancer cells, after the conjugation process. This shows that there was higher cytotoxic effect even at low concentrations, which can reduce the undesired side effects of the drug. The enhanced cytotoxicity can be attributed to the high cellular accessibility of fullerene-based systems, followed by the drug release by intra-cellular esterases and acidic microenvironment. The partial P-gp efflux inhibition by fullerenes might have also contributed to the enhanced efficacy [Citation43]. The results from confocal laser microscopy were also in consonance with the cell viability assay, as the conjugate was able to get the access of cytoplasm as well as nucleoplasm, the target site of drug. Unlike naïve fullerenes, fullerenols are not able to penetrate the biological membranes, as such. However, the carriers like designed conjugate (molecular mass >500 Da; approximately 2841 Da) are engulfed inside the cells by phagocytosis, as reported in literature. The negatively charged nanocarriers like the present one, are generally phagocytized by clathrin-independent mechanisms, where the engulfed system is exposed to the acidic microenvironment of endosomes [Citation44]. The esterases and the acidic vicinity might have assisted the cleavage of ester linkage to release DTX inside the cytoplasm [Citation45]. This provides a sound explanation by which the developed conjugate helped in enhancing the efficacy of DTX [Citation30].
The nanoconjugate was able to improve the pharmacokinetic performance of drug, not only by enhancing the bioavailability, but also by increasing the biological residence. The findings are in close agreement with the inferences drawn from micromeritics and zeta potential. Higher plasma levels for prolonged durations provides a fair chance to drug to get easy access of the target site and also offers a huge promise in dose reduction, one of the most desirables in cancer chemotherapy [Citation46].
Acknowledgements
M/s Frensius Kabi., Ltd., Gurgaon, Haryana, India is acknowledged for the generous gift sample of docetaxel.
Disclosure statement
The authors report no conflict of interest.
Additional information
Funding
References
- Zhang H, Li R, Lu X, et al. Docetaxel-loaded liposomes: preparation, pH sensitivity, pharmacokinetics, and tissue distribution. J Zhejiang Univ Sci B. 2012;13:981–989.
- Prakash O, Kumar A, Kumar P, et al. Anticancer potential of plants and natural products: a review. Am J Pharmacol Sci. 2013;1:104–115.
- Guenard D, Gueritte-Voegelein F, Potier P. Taxol and taxotere: discovery, chemistry, and structure-activity relationships. Acc Chem Res. 1993;26:160–167.
- Raza K, Thotakura N, Kumar P, et al. C60-fullerenes for delivery of docetaxel to breast cancer cells: a promising approach for enhanced efficacy and better pharmacokinetic profile. Int J Pharm. 2015;495:551–559.
- Kumar P, Raza K, Kaushik L, et al. Role of colloidal drug delivery carriers in taxane-mediated chemotherapy: a review. Curr Pharm Des. 2016;22:5127–5143.
- Wang H, Xu Y, Zhou X. Docetaxel-loaded chitosan microspheres as a lung targeted drug delivery system: in vitro and in vivo evaluation. Int J Mol Sci. 2014;15:3519–3532.
- Shi J, Zhang H, Wang L, et al. PEI-derivatized fullerene drug delivery using folate as a homing device targeting to tumor. Biomaterials. 2013;34:251–261.
- Arora S, Saharan R, Kaur H, et al. Attachment of docetaxel to multiwalled carbon nanotubes for drug delivery applications. Adv Sci Lett. 2012;17:70–75.
- Musumeci T, Ventura CA, Giannone I, et al. PLA/PLGA nanoparticles for sustained release of docetaxel. Int J Pharm. 2006;325:172–179.
- Prylutska SV, Skivka LM, Didenko GV, et al. Complex of C60 fullerene with doxorubicin as a promising agent in antitumor therapy. Nanoscale Res Lett. 2015;10:499.
- Raza K, Kumar M. C60-fullerenes as drug delivery carriers for anticancer agents: promises and hurdles. Pharm Nanotechnol. 2017;5:1–1.
- Olah GA, Bucsi I, Aniszfeld R, et al. Chemical reactivity and functionalization of C60 and C70 fullerenes. Carbon NY. 1992;30:1203–1211.
- Maggini M, Scorrano G, Bianco A, et al. Addition reactions of C60 leading to fulleroprolines. J Chem Soc Chem Commun. 1994;45:305.
- Hirsch A, Brettreich M. Fullerenes: chemistry and reactions. Weinheim (Germany): Wiley-VCH; 2005.
- Avent AG, Boltalina OV, Lukonin AY, et al. Isolation and characterisation of C60F16; a key to understanding fullerene addition patterns. J Chem Soc Perkin Trans. 2000;2:1359–1361.
- Savage N, Diallo MS. Nanomaterials and water purification: opportunities and challenges. J Nanopart Res. 2005;7:331–342.
- Martín N, Elliott B, Hudson JS, et al. New challenges in fullerene chemistry. Chem Commun. 2006;12:2093–2104.
- Injac R, Prijatelj M, Strukelj B. Fullerenol nanoparticles: toxicity and antioxidant activity methods. Mol Biol. 2013;1028:75–100.
- Aoshima H, Saitoh Y, Ito S, et al. Safety evaluation of highly purified fullerenes (HPFs): based on screening of eye and skin damage. J Toxicol Sci. 2009;34:555–562.
