Abstract
Non-viral gene delivery methods are considered due to safety and simplicity in human gene therapy. Since the use of cationic peptide and niosome represent a promising approach for gene delivery purposes we used recombinant fusion protein and cationic niosome as a gene carrier. A multi-domain fusion protein including nuclear localization motif (NLS) and two DNA-binding (Mu) domains, namely NLS-Mu-Mu (NMM) has been designed, cloned and expressed in E. coli DE3 strain. Afterward, the interested protein was purified by affinity chromatography. Binary vectors based on protein/DNA and ternary vectors based on protein/DNA/niosome were prepared. Protamine was used as a control. DNA condensing properties of NMM and protamine were evaluated by various experiments. Furthermore, we examined cytotoxicity, hemolysis and transfection potential of the binary and ternary complexes in HEK293T and MCF-7 cell lines. Protamine and Lipofectamine™2000 were used as positive controls, correspondingly. The recombinant NMM was expressed and purified successfully and DNA was condensed efficiently at charge ratios that were not harmful to cells. Peptidoplexes showed transfection efficiency (TE) but ternary complexes had higher TE. Additionally, NMM ternary complex was more efficient compared to protamine ternary vectors. Our results showed that niosomal ternary vector of NMM is a promising non-viral gene carrier to achieve an effective and safe carrier system for gene therapy.
Introduction
Nowadays, one of the most efficient strategies to deal with genetic disorders is gene-based therapeutic technologies that will be acceptable with the purposeful delivery of exogenous genes into target cells and their successful expression [Citation1,Citation2]. According to previous studies, there are currently two major categories of nucleic acid delivery, which includes viral- and non-viral-based approaches. The viral vectors, despite having their advantages like high transfection efficiency, can result in some adverse clinical complications for instance immunogenicity, mutagenesis and pro-inflammatory responses [Citation3]. For successful non-viral gene delivery, numerous groups of compounds such as cationic lipids, polymers, peptides and carbohydrate analogs play central roles as the delivery vectors [Citation4–7]. These non-viral-based gene delivery vectors are required to severely condense DNA and conserve it against nuclease enzyme, targeted cell-surface receptors disrupt the endosome membrane and finally transferring the loaded DNA to the nucleus [Citation8] Thus, such kind of vectors will overcome a lot of delivery barriers.
Innovative peptides have shown to be the most efficient, among the non-viral approaches. The nucleus targeting is the key objective of the use of peptide; nuclear localization signal (NLS) are amino acid sequences that label a protein to import it into the nucleus through the nuclear membrane. To enhance gene expression, NLS motifs have been conjugated with different cationic peptides in several investigations [Citation9–11]. Another significant advantage of peptide-based vectors over other non-viral strategies is the power of condensing DNA. As cationic peptides rich in basic residues such as lysine and/or arginine are able to efficiently condense DNA [Citation7,Citation12,Citation13].
There are many effective cationic peptides like poly-l-lysine (pLL), protamine sulfate (PA) and Mu peptide (µ) [Citation9,Citation14,Citation15]. Protamine as a cationic peptide has the potent capability in DNA condensing and nuclear targeting that recently has been widely employed in gene delivery [Citation16–18]. FDA has accepted protamine as a safe cationic peptide; however, there are some reports about low transfection efficiency of protamine/DNA complex in gene delivery [Citation19–21]. One possible reason may lie in the strong hydrophilicity of protamine, which makes it difficult to cross the cellular membrane. In another study, the addition of hydrophobic stearyl group into hydrophilic protamine showed better transfection efficiency than protamine without modification through enhanced internalization which improved nuclear delivery of plasmid DNA (pDNA) as well [Citation19]. Furthermore, some researchers have shown that addition of stearyl moiety to octaarginig peptide improved its cell entry and transfection efficiency through increase hydrophobicity of the binary complex [Citation20]. Mu, as a peptide with 19 amino acids residue (MRRAHHRRRRASHRRMRGG), has been shown promising transfection enhancing properties in different in-vitro and in-vivo models [Citation9,Citation10,Citation22].
Though cationic peptide vectors have advantages in gene therapy; their efficiency should be improved to obtain clinical benefits [Citation21,Citation23]. One of the possible strategies to eliminate obstacles and enhance the transfection is the hybridized utilization of non-viral vectors. Researchers have demonstrated the possibility of solving several problems in gene therapy by use of ternary complexes such as the combination of cationic liposomes with peptides as well as protein-linked lipid chains [Citation23]. The ternary nanoparticles have revealed higher transfection performance compared to the binary complexes [Citation17,Citation24,Citation25]. Both liposomes and niosomes have significance role in the hybridization of cationic vesicles and peptide. Niosomes are composed of bilayer-structure non-ionic surfactants, which are considered as carriers which enhance the delivery of chemical drugs, peptides, DNA, hormones and other bioactive agents [Citation26]. In fact, niosomes represent an alternative to liposomes for cargo delivery, where the phospholipids of the liposomes have been substituted by non-ionic surfactants and are lower in cost and is a superior chemical with storage stabilities compared to liposomes [Citation27]. However, there are only a few reports on their application for gene delivery purposes [Citation17,Citation28].
Due to to the limited information on niosomal gene delivery system and low transfection efficiency of peptide-based gene delivery vectors, we decided to conduct the current study to assess the transfection efficiency of a multi-domain cationic peptide in binary and ternary systems. Here, we produced a new multi-domain His-tag fusion protein in bacteria, namely NLS-Mu-Mu (NMM). We also hypothesized the incorporation of NMM and protamine into the niosome structure, at an optimized formulation, which may lead to the design of novel non-viral ternary vectors, with a low toxic profile, that will enhance the cell transfection effciency in an in vitro model.
Materials and methods
Strains and chemicals
Cell lines were obtained from Pasteur Institute of Iran (Tehran, Iran). Plasmid encoding Cherry fluorescent protein (pmCherry-C1) containing a CMV promoter was prepared as previously described by Eslaminejad et al. [Citation29]. Restriction enzymes, T4 DNA ligase, PicoGreen dye, Opti-MEM medium were obtained from Thermo scientific (Carlsbad, CA,). DC-cholesterol, cholesterol, Span 80, Tween 80, nickel chloride, ethidium bromide (EtBr), protamine sulfate, isopropyl b-D-1- thiogalactopyranoside (IPTG)and primary 6x-his tag monoclonal antibody were provided by Sigma (Steinheim, Germany). Ni-NTA Superflow was provided from QIAGEN Company (Hilden, Germany). All other needed reagents were obtained from valid commercial sources.
