?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
Chronic osteomyelitis and infected bone defects are substantial challenges faced by orthopaedic surgeons. In this study, vancomycin was loaded into mesoporous bioactive glass (MBG) to form a local antibiotic delivery system and then a bone tissue-engineering scaffold combining MBG and poly-(L-lactic-co-glycolic acid) (PLGA) was prepared by freeze-drying fabrication. In vitro degradation and water contact angle analysis suggested that the MBG-incorporated PLGA scaffold exhibited controlled degradability, stabilizing the pH of the surrounding environment and improved the hydrophilicity. Moreover, the presence of MBG provides a well-interconnected pore structure, to which human bone marrow-derived mesenchymal stem cells can attach, spread and proliferate, promoting upregulation of the expression of osteogenic markers. Thus, MBG/PLGA scaffolds exhibit better cytocompatibility and osteoblastic difierentiation properties compared with pure PLGA scaffolds. Vancomycin-loaded scaffolds have been found to yield sustained release that lasts for more than eight weeks in vitro. We tested the antibacterial performance of vancomycin-loaded scaffolds against Staphylococcus aureus, the most common bacteria isolated from infected bone. In vitro experiments demonstrated that loading vancomycin onto the scaffold promoted antibacterial activity and inhibited biofilm formation without deleterious effect on cytocompatibility. In conclusion, the novel inorganic-organic composites are considered potential materials for the treatment of infected bone defects.
Introduction
The development of synthetic scaffolds presents an advantageous approach in the field of bone tissue engineering, with considerable potential for repairing bone defects bone [Citation1,Citation2]. The highly-porous scaffold is characterized by the formation of tissue matrices that promote cell adhesion and proliferation, with an interconnected vesicle structure that allows transport of nutrients to cells in vitro and in vivo [Citation3]. Biocompatible and biodegradable polymers are the most widely-studied scaffolds because of their excellent biocompatibility and capacity to retain long-term physical and mechanical properties, which allows bone tissue growth [Citation4,Citation5]. However, polymer applications remain a major challenge in the fabrication of an ideal material for bone repair. Compared with natural extracellular matrix (ECM), polymers possess relatively low hydrophilicity, rendering them suboptimal as substrates because of inferior bioactivity and osteoconductivity [Citation6,Citation7].
Recently, the addition of bioactive glasses has been suggested to improve the biological performance of composite scaffolds because of certain properties, including high levels of porosity, appropriate pore size and an interconnected pore network [Citation8,Citation9]. Bioactive materials form a biologically-active layer on the surface of the implant to form a bonding interface with the host bone, whereas the dissolution products (including silicon (Si), sodium (Na), calcium (Ca) and phosphate ions) enhance bone ingrowth and regeneration [Citation10–13]. Our research institutes successfully fabricated three-dimensional porous mesoporous bioactive glass (MBG) scaffolds with strontium or calcium sulfate and found that these scaffolds show enhanced mechanical strength and excellent bone regeneration [Citation8,Citation9,Citation14]. Mesoporous silica materials, with pore size ranging from 2 to 50 nm, have been used in drug delivery systems and scaffolds after the introduction of functional polymers [Citation11]. This utility is due to their massive surface areas and porosity, which promote the response of osteoblasts, produce enhanced osteointegration and provide excellent drug-loading capabilities. More importantly, mesoporous materials combined with biodegradable polymers have been developed as functional mesoporous composites for bone regenerative treatment [Citation15,Citation16]. Zhu et al. [Citation16] demonstrated that mesoporous bioactive glass-coated poly (L-lactic acid) scaffolds can enhance cell attachment, spreading and proliferation in a rabbit bone marrow stromal cell model in vitro. Thus, such inorganic-organic composite scaffolds can mimic the natural bone ECM and favor bone repair, thereby maximizing the advantages and minimizing the drawbacks of each material alone.