- Johnson-Lyles DN, Peifley K, Lockett S, et al. Fullerenol cytotoxicity in kidney cells is associated with cytoskeleton disruption, autophagic vacuole accumulation, and mitochondrial dysfunction. Toxicol Appl Pharmacol. 2010;248:249–258.
- Misra C, Thotakura N, Kumar R, et al. Improved cellular uptake, enhanced efficacy and promising pharmacokinetic profile of docetaxel employing glycine-tethered C 60-fullerenes. Mater Sci Eng C. 2017;76:501–508.
- Raza K, Kumar N, Misra C, et al. Dextran-PLGA-loaded docetaxel micelles with enhanced cytotoxicity and better pharmacokinetic profile. Int J Biol Macromol. 2016;88:206–212.
- Zhang JM, Yang W, He P, et al. Efficient and convenient preparation of water-soluble fullerenol. Chin J Chem. 2010;22:1008–1011.
- Narang CK, Iyer V, Mathur NK. Microdetermination of hydroxyl groups via esterification with succinic and o-sulfobenzoic anhydrine. Microchem J. 1965;9:408–410.
- Thakur CK, Thotakura N, Kumar R, et al. Chitosan-modified PLGA polymeric nanocarriers with better delivery potential for tamoxifen. Int J Biol Macromol. 2016;93:381–389.
- Raza K, Kumar D, Kiran C, et al. Conjugation of docetaxel with multiwalled carbon nanotubes and co-delivery with piperine: implications on pharmacokinetic profile and anti-cancer activity. Mol Pharmaceutics. 2016;13:2423–2432.
- Kumar P, Kumar R, Singh B, et al. Biocompatible phospholipid-based mixed micelles for tamoxifen delivery: promising evidences from –in-vitro anticancer activity and dermatokinetic studies. AAPS Pharm Sci Tech. 2017;18:2037–2044.
- Madhwi Kumar R, Kumar P, et al. In vivo pharmacokinetic studies and intracellular delivery of methotrexate by means of glycine-tethered PLGA-based polymeric micelles. Int J Pharm. 2017;519:138–144.
- Joshi M, Kumar P, Kumar R, et al. Aminated carbon-based “cargo vehicles” for improved delivery of methotrexate to breast cancer cells. Mater Sci Eng C. 2017;75:1376–1388.
- Kumar P, Sharma G, Kumar R, et al. Vitamin-derived nanolipoidal carriers for brain delivery of dimethyl fumarate: a novel approach with preclinical evidence. ACS Chem Neurosci. 2017;8:1390–1396.
- Zhang J, Chen XG, Huang L, et al. Self-assembled polymeric nanoparticles based on oleic acid-grafted chitosan oligosaccharide: biocompatibility, protein adsorption and cellular uptake. J Mater Sci Mater Med. 2012;23:1775–1783.
- Kumar P, Sharma G, Kumar R, et al. Enhanced brain delivery of dimethyl fumarate employing tocopherol-acetate-based nanolipidic carriers: evidence from pharmacokinetic, biodistribution, and cellular uptake studies. ACS Chem Neurosci. 2017;8:860–865.
- Daniel WW, Cross CL. Biostatistics: a foundation for analysis in the health sciences (Wiley Series in Probability and Statistics). Hoboken, NJ: John Wiley & Sons Inc; 2013.
- Dash S, Murthy PN, Nath L, et al. Kinetic modeling on drug release from controlled drug delivery systems. Acta Pol Pharm. 67:217–223.
- Tang X, Cai S, Zhang R, et al. Paclitaxel-loaded nanoparticles of star-shaped cholic acid-core PLA-TPGS copolymer for breast cancer treatment. Nanoscale Res Lett. 2013;8:420.
- Pavia DL, Lampman GM, Kriz GS, et al. Introduction to spectroscopy. Stamford (CT): Brooks/Cole, Cengage Learning; 2009.
- Sathish M, Miyazawa K, Mukhopadhyay P, et al. Selective precipitation of tubular-like short fullerene (C60) whiskers at liquid–liquid interface. Cryst Eng Comm. 2010;12:4146.
- Sathish MK, Miyazawa A, Sasaki T. Nanoporous fulleren nano-whiskers. Chem Mater. 2007;19:2398–2400.
- Clausell JV, Bastida J, Serrano FJ, et al. A new FESEM procedure for assessment of XRD microstructural data of kaolinites. Appl Clay Sci. 2007;37:127–132.
- Nimesh S, Chan R, Gupta N. Advances in nanomedicine for the delivery of therapeutic nucleic acids. India: Elsevier Science & Technology; 2017.
- Scheife RT. Protein binding: what does it mean? DICP. 1989;23:S27–S31.
- Fröhlich E. Hemocompatibility of inhaled environmental nanoparticles: potential use of in vitro testing. J Hazard Mater. 2017;336:158–167.
- Xu X, Li R, Ma M, et al. Multidrug resistance protein P-glycoprotein does not recognize nanoparticle C60: experiment and modeling. Soft Matter. 2012;8:2915.
- Bareford LM, Swaan PW. Endocytic mechanisms for targeted drug delivery. Adv Drug Deliv Rev. 2007;59:748–758.
- Nielsen GD, Roursgaard M, Jensen KA, et al. In vivo biology and toxicology of fullerenes and their derivatives. Basic Clin Pharmacol Toxicol. 2008;103:197–208.
- Shargel L, Wu-Pong S, Yu A. Applied biopharmaceutics and pharmacokinetics. 5th ed. USA: McGraw Hill Professional; 2004.