Plasmid construction and sub-cloning
The NMM gene () was synthesized and inserted into the Nco1/Xho1 sites of the PUC57 plasmid. Then, the gene was sub-cloned into the PET28a plasmid, an expression vector, at the Nco1 and Xho1 positions of its multiple cloning sites. The C-terminal of NMM gene had polyhistidine tag.
NMM protein overexpression and purification
The expression of recombinant NMM protein in E. coli BL21 strain was induced by 0.7 mM IPTG at 25 °C according to initial expression experiments. The cells were harvested by centrifugation at 6000 × g, at 4 °C for 16 h after IPTG (0.7 mM) induction. The collected cells were resuspended in lysis buffer containing 8 mM urea and then disrupted by sonication 15 times for 20 s on ice with 30 s break times. The supernatant was collected by centrifugation at 18,000 × g at 4 °C for 30 min. Recombinant NMM protein was purified from bacterial pellets followed by the use of metal affinity matrix Ni-nitrilotriacetic acid-Sepharose (Ni-NTA) column according to manufacturer’s protocol as described previously [Citation30]. For urea removal PD-10 desalting columns were used subsequently. Finally, for investigation of protein purity, eluted fractions were loaded on SDS-PAGE gel. Western blotting of the purified protein was carried out based on our previous work [Citation31] using mouse monoclonal antibody against the 6xhis-tag of recombinant protein (Sigma-Aldrich, St. Louis, MO) followed by binding to the goat anti-mouse HRP-secondary antibody (Sigma-Aldrich, St. Louis, MO).
Binary and ternary complex preparation
The mCherry-C1plasmid was purified by using the YTA Maxi-prep Kit (Yekta Tajhiz Azma Co, Tehran, Iran) according to the manufacturer’s protocol. Binary complexes were prepared in PBS buffer (10 mM, pH 7.4) by mixing 100 µg of plasmid DNA and various amounts of the peptide/protein to obtain the desired charge ratio and incubated for 1 h at room temperature (RT).
For ternary complex preparation Span 80, Tween 80 and DC-cholesterol were dissolved in chloroform in 4:4:2 molar ratio percent. The concentration of surfactants was 40 mM. Chloroform was evaporated by rotary evaporator (Laboroa 4003, Heidolph, Germany) under reduced pressure at 50 °C. After complete removal of chloroform, the hydration of the thin film was performed under stirring at 37 °C for 30 min with 1 ml of aqueous solution of previously prepared peptide/DNA in 1:7:3, 1:7:5 and 1:7:15 (w/w/w) ratios of DNA/peptide/niosome complex.
Electrophoretic mobility shift assay (EMSA)
To evaluate the ability of NMM to condense pDNA, gel retardation assay was done. NMM and protamine was incubated with 1 µg of pmCherry-C1 vector at different protein/pDNA molar ratios (1, 3, 5, 7, 9 and 11) in a final volume of 50 μL. The samples were incubated for 1 h at RT and then were run on a 0.8% agarose gel and visualized by EtBr staining [Citation32].
DNase I protection assay and DNA release study
To evaluate DNase protection, the previously made binary complexes (DNA, 1 µg/reaction) were incubated in 5 mM HEPES buffer (pH 7.4) containing 1 units of DNase I for 30 min at 37 °C. After inactivation of DNase I with EDTA, samples were run on a 0.8% agarose gel and visualized by EtBr staining. To confirm the pDNA release from binary complex, vectors were treated with heparin by the following procedure. NMM/pDNA complexes were prepared in PBS (10 mM, pH 7.4) at various molar ratios, ranging from 1:1 to 1:11 and then complexes were incubated with heparin (10 U) for 30 min at RT.
Ethidium Bromide (EtBr) exclusion assay
The DNA binding capacity of cationic peptide/protein for anionic DNA was validated via EtBr exclusion assay. A PBS solution (10 mM, pH 7.4) containing the EtBr (400 ng/mL) and DNA (1 μg) was incubated at RT for 10 min to form a stable complex. To this, pmCherry-C1plasmid was added to a buffer containing EtBr and fluorescence emission due to EtBr interaction was measured at RT. The EtBr excitation and emission wavelengths were 510 and 590 nm, respectively. Then, aliquots of a given peptide/protein suspension (based on various N/P ratios(nitrogen to phosphate ratio)) were added into preformed EtBr/plasmid DNA complex in PBS (10 mM, pH 7.4) buffer.
Circular dichroism (CD)
Far-UV CD spectra were recorded on an Aviv model-215 spectropolarimeter (USA) with the NMM protein concentration of 0.2 mg/ml in PBS buffer (10 mM, pH 7.4) at RT. Results were expressed as molar ellipticity [θ] (deg cm dmol−1). The raw data was processed by CDS software and to determine secondary structure deconvolution contents of NMM protein before and after incubation with pDNA, the data was analyzed by CDNN software.
Atomic force microscopy (AFM)
According to gel retardation results, NMM/pDNA complex at N/P = 9 molar ratio was prepared and compared with naked plasmid as a control group. Complexes were prepared in 5 mM Tris-EDTA (TE) buffer pH 7.4 in a final DNA concentration of 500 pg/μl following incubation at RT for 1 h. After complex formation, 20 mM NiCl2 was poured on freshly cleaved muscovite mica (Ted Pela, CA) and incubated for 1 min at RT. After adsorption, the surface was washed quickly with ultrapure water and dried in air at RT. later, 20 μl of the binary complex solution was added. The AFM imaging was performed in air at RT. The topography images were treated using the SPIP 6.6.4 software (www.imagemet.com).
PicoGreen assay
Fluorescent probes that can interact with nucleic acids have an important role in biophysical studies of DNA and their complexes. For quantitating the unbound pDNA in each complex, binary vectors at different N/P ratios (0, 1, 3, 5, 7 and 9) were prepared as described in above. Then, 200 µl of PicoGreen reagent, diluted in TE buffer, was added and the mixtures were incubated for 5 min at RT. PicoGreen fluorescence was measured using a Varian Cary Eclipce Fluorescence Spectrophotometer (ex.488 nm, em. 520 nm).