Chronic osteomyelitis with the destruction of bone tissues is a serious complication, with possible devastating consequences including poor functional outcomes, prolonged hospitalization and patient dissatisfaction [Citation17,Citation18]. Over decades, the in situ implantation of a local antibiotic delivery system has been proven as a reliable alternative method to systemic antibiotic administration [Citation19]. Several groups reported successful fabrication of gentamicin-loaded MBG scaffolds to prolong the duration of drug delivery in bone repair [Citation11,Citation15]. These scaffolds, which were designed to reduce considerable dead space and obliterate the propagation of regional bacteria and the formation of bacteria biofilm, are proven to be effective in preventing and treating infection [Citation19–21]. However, antibiotic-dependent treatment inevitably engendered serious complications, such as drug resistance. The prevalence of antibiotic-resistant bacterial strains continues to be a major problem [Citation22–24]. More effective antibacterial drugs, especially drugs aimed at antibiotic-resistant organisms, are necessary to improve the efficiency of prophylaxis and treatment of infection. Vancomycin is a wide-spectrum antibiotic that is usually selected as a local delivery drug to successfully prevent and treat methicillin-resistant Staphylococcus aureus (MRSA) infection and osteomyelitis [Citation25–27]. In another study, investigators established a borate glass loaded with vancomycin which has excellent biocompatibility and compressive strength; the material successfully eradicated osteomyelitis induced by MRSA in a rabbit model [Citation18].
The development of inorganic-organic composites that can provide both effective antibacterial activity and promotion of bone ingrowth would be particularly advantageous for bone tissue engineering [Citation16,Citation28]. To date, there have been several reports of fabricated MBG as a nanocarrier of antibiotics [Citation11,Citation15,Citation16,Citation28–31]. However, to the best of our knowledge, investigation on MBG has been limited to its use as a drug delivery system for vancomycin and the potential of vancomycin-laden MBG incorporated into poly-(L-lactic-co-glycolic acid) (PLGA) for the treatment of infected bone defects is unknown. In this study, we fabricated a novel mesoporous composite scaffold loaded with vancomycin. The aim of this study was to investigate the influence of MBG and vancomycin on the physical and chemical properties and antimicrobial activity of this scaffold in vitro. The current work is expected to provide valuable information for the development of anti-infective bone regeneration scaffolds.
Materials and methods
Materials
Tetraethyl orthosilicate (TEOS, 99%), ethyl alcohol (99%), hydrochloric acid (HCl, ≥ 36%), EO20-PO70-EO20 ( Pluronic P123, MW = 5800), calcium nitrate tetrahydrate (99%) and triethylphosphate (TEP, 99.8%) were purchased from Sinopharm Chemical Reagent Co., Ltd, (Shanghai, China). Vancomycin hydrochloride was obtained from Sigma-Aldrich (St Louis, MO). Commercial PLGA (Mw = 10 kDa with lactide:glycolide molar ratio 50:50) was supplied by Daigang Company (Jinan, China). These chemicals were used as received without further purification. Minimum essential medium alpha (MEM-A), fetal bovine serum (FBS), penicillin, streptomycin and phosphate-buffered saline (PBS) were purchased from Gibco (Grand Island, NY).
Synthesis and characterization of MBG
Highly-ordered MBGs were synthesized using nonionic block copolymer P123 as the template agent and TEOS as the silica source. In brief, 8 g of P123 was dissolved in 120 g of ethanol, and the solution was stirred at room temperature for 2 h. After the addition of 13.4 g of TEOS and 2 g of 0.5 M HCl to the mixture, 2.8 g of calcium nitrate tetrahydrate and 1.46 g of triethyl phosphate were added as calcium and phosphorus oxide precursors, respectively. The slurry was mixed on a magnetic stirrer overnight at room temperature. The precipitate was then filtered and washed exhaustively with deionized water. After centrifugation, the dry powders were obtained for evaporation-induced self-assembly (EISA) at 40 °C under vacuum for 24 h. The samples were then calcined at 600 °C for 6 h at a heating rate of 1 °C/min to remove surfactant and form the final MBG powder (Si/Ca/P molar ratio: 80/15/5).
The morphology and microstructure of the MBGs were characterized by high- resolution transmission electron microscopy (TEM) (JEM-2010; JEOL Ltd., Tokyo, Japan) at an acceleration voltage of 200 kV and small-angle X-ray diffraction (SAXRD). To determine the specific surface area, nanopore size distribution and nanopore volume, we used Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halenda (BJH) methods with N2 adsorption-desorption isotherms (TRISTAR 3000, Micrometrics Instrument Corp., Norcross, GA).
Drug loading and release profile of MBG
Typically, vancomycin hydrochloride was first dissolved in deionized water at a concentration of 1 mg/ml. Subsequently, 0.5 g of MBG sample was added to 40 ml of vancomycin solution and mixed by gentle shaking for 24 h at room temperature. Afterwards, all samples were centrifuged at 3000 rpm for 25 min to separate the MBG and supernatant. The drug-loaded MBG was then dried at 70 °C in a vacuum oven for two days. The final obtained product was termed as vancomycin-laden MBG that was used for further experiments.
To estimate loading efficiency, the difference of vancomycin concentration in the loading medium before (0.6, 1.0, and 1.4 mg/mL) and after loading was analyzed using a UV/VIS spectrophotometer (UV-2300, Techcomp, Kowloon, Hong Kong) at a wavelength of 280 nm. The amount of drug loaded was determined at room temperature, with distilled water as reference. Drug-loading efficiency was calculated using the EquationEquation (1)(1)
(1) :
(1)
(1)
For in vitro drug releasing assay, 0.5 g of vancomycin-loaded MBG was soaked in 10 ml PBS at a pH of 7.4 and shaken at constant speed of 120 rpm in a 37 °C water bath. At each pre-set time point, 1 ml of supernatant was withdrawn and replaced with the same volume of fresh PBS. Hence, the cumulative release of the loaded drug was calculated. The vancomycin concentration was determined at a wavelength of 280 nm using UV/VIS spectrophotometry. Five samples under each condition were used for the in vitro drug-release tests.
Fabrication and characterization of the MBG/PLGA scaffolds
PLGA was dissolved in 1,4-dioxane (10 wt %) and stirred for 24 h to form a stable sol. MBG powders loaded with vancomycin were added into the aforementioned mixture and continuously stirred for 24 h to disperse the nanocomposites uniformly. The solution was then flash-frozen at −80 °C overnight. Finally, the solvent was removed by freeze-drying for 48 h. Vacuum drying was used to completely remove any possible solvent left in the scaffolds and they were then stored in a desiccator until use. PLGA scaffolds with or without vancomycin and MBG/PLGA scaffolds without vancomycin were also prepared using the same procedure which served as the controls. Prior to cells seeding, the fabricated scaffolds were sterilized by 20 kGy of 60Co radiation.
The pore structure of the porous scaffolds was observed using scanning electron microscopy (SEM) (JEOL JSM-5910 LV, JEOL USA, Inc.,Peabody, MA) at an accelerating voltage of 20 keV. The surface hydrophilicity and energy of the samples were investigated by static contact angle measurements on a drop-shape analysis system (JC 2000D3, Shanghai Zhongcheng Digital Technology Co., Ltd., China) at indoor humidity (60%) and room temperature (20 °C). The water droplets were placed at six different positions for each specimen. The surface energy was calculated according to the surface tension and the contact angle.
In vitro drug release from scaffolds
To determine vancomycin release from drug-loaded scaffolds in vitro, we immersed 5 × 5 × 10 mm3 of 15 ml of fresh PBS at pH 7.4 at 37 °C and vibrated at 120 rpm. At predetermined time intervals, vancomycin released in the medium was analyzed using the above-mentioned UV/VIS spectroscopy at a wavelength of 280 nm. The cumulative percentage of vancomycin release was normalized to the total amount of drug.
In vitro degradation of scaffolds
The weight loss ratio of the samples was calculated (with PLGA scaffold as control) over 42 weeks to determine the in vitro degradation of MBG/PLGA scaffolds. Cylindrical samples were prepared with Φ10 mm ×10 mm and original weight (W0) was obtained. Then, the specimens were immersed in 30 ml PBS at 37 °C under constant shaking (100 rpm). At predetermined time points, samples were washed three times with deionized water, dried overnight at 60 °C and weighed to determine the weight at each time-point (Wt). The weight loss ratio was calculated using the EquationEquation (2)(2)
(2) :
(2)
(2)
Five samples were used for in vitro testing of scaffold degradation.
The assessment of pH stability of scaffolds was performed in PBS with an initial pH of 7.3. The pH of the degradation medium over time was measured using a pH meter (S20-K, Mettler-Toledo, Columbus, OH) at the start of the assay and at every time-point.
The scaffolds were soaked in 50 ml PBS at 37 °C, followed by successive incubation for up to 28 weeks. At each incubation time-point, extracts were obtained by filtration (1 µm) and the released ions, including Si, Ca and phosphorous (P), were measured using inductively-coupled plasma optical emission spectrometry (ICP-OES, Varian Liberty 150, Agilent Technologies, Santa Clara, CA).