Particle size and zeta potential measurements
Zeta potential measurements were performed to assess the surface charge of binary complexes formed by NMM and protamine. Complexes were formed as described formerly. Samples were measured using VASCOTM Size particle Analyzer and WALLISTM Zeta Potential (Cordouan, France). Particle size measurements were performed to evaluate the changes in size of complexes at different N/P molar ratio. The size and zeta potential of the complexes were measured and reported as mean ± SD (n = 3).
Hemolysis assay
For this experiment 2 ml of sheep red blood cells (prepared from Pasteur Institute of Iran, Tehran, Iran) was washed several times by NaCl with physiological ionic strength, counted and then resuspended in the same buffer and a given number at pH 7.4. The cell suspension in buffer was added with different N/P ratios (0, 1, 3, 5, 7, 9 and 11). The mixtures were incubated at 37 °C for 1 h and centrifuged at 2000 × g for 10 min at 4 °C. The hemolytic activity was estimated from the absorbance of the supernatant at 541 nm.
Binary and ternary complexes cytotoxicity
The HEK293T and MCF-7 cells were cultured in high glucose Dulbecco's Modified Eagle's Medium (DMEM) medium with 10% fetal bovine serum and 1% antibiotics (streptomycin-penicillin). The MTT assay was conducted essentially as described elsewhere [Citation33]. Data is reported as mean ± SD (n = 3). The statistical significance was tested using one-way ANOVA tests (p < .05).
Transfection of cells with binary and ternary complexes
To evaluate gene transfection, different N/P ratios with and without cationic niosome were mixed into 100 μl serum-free media and complexes were incubated for 1 h at RT. The complexes were gently added to the cells and incubated for 2 h at 37 °C in 5% CO2 atmosphere. The specified amount of each binary and ternary complex was incubated with HEK293T and MCF-7 cells in 96-well plates and expression of mCherry (mCh) protein was detected by fluorescence microscopic analysis (Nikon, Diaphot 300). After 48 h, reporter gene expressions were monitored and quantified by Becton and Dickinson flow cytometer (BD Company, Franklin Lakes, NJ). The fluorescent protein was excited at 587 nm and emission was detected using a 610/20 filter. The lipid-based positive control Lipofectamine™2000-mediated transfections were performed based on the manufacturer's protocol and protamine sulfate was used as a peptide-based positive control.
Statistical analysis
The data were presented as mean ± SD and analyzed by SPSS software version 16. The statistical significance was determined using one-way ANOVA and Tukey post-hoc analysis. p values < .05 was considered significant.
Results and discussion
Construction, overexpression and purification of recombinant NMM
In 1996, Gao et al. [Citation34] introduced a new technique to promote the transfection efficacy of cationic lipid/DNA complexes. In this approach, before encapsulating or binding by a cationic liposome, the DNA was condensed with a polycationic peptide resulting in size reduction and increased nuclease stability. These ternary complexes mimic viral carriers containing condensed DNA and a lipid bilayer that surrounds it. The schematic structure of NMM protein and its sequences compared to protamine has been shown in and , respectively. Moreover, sequencing results are presented in . The gene encoding of NMM protein is placed under the control of a T7 promoter and its SDS-PAGE analysis of the soluble and insoluble fractions after IPTG induction is depicted in . According to this figure, most of the expressed protein was found to be in pellet (insoluble) fraction. Hence, in the next experiment, the cell pellet was solubilized in 8 M urea and the recombinant protein was purified with 250 mM imidazole as elution buffer with Ni-NTA affinity chromatography column. The fractions were analyzed on 15% SDS-PAGE gel (). Finally, the denaturant and imidazole were removed with PD-10 columns (lane 9 in ). confirms the presence of his-tag epitope by anti-his tag western blotting. The molecular weight of NMM was 13 kDa based on a protein marker in the SDS-PAGE analysis.
Figure 2. SDS-PAGE analysis of NMM expression in E. coli DE3 strain. (a) Soluble and insoluble fractions on 15% SDS-PAGE gel after IPTG induction. (b) The eluted fractions from the Ni-NTA-sepharose column was analyzed with 15% SDS-PAGE. Lane 1: The eluted fraction belongs to total protein content after cell lysis. Lane 2: flow-though, lanes 3 and 4: first and last washing, lanes 5–8: eluted fractions and lane 9: desalted fraction, M is protein marker. (c) NMM fusion protein was analyzed by SDS-PAGE, transferred to 0.2 µm PVDF membrane and detected by polyclonal anti-his tag antibody. Lane 1: total protein after cell lysis, lane 2: flow through and lane 3–8: eluted fractions. Ins: insoluble fraction; Sol: soluble fraction; NMM: NLS-Mu-Mu.
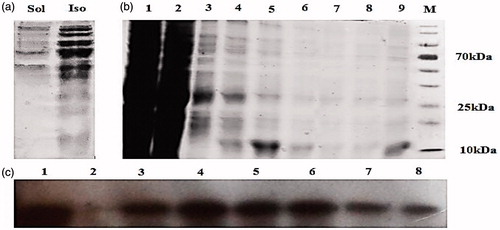
Table 1. Some properties of NLS-Mu-Mu and protamine used in this study.
Interaction of peptide/protein with pDNA and DNase I protection
EMSA assay
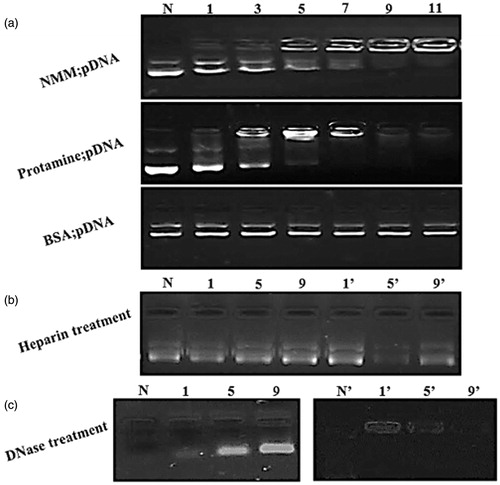
The EMSA results were achieved by incubation a fixed quantity of pDNA with increasing amounts of NMM and protamine (). The complete retardation of supercoiled and relaxed forms of pDNA were found at above 5 N/P ratios of NMM and protamine suggesting that both peptide/protein may have similar capacities to neutralize the charge of pDNA. As shows, the N/P ratio with the higher than 5 for protamine cannot be visualized with EtBr as complete masking of EtBr binding sites in pDNA by protamine condensing is observed. We hypothesized that BSA cannot interact with pDNA and could be used as a negative control. Protamine, an arginine-rich peptide derived from fish sperm, has excellent properties for gene delivery since it condenses DNA [Citation21]. Based on our results, the NMM appears to act similar to protamine though it seems to be less efficient in pDNA condensation, but is more efficient in gene mediated delivery (which will be discussed later). While both NMM and protamine are composed of the same numbers of positive residues, but the reason for protamine to be more efficient in pDNA condensation according to the previous studies is due to the effectiveness of arginine-rich peptides than lysine-rich peptides at pDNA charge neutralization and condensationn [Citation35,Citation36]. With respect to the importance of arginine residues, in protamine, all positive charge are arginine residues but in NMM there are four lysine residues. Thus protamine is more efficient in pDNA condensation.
Figure 3. (a) (a) EMSA assay for different NMM and protamine/pDNA ratios: lane 1: naked plasmid, lane 2–7: six N/P ratios (1, 3, 5, 7, 9, and 11). Each lane indicates a stepwise increase in the peptide or protein/pDNA. The pDNA is retained from N/P 7 onward completely in both protamine and NMM complexes. BSA was used as a negative control. (b) Integrity of pDNA released from the NMM and protamine complexes after incubating them with heparin. Lanes N: naked plasmid with heparin; lanes 1–9 and 1’–9′: NMM and Protamine at N/P 1–9, respectively. (c) The effect of DNase1 on the stability of condensed plasmid. Lanes N and N?: naked plasmid; lanes 1–9 and 1′–9′: NMM and Protamine at N/P 1–9, respectively. NMM: NLS-Mu-Mu; N/P ratio: nitogen to phosphate ratio.
DNase I protection and release study
Our results display that DNA condensation by NMM and protamine peptide is reversible and DNA can be released from the complexes. Subsequence release of DNA leads to gene expression in the cells. For this, cationic peptide/pDNA complex should be incubated with different cellular or synthetic negatively charged molecules such as sulfated anionic polymers, dextran sulfate and heparin that could displace cationic peptide from the complexes [Citation37]. After adding negatively charged molecules to binary complexes, the pDNA migrates on the gel where expected. In this study, previously made complexes with different charge ratios were tested for stability in the presence of heparin. The results showed that all N/P ratio complexes can easily dissociate and release pDNA (). Furthermore, the nuclease stability of the each complex was investigated. We showed that condensing can protect DNA against degradation in an N/P dependent manner. The protamine:DNA and NMM:DNA complexes (1, 5, and 9 N/P ratios) were treated with 1 U of DNase I per µg DNA for 30 min at 37 °C. The lanes treated with nuclease showed decreasing sensitivity to nuclease as the peptide/protein: DNA ratio increased. When no peptide/protein was present (N and N’ in ) the naked DNA is completely digested by DNase I. At the N/P = 1 in both binary complexes (lanes 1 and 1’) there is significant digestion of the pDNA. The lanes 5 and 5’ were more stable in the presence of the nuclease (), that show pDNA condensing by NMM and protamine have stability against nuclease activity. Our results were in agreement with previous studies regarding Mu protection against nuclease [Citation9,Citation38].
EtBr exclusion results
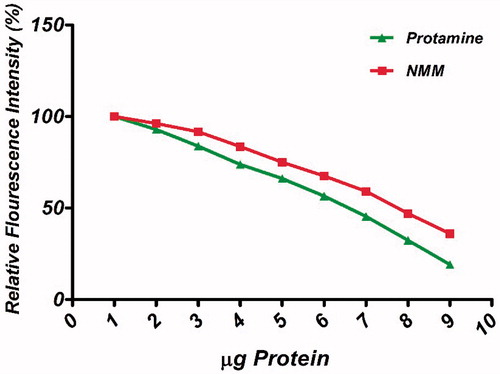
EtBr is a universal DNA intercalation dye and its fluorescence intensity increases substantially after binding to DNA. In the presence of another molecule, there would be competition between the new molecule and EtBr for binding to DNA. Such competitive binding studies have been used to identify the new DNA binding protein or drug. Our result confirmed that NMM similar to protamine has the ability to displace EtBr (). Therefore, NMM protein is likely to be an intercalator of DNA. Although, the NMM was also able to induce a decrease in EtBr fluorescence but this effect was more obvious in presence of protamine. Similar results regarding protamine EtBr exclusion are also reported by De Ilarduya et al. [Citation39].
AFM studies
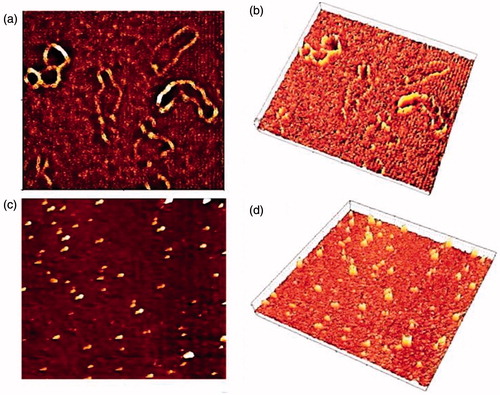
AFM is a powerful tool for observing DNA condensation on a single molecule scale and allowed us to directly visualize the effect of the DNA-binding and pDNA condensation [Citation40]. As it can be observed in , NMM (N/P = 9) showed a strong enough capacity to condense the pDNA in contrast to the naked plasmid. In fact, images collected with this technique illustrate that NMM condenses double stranded DNA in compact pieces which can probably cross the cell membrane.