Culture of human bone marrow stem cells (hBMSCs)
This study was approved by our institutional review board and informed consent was obtained from all patients. Human bone marrow stem cells (hBMSCs) were isolated from the marrow of the femoral head of patients undergoing arthroplasty. In brief, human bone marrow was flushed out and suspended in α-MEM medium supplemented with 10% FBS, 1% penicillin and streptomycin sulfate. The cells were incubated at 37 °C in humidified air containing 5% carbon dioxide. The culture medium was changed every 2–3 days and non-adherent cells were removed after 48 h. After being detached by 0.25% trypsin and resuspended, the hMSCs were passaged three times before use in subsequent experiments.
Cell proliferation and morphology on the scaffolds
Each well containing scaffold (Φ10 mm × 2 mm) was seeded with 1 ml cell suspension containing 2 × 104 viable cells. After seeding, the cells were incubated at 37 °C in a humidified atmosphere to allow for cell attachment. At 6 h after cell seeding, the cells incubated on the scaffolds were stained with 4′, 6′-diamidino-2-phenylindole (DAPI) and then seeding efficiency was assessed using a fluorescence microscope (TE2000U, Nikon, Tokyo, Japan).
A 3-(4, 5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) assay kit (Beyotime Institute of Biotechnology, Shanghai, China) was used to examine the proliferation of hMSCs on the scaffolds. At different time points (3, 7 and 14 days) 0.1 ml of MTT solution (5 mg/ml in Hank’s balanced salt solution) was added to the cultured cells and incubated at 37 °C for 2 h. The culture medium was then removed and an equivalent volume of dimethyl sulfoxide was added to dissolve formazan crystals. After centrifugation, the supernatant of each sample was transferred to a new 96-well plate for measurement. Finally, the absorbance was measured using a micro plate reader (Bio-Tek Instruments Inc.,Winooski, VT) at a wavelength of 570 nm.
For cytoskeleton staining, 5 × 104 hMSCs were seeded onto each scaffold and cultured for one or three days. Then, cells were washed gently three times with PBS and fixed with 4% paraformaldehyde for 2 h. Cells were lysed using 0.1% Triton X-100 solution and incubated for another 15 min. After washing three times with PBS, the cells were incubated with fluorescein isothiocyanate (FITC)-phalloidin (Sigma-Aldrich) for 1 h. Cell nuclei were stained with DAPI( Sigma-Aldrich) for 10 min after a PBS wash. The cell morphology and spread of hMSCs cultured on scaffolds were investigated through visualization of the actin cytoskeleton and nuclei using confocal laser scanning microscopy (CLSM, Nikon, Tokyo, Japan).
Osteogenic differentiation of hBMSCs on scaffolds
To assess the biological effect of scaffolds on early osteogenic differentiation, alkaline phosphatase (ALP) activity was assayed after hBMSC seeding on each scaffold at a density of 3 × 104 cells/well and cultured in a 48-well plate for 24 h. When the cells reached 80% confluence, the culture medium was replaced with osteogenic induction medium (OIM), consisting of α-DMEM (Dulbecco's Modified Eagle's medium), 10% FBS, 0.1 μM dexamethasone (Sigma-Aldrich), 10 mM β-glycerophosphate sodium (Sigma-Aldrich) and 50 μM ascorbic acid (Sigma-Aldrich). The medium was exchanged every two days throughout the experiment. The hBMSC suspension was seeded onto scaffolds in a 48-well plate and cultured at 37 °C under 5% CO2 for 3, 7, or 14 days. After each time-point, the cells were rinsed twice with PBS and treated with 0.2% Triton X-100. Lysates were sonicated and centrifuged for 15 min at 4 °C. For ALP measurement, the conversion of p-nitrophenol phosphate (Sigma-Aldrich) into p-nitrophenol was determined through the measurement of absorbance at 405 nm using a VarioSkan Flash spectral scanning multimode reader (Thermo Fisher scientific, Waltham, MA). Total protein in the supernatant was determined using a MicroBCA Protein Assay Kit from Thermo Fisher Scientific. The relative ALP activity was calculated as enzyme concentration/protein content ratio per sample.
To evaluate the effects of scaffolds on the osteogenic difierentiation of hBMSCs, the transcript levels of ALP, runt-related transcription factor 2 (Runx2), osteocalcin (OCN) and osteoprotein (OPN) were assessed by quantitative real-time reverse transcription polymerase chain reaction (RT-PCR). After seven and 14 days, total RNA was isolated using Trizol reagent (Invitrogen, Carlsbad, CA) and first-strand complementary DNA (cDNA) was synthesized using M-MLV reverse transcriptase and a cDNA synthesis Kit (Takara Bio Inc., Shiga, Japan) according to the manufacturer’s recommended protocol. Subsequently, mRNA expression was quantified using a Thermal Cycler Dice TP800 (Takara Bio Inc., Kusatsu, Japan) with a real-time PCR kit (SYBR Premix EX Taq, Takara Bio Inc.). The following primers were used in the study: GAPDH sense: 5′-CCTGCACCACCAACTGCTTA-3′; GAPDH antisense: 5′- AGGCCATGCCAGTGAGCTT-3′; OCN sense: 5′-GGCGCTACCTGTATCAATGGC-3′; OCN antisense: 5′-TGCCTGGAGAGGAGCAGAACT-3′; OPN sense: 5′-CTGAACGCGCCTTCTGATTG-3′; OPN antisense: 5′- ACATCGGAATGCTCATTGCTCT-3′; Runx2 sense: 5′- CCAACCCACGAATGCACTATC-3′; Runx2 antisense: 5′-TAGTGAGTGGTGGCGGACATAC-3′; ALP sense: 5′-TTGACCTCCTCGGAAGACACTC-3′ and ALP antisense: 5′-CCATACAGGATGGCAGTGAAGG-3′. The reference gene GAPDH was used as a control of the amount of template for each sample and the relative gene expression was analyzed using the 2−ΔΔCt method. Relative mRNA expression of the osteogenesis-related genes was normalized to the PLGA group.
Bacterial culture
S. aureus (ATCC25923) is commonly used as a control strain for testing susceptibility to antibiotics and was purchased in freeze-dried form from the American Type Culture Collection (Manassas, VA). The bacteria were grown in a trypticase soy broth (TSB; BD Bioscience, San Jose, CA) medium with agitation at 120 rpm at 37 °C for 16 h. A bacterial suspension (500 μL) containing 1 × 106 CFU/mL was added into wells containing pure PLGA, MBG/PLGA and van-MBG/PLGA scaffolds. For the subsequent antimicrobial test, the bacteria-scaffold mixtures were co-incubated with orbital shaking at 80 rpm at 37 °C.
Antibacterial assays by spread plate method
The absolute number of bacteria present in the biofilms, which was used to evaluate the antibacterial property of the aforementioned scaffolds, was analyzed for S. aureus using the spread plate method as described in previous reports [Citation11,Citation32]. After bacterial culture for 4 h, the scaffolds were gently washed with PBS three times to remove the planktonic bacteria and were placed into glass tubes with 0.5 ml TSB. The adherent bacteria were detached by ultrasonication (10 min) in an ultrasonic bath (B3500S-MT, Branson Ultrasonics Co., Shanghai, China) operated at a frequency of 40 Hz and then centrifuged (Thermo Fisher Scientific) at 4000 rpm for 5 min. Later, the solution was serially diluted 10-fold with fresh PBS, plated in triplicate onto tryptic soy agar (TSA) and incubated overnight at 37 °C for colony counts. The ultimate number of colony forming units (CFUs) on the TSA was calculated and expressed relative to the PLGA group.
Antibacterial assays by crystal violet staining
To assess the biofilm density of scaffolds, cells cultured in multi-well plates were gently washed three times with PBS to remove planktonic cells after 24 h of biofilm formation at a temperature of 37 °C. Each well was stained with 1 ml of 1% (w:v) crystal violet (CV, Sigma-Aldrich) for 15 min at room temperature. Then, the samples were rinsed three times with distilled water. Biofilm formation was quantified by solubilization of the CV stain on incubation with 2 ml of 95% ethanol with shaking for 15 min. The concentration of CV was determined using an automated plate reader (uQuant, Bio-tek, Instruments Inc., Winooski, VT) at a wavelength of 570 nm. The relative absorption of CV was normalized to the 2 h PLGA group. The pure PLGA and MBG/PLGA groups were included as positive controls.