CD analysis
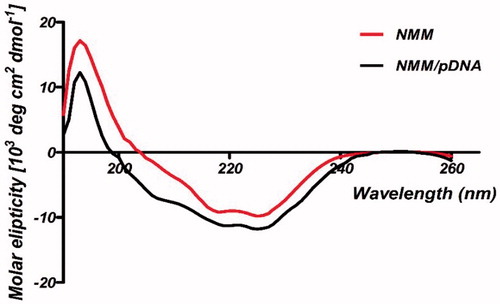
CD spectroscopy is an acceptable technique to monitor conformational changes in DNA and in the “far-UV” spectral region (190–250 nm) [Citation41]. Typical α-helical proteins display negative bands at 222 and 208 nm and a positive band at 193 nm, respectively [Citation41]. Free NMM protein presented almost similar pattern to this spectrum. Thus, NMM spectrum indicated the α-helix-like secondary structure. Furthermore, results of CD analysis for NMM indicated that addition of the pDNA to protein solution had some effects on the secondary fold of NMM protein (). Our data showed that binding of pDNA to the NMM caused an increase in α-helical secondary structure. The mechanism of this induced secondary structure is based on a basic region of NMM protein that interacts to pDNA, leading to stabilize the helix formation. The protamine CD studies have shown that the secondary structure changes from a random coil in solution to a structure containing four α-helical regions upon binding to nucleic acids [Citation16].
Figure 4. Ethidium bromide (EtBr) exclusion assay of NMM and protamine. Titration curves depicting the release of EtBr from pDNA upon binding NMM (solid squares) and protamine (solid triangles). The assay was conducted in PBS (10 mM, pH 7.4). Graphs represent gradual decreasing in % fluorescent intensity of EtBr due to EtBr exclusion across N/P charge ratios ranging 1 to 11. NMM: NLS-Mu-Mu; N/P ratio: nitrogen to phosphate ratio.
PicoGreen assay
In the present study, EMAS as a direct technique and a PicoGreen assay as an indirect method were chosen for complex formation examination. PicoGreen fluorescence emission was found to decrease at higher N/P ratios in both NMM and protamine peptide/protein (). In the PicoGreen experiments, we observed almost similar pattern of PicoGreen fluorescence signal by condensation in both NMM and protamine binary complexes. PicoGreen data confirmed protamine/pDNA complexes were stronger than NMM complexes and the amount of unbound DNA was lower in protamine complexes. The principle of this assay is based on that non-complexed amount of the DNA exposed to the PicoGreen dye and the fluorescence signal is related to the degree of binary complexes formation. We observed the continuous decreasing of the PicoGreen dye fluorescence with increase in the charge ratio that corresponds to the higher amount of DNA bound in binary vectors.
Figure 5. Visualization of NMM:pDNA and naked pDNA by AFM. (a) and (b) height image of naked pDNA and 3D rendering of AFM image, respectively; (c) and (d) height image of NMM/pDNA complexes formed at 9 N/P ratios and 3D rendering of AFM image, respectively. Each image represents a 2 × 2 mm scan. NMM: NLS-Mu-Mu; N/P ratio: nitrogen to phosphate ratio.
Characterization of the NMM/pDNA complexes
Zeta potential and size measurements
Since the cell entry of vectors is related to their size, the particle size of complexes is an important property for gene delivery system. Hence, particle size should be limited to less than 150 nm in diameter for endocytosis [Citation42]. Surface charge is another valuable property for gene delivery system because it can affect stability, cell adhesion and transfection efficiency. The presence of positive surface charge on the complexes facilitates the attachment of vector to cells with the anionic surface charge at a neutral pH value [Citation43]. Therefore, evaluations of these criteria are necessary in gene delivery. The particle size and zeta potential of the different N/P molar ratios are presented in . The size of NMM and protamine binary complexes were found to have a tendency to decrease with an increase in the N/P ratio. Therefore, it could be concluded that the formation of the nanoparticles was successful with both cationic peptide/protein. On the other hand, zeta potential of the nanoparticles at the ratio of 1 was negative which tended to become positive zeta potential above N/P 3 ratio. The shift in zeta potentials from negative to positive indicated the condensed structure of NMM/pDNA and protamine/pDNA nanoparticles ().
Figure 6. CD curves for NMM protein and NMM/pDNA complex at 9 N/P. Differences between the spectra probably are belong to the binding of NMM to pDNA. The results were analyzed by CDNN software which showed the fit mechanism for NMM/pDNA interaction that enhanced α-helices. NMM: NLS-Mu-Mu; N/P ratio: nitrogen to phosphate ratio.
Hemolysis and cytotoxicity
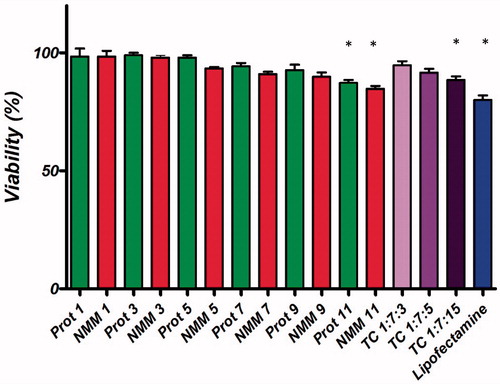
showed the relative hemolysis (%) induced by the various N/P ratios. As can be seen, in all cases the hemolysis induced by both binary and ternary complexes is low. The Triton X-100 induced hemolysis was considered as 100% hemolysis and PBS induced as 0% of hemolysis. As shown in , cytotoxicity assays were performed using HEK293T cell line (MCF-7 viability results not shown). In the presence of 10% FBS, the cells were allowed to grow to 80% confluency. Then, the cells were treated with the binary and ternary complexes at various N/P molar ratios for 48 h. As it shows protamine and NMM binary complexes had low cytotoxic effects except at high concentrations (N/P = 11). Besides, ternary complexes demonstrated low cytotoxic effect, as well. Groups with significant difference are determined in . The results from cytotoxicity and hemolytic section showed that the binary and ternary vectors were safe in the in-vitro system.
Figure 7. PicoGreen assay for the quantification of the DNA bound in NMM and protamine complexes at different molar ratios ranging from 1 to 9 N/P molar ratios. Results are represented as mean ± SD (n = 3). NMM: NLS-Mu-Mu; N/P ratio: nitrogen to phosphate ratio; Prot: protamine.
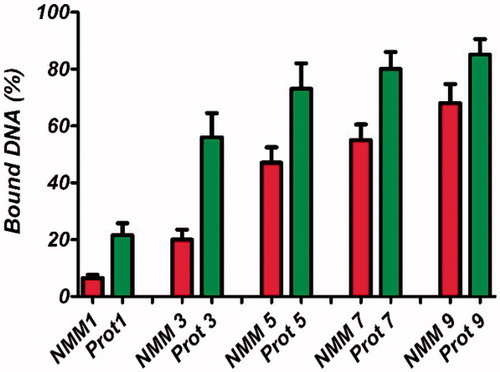
Figure 8. (a) Particle size and zeta potential of NMM binary complexes at N/P ratios ranging from 1 to 11. (b) Particle size and zeta potential of protamine binary complexes at N/P ratios ranging from 1 to 11. The results are presented as mean ± SD (n = 3). NMM: NLS-Mu-Mu; N/P ratio: nitrogen to phosphate ratio; Prot: protamine.
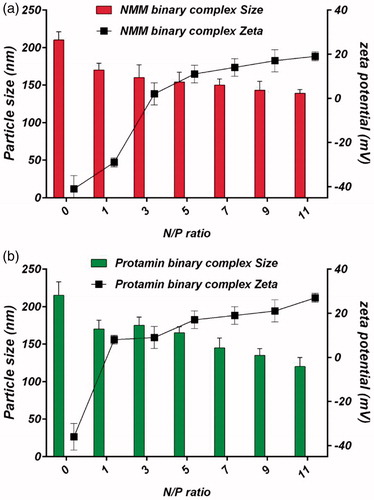
Figure 9. Hemolysis of binary and ternary complexes at different N/P molar ratios. Low levels of hemolysis are observed with the NMM/pDNA complexes. All results are expressed in relation to 100% hemolysis observed in the presence of Triton X-100. The results are presented as mean ± SD (n = 3). NMM: NLS-Mu-Mu; N/P ratio: nitrogen to phosphate ratio; Prot: protamine; TC: ternary complex with NMM.
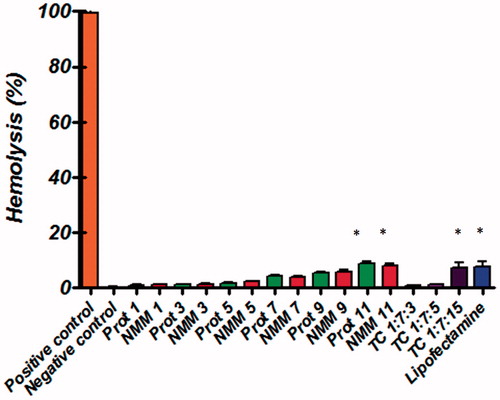
Figure 10. Comparison of the cell viability of NMM/DNA and Prot/DNA complexes at various N/P ratios. Each value represents the mean ± SD of three wells. *Difference values were statistically significant (p < .05). Each group was compared to control. NMM: NLS-Mu-Mu; N/P ratio: nitrogen to phosphate ratio; Prot: protamine; TC: ternary complex with NMM.
Gene delivery mediated by binary and ternary complexes
Transfection efficiency
The transient expression efficiency of the binary and ternary vectors was evaluated in the transfection assay. The pmCherry-C1 plasmid encoding Cherry fluorescent protein was used. Transfection efficiency of the binary complexes was tested at various N/P ratios varying from 1 to 11. Maximum transfection was obtained when cells were transfected with N/P = 7 for NMM/pDNA complexes and N/P = 5 for protamine complexes (data not shown). Transfection efficiency over time were studied in HEK293T and MCF-7 cell lines. As observed in , the percentage of transfected cells was higher with the NMM binary vectors compared to vectors containing protamine and naked plasmid. In addition, transfection efficiency of ternary vectors was higher than both binary vectors and the difference between NMM ternary efficiency was significant compared to protamine ternary (p < .001). At 48 h post-transfection, the maximum percentage of transfected cells for NMM/DNA and protamine/DNA vectors were 18 and 13%, respectively. Both values were inferior to their related ternary vectors (NMM ternary 43% and protamine ternary 32%). Representative fluorescent images of transfection efficiency in HEK293T cell line and quantified results for both cell lines have been shown in and , respectively.
Figure 11. Representative fluorescent images of transfection efficiency in HEK293T cell line. Naked plasmid (a), DP transfection (b), DN transfection (c), DPN transfection (d) and DNN transfection (e) in HEK293T cells detected by red fluorescence protein (mCherry) using a fluorescence microscope at 48 h after transfection. Transfections were performed in 96-well plates using 1 µg of plasmid per well. DP: DNA/protamine; DN: DNA/NMM; DPN: DNA/protamine/noisome; DNN: DNA/NMM/niosome.
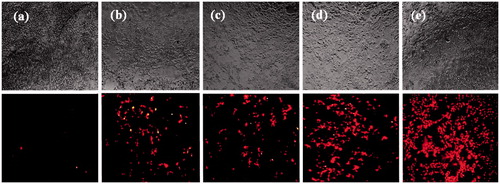
Figure 12. Transfection efficiency of m-Cherry gene with the binary and ternary complexes in different cell lines. The binary complexes were made and further encapsulated into cationic niosome vesicles. These particles were incubated with HEK293T and MCF-7 cells in serum free medium and gene expression was evaluated. Statistical analysis was performed with one-way analysis of variance (ANOVA) followed by Tukey's post-hoc test. #p < .05. D: DNA; DP: DNA/protamine, DN: DNA/NMM; DPN: DNA/protamine/noisome; DNN: DNA/NMM/niosome; DL: DNA/Lipofectamine™2000.
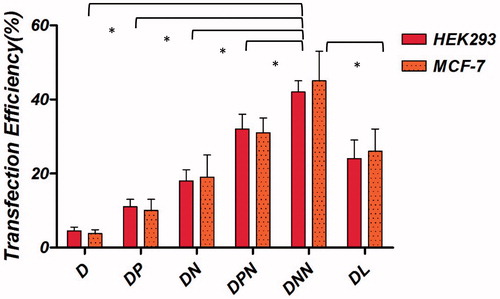
The results showed that ternary complex was efficient in both cell lines. For transfection evaluation, we chose HEK293T and MCF-7 cells. There are some studies regarding the cell entry of Mu peptide suggesting endocytic uptake mediated the complexes due to its rapid cell entry [Citation38]. Rajagopalan et al. have evaluated the cell entry of Mu, Mu-Mu and TAT-Mu peptides and the result was that Mu-Mu has shown maximum internalization even in the absence of lipid [Citation38]. Our results indicated superior properties of NMM binary in transfection than protamine binary. As it has been explained that, the possible reason could be the difference in the hydrophobicity of each peptide that influences peptide membrane interactions and cell entry. Based on this property NMM protein can more easily come close to the membrane and pass through it [Citation19,Citation20]. On the other hand, some researchers have proposed that in spite of the high DNA condensation capacity of protamine and its intrinsic NLS signal, this does not always lead to high transfection and it may impair the previous steps due to the multistage nature of the transfection process [Citation44].
Some studies used niosomes as a gene [Citation45] or drug carrier [Citation46] and some others have examined the cellular entry mechanism of niosomes [Citation47]. For example, Zhou et al. have investigated the cell entry mechanisms of niosomes using the specific endocytosis pathway inhibitors. They have reported that Span 80 niosomes enter the cell mainly through a caveolae-mediated endocytosis (60%); while lipofectamine had mainly clathrin-mediated endocytosis (62%) [Citation47]. It well understands that transfection vectors that enter the cell through clathrin-mediated endocytosis usually are trapped in endosomes and then are followed by enzymatic degradation in lysosomes [Citation48]. In contrast, caveolae-mediated endocytosis escaping from lysosomal degradation is a favorable route for cargo delivery [Citation48,Citation49]. Therefore, Span 80 containing niosomes facilitates cargo escaping from the endosomes/lysosomes, leading to increase gene expression in the cells.
Conclusion
Our results proposed that NMM as a cationic multifunctional could be expressed and purified successfully. Besides, NMM protein is able to condense DNA efficiently at charge ratios that are not toxic to cells. We also demonstrated that this recombinant protein transfects HEK293T cells acceptably and the transfection efficiency improve when ternary complexes were made with cationic niosomes. Moreover, the other NMM properties such as protection from DNases, release by heparin and low hemolysis induction suggest that this type of binary gene carrier is promising approach for in vivo gene delivery. We also conducted a comparison between NMM ternary complexes and protamine ternary vectors. The data showed that the cationic niosome could improve transfection efficiency two fold more than binary vector in pDNA delivery. To our best knowledge, this is the first report on the use of ternary complex containing a recombinant fusion protein (NMM) as a condensing agent and the cationic niosome for delivery of pDNA suggesting that niosomes and NMM protein can be used together as a ternary complex to deliver pDNA in the cells. The results of present study showed that niosomal ternary vector containing NMM is a promising non-viral gene carrier to achieve an efficient and safe carrier system for gene therapy and in vivo complementary study should be designed.
Disclosure statement
No potential conflict of interest was reported by the authors.
Funding
This study was supported by a grant (Grant No. 930251) from Kerman University of Medical Sciences and is the part of a PhD thesis. The authors thankfully acknowledge the support for this research.
References
- Deng W, Fu M, Cao Y, et al. Angelica sinensis polysaccharide nanoparticles as novel non-viral carriers for gene delivery to mesenchymal stem cells. Nanomedicine. 2013;9:1181–1191.
- Dincer S, Türk M, Piişkin E. Intelligent polymers as nonviral vectors. Gene Ther. 2005;12:S139–S145.
- Nayerossadat N, Maedeh T, Ali PA. Viral and nonviral delivery systems for gene delivery. Adv Biomed Res. 2012;1:27.
- Pack DW, Hoffman AS, Pun S, et al. Design and development of polymers for gene delivery. Nat Rev Drug Discov. 2005;4:581–593.
- La¨chelt U, Wagner E. Nucleic acid therapeutics using polyplexes: a journey of 50 years (and beyond). Chem Rev. 2015;115:11043–11078.
- Wasungu L, Hoekstra D. Cationic lipids, lipoplexes and intracellular delivery of genes. J Control Release. 2006;116:255–264.
- Martin ME, Rice KG. Peptide-guided gene delivery. AAPS J. 2007;9:E18–E29.
- Jones CH, Chen CK, Ravikrishnan A, et al. Overcoming nonviral gene delivery barriers: perspective and future. Mol Pharm. 2013;10:4082–4098.
- Xavier J, Singh S, Dean DA, et al. Designed multi-domain protein as a carrier of nucleic acids into cells. J Control Release. 2009;133:154–160.
- Akita H, Tanimoto M, Masuda T, et al. Evaluation of the nuclear delivery and intra-nuclear transcription of plasmid DNA condensed with µ (mu) and NLS-µ by cytoplasmic and nuclear microinjection: a comparative study with poly-L-lysine. J Gene Med. 2006;8:198–206.
- Keller M, Harbottle RP, Perouzel E, et al. Nuclear localisation sequence templated nonviral gene delivery vectors: investigation of intracellular trafficking events of LMD and LD vector systems. Chembiochem. 2003;4:286–298.
- Lundberg P, El-Andaloussi S, Sütlü T, et al. Delivery of short interfering RNA using endosomolytic cell-penetrating peptides. The FASEB J.. 2007;21:2664–2671.
- Vives E. Present and future of cell-penetrating peptide mediated delivery systems: “is the Trojan horse too wild to go only to Troy?” J Control Release. 2005;109:77–85.
- Zhang X, Oulad-Abdelghani M, Zelkin AN, et al. Poly (L-lysine) nanostructured particles for gene delivery and hormone stimulation. Biomaterials. 2010;31:1699–1706.
- Arangoa MA, Düzgüneş N, De Ilarduya CT. Increased receptor-mediated gene delivery to the liver by protamine-enhanced-asialofetuin-lipoplexes. Gene Ther. 2003;10:5–14.
- Ando T, Yamasaki M, Suzuki K. Protamines: isolation-characterization-structure and function. Vol. 12. New York: Springer Science & Business Media; 2012.
- Puras G, Martínez-Navarrete G, Mashal M, et al. Protamine/DNA/niosome ternary nonviral vectors for gene delivery to the retina: the role of protamine. Mol Pharmaceutics. 2015;12:3658–3671.
- Abes S, Moulton H, Turner J, et al. Peptide-based delivery of nucleic acids: design, mechanism of uptake and applications to splice-correcting oligonucleotides. Biochem Soc Trans. 2007;35:53–55.
- Liu J, Guo S, Li Z, et al. Synthesis and characterization of stearyl protamine and investigation of their complexes with DNA for gene delivery. Colloids Surf B Biointerfaces. 2009;73:36–41.
- Khalil IA, Futaki S, Niwa M, et al. Mechanism of improved gene transfer by the N-terminal stearylation of octaarginine: enhanced cellular association by hydrophobic core formation. Gene Ther. 2004;11:636–644.
- Sorgi FL, Bhattacharya S, Huang L. Protamine sulfate enhances lipid-mediated gene transfer. Gene Ther. 1997;4:961–968.
- Murray KD, Etheridge CJ, Shah SI, et al. Enhanced cationic liposome-mediated transfection using the DNA-binding peptide [mu](µ) from the adenovirus core. Gene Ther. 2001;8:453.
- Zhang S, Zhao Y, Zhao B, et al. Hybrids of nonviral vectors for gene delivery. Bioconjug Chem. 2010;21:1003–1009.
- Li SD, Huang LY. In vivo gene transfer via intravenous administration of cationic lipid-protamine-DNA (LPD) complexes. Gene Ther. 1997;4:891–900.
- He SN, Li YL, Yan JJ, et al. Ternary nanoparticles composed of cationic solid lipid nanoparticles, protamine, and DNA for gene delivery. Int J Nanomedicine. 2013;8:2859.
- Pardakhty A, Moazeni E. Nano-niosomes in drug, vaccine and gene delivery: a rapid overview. Nanomedicine. 2013;1:1–12.
- Uchegbu IF, Florence AT. Non-ionic surfactant vesicles (niosomes): physical and pharmaceutical chemistry. Adv Colloid Interface Sci. 1995;58:1–55.
- Puras G, Mashal M, Zárate J, et al. A novel cationic niosome formulation for gene delivery to the retina. J Control Release. 2013;174:27–36.
- Eslaminejad T, Nematollahi-Mahani SN, Ansari M. Cationic β–cyclodextrin-chitosan conjugates as potential carrier for pmCherry-C1 gene delivery. Mol Biotechnol. 2016;58:287–298.
- Wingfield PT, Palmer I, Liang SM. Folding and purification of insoluble (inclusion body) proteins from Escherichia coli. Curr Protoc Protein Sci. 2001;78:6.5.1–30.
- Mehrabani M, Najafi M, Kamarul T, et al. Deferoxamine preconditioning to restore impaired HIF-1α-mediated angiogenic mechanisms in adipose-derived stem cells from STZ-induced type 1 diabetic rats. Cell Prolif. 2015;48:532–549.
- Hellman LM, Fried MG. Electrophoretic mobility shift assay (EMSA) for detecting protein-nucleic acid interactions. Nat Protoc. 2007;2:1849–1861.
- Igder S, Asadikaram GR, Sheykholeslam F, et al. Opium induces apoptosis in Jurkat cells. Addict Health. 2013;5:27–34.
- Gao X, Huang L. Potentiation of cationic liposome-mediated gene delivery by polycations. Biochemistry. 1996;35:1027–1036.
- Preuss M, Tecle M, Shah I, et al. Comparison between the interactions of adenovirus-derived peptides with plasmid DNA and their role in gene delivery mediated by liposome-peptide-DNA virus-like nanoparticles. Org Biomol Chem. 2003;1:2430–2438.
- Schwartz B, Ivanov MA, Pitard B, et al. Synthetic DNA-compacting peptides derived from human sequence enhance cationic lipid-mediated gene transfer in vitro and in vivo. Gene Ther. 1999;6:282–292.
- Zelphati O, Szoka FC. Mechanism of oligonucleotide release from cationic liposomes. Proc Natl Acad Sci USA. 1996;93:11493–11498.
- Rajagopalan R, Xavier J, Rangaraj N, et al. Recombinant fusion proteins TAT-Mu, Mu and Mu-Mu mediate efficient non-viral gene delivery. J Gene Med. 2007;9:275–286.
- De Ilarduya CT, Arangoa MA, Moreno-Aliaga MJ, et al. Enhanced gene delivery in vitro and in vivo by improved transferrin-lipoplexes. Biochim Biophys Acta. 2002;1561:209–221.
- Balhorn R, Brewer L, Corzett M. DNA condensation by protamine and arginine-rich peptides: Analysis of toroid stability using single DNA molecules. Mol Reprod Dev. 2000;56:230–234.
- Greenfield NJ. Using circular dichroism spectra to estimate protein secondary structure. Nat Protoc. 2006;1:2876–2890.
- Guy J, Drabek D, Antoniou M. Delivery of DNA into mammalian cells by receptor-mediated endocytosis and gene therapy. Mol Biotechnol. 1995;3:237–248.
- Hu Y, Xu B, Ji Q, et al. A mannosylated cell-penetrating peptide-graft-polyethylenimine as a gene delivery vector. Biomaterials. 2014;35:4236–4246.
- Delgado D, del Pozo-Rodríguez A, Má S, et al. Understanding the mechanism of protamine in solid lipid nanoparticle-based lipofection: the importance of the entry pathway. Eur J Pharm Biopharm. 2011;79:495–502.
- Yazdi Rouholamini SE, Moghassemi S, Maharat Z, et al. Effect of silibinin-loaded nano-niosomal coated with trimethyl chitosan on miRNAs expression in 2D and 3D models of T47D breast cancer cell line. Artif Cells Nanomed Biotechnol. 2017;45. [cited 2017 May 16]. doi: 10.1080/21691401.2017.1326928.
- Behnam B, Rezazadehkermani M, Ahmadzadeh S, et al. Microniosomes for concurrent doxorubicin and iron oxide nanoparticles loading; preparation, characterization and cytotoxicity studies. Artif Cells Nanomed Biotechnol. 2017;45. [cited 2017 May 16]. doi: 10.1080/21691401.2017.1296850.
- Zhou C, Mao Y, Sugimoto Y, et al. SPANosomes as delivery vehicles for small interfering RNA (siRNA). Mol Pharmaceutics. 2011;9:201–210.
- Khalil IA, Kogure K, Akita H, et al. Uptake pathways and subsequent intracellular trafficking in nonviral gene delivery. Pharmacol Rev. 2006;58:32–45.
- Love KT, Mahon KP, Levins CG, et al. Lipid-like materials for low-dose, in vivo gene silencing. Proc Natl Acad Sci USA. 2010;107:1864–1869.