Antibacterial assays by CLSM
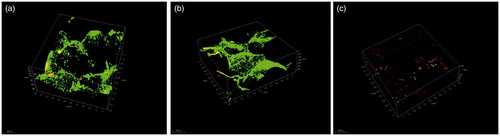
Samples were prepared for the qualitative evaluation of bacterial adhesion on the surface by CLSM. After 24 h of incubation, the above scaffolds were lightly washed with sterile PBS, placed in a new 24-well plate and stained with 500 μl of combination dye (DEAD/LIVE BacLight Kit, Invitrogen, Carlsbad, CA) for 20 min. Adherent bacteria on the scaffolds were visualized using CLSM (LSM 5 PASCAL; Carl Zeiss, Jena, Germany). Three-dimensional images were acquired from random positions within the biofilm that was formed on the scaffolds. Dead and viable cells could be distinguished because viable bacteria with an intact cell membrane were stained fluorescent green, whereas dead bacteria with damaged cell membrane were fluorescent red.
Statistical analysis
All quantitative data are presented as mean ± standard deviation. Statistical analysis was performed using one-way analysis of variance and Tukey post hoc tests by SPSS software (SPSS Inc., Chicago, IL). A value of p < .05 was considered to be statistically significant.
Results
Characterization of MBG
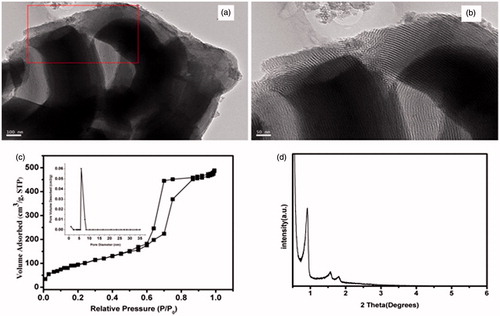
TEM images of the MBG clearly revealed well-ordered hexagonal mesoporous channels with uniform pore size (). The N2 adsorption-desorption isotherm and corresponding BJH pore size distribution curve of synthesized MBG powder showed that it possessed a highly-ordered mesoporous channel and average pore size of about 5 nm (). The relatively large surface area and single-point pore volume of MBGs were approximately 552.8 m2/g and 1.6 cm3/g, respectively. The MBGs presented type IV isotherms and steep type H1 hysteresis at high relative pressure, which are typical characteristics of mesoporous materials with narrow size distributions. SAXRD patterns show that the apparent diffraction of MBG scaffolds peaks at 2θ = 1.25°, while the other two peaks are relatively weak (). This phenomenon indicates that MBG possesses a highly-ordered mesoporous wall with two-dimensional hexagonal structure.
Characterization of scaffolds
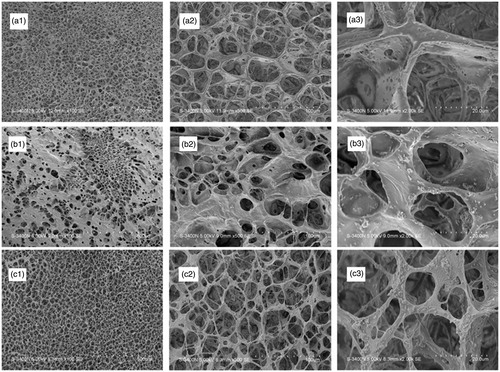
SEM micrographs of the cross-sections of pure PLGA scaffolds, MBG/PLGA, and van-MBG/PLGA scaffolds are shown in . We observed the morphology of scaffolds and the distribution of MBG within the polymer matrix of the scaffolds. All scaffolds present an interconnected porous architecture with pore sizes varying from 200–400 μm. The homogeneously-distributed MBG particles are primary particles in the polymer matrix of the scaffolds. Furthermore, no vancomycin crystallization was observed in the composite scaffolds. As a result, the incorporation of MBG promoted the formation of an interconnected porous structure, whereas vancomycin loading exerted no significant effect on the pore size and interconnection between the pores of the scaffolds.
Figure 2. Scanning electron microscope images of pure PLGA (a1–a3), MBG/PLGA (b1–b3) and van-MBG/PLGA (c1–c3) scaffolds.
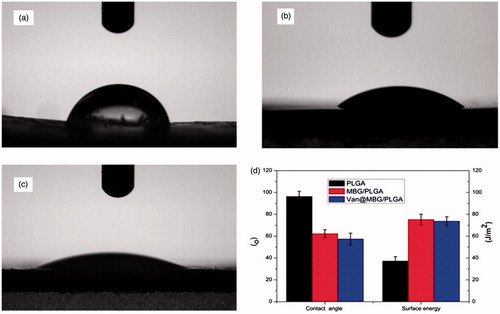
The contact angle of PLGA against water (94.6°) was relatively higher than that of MBG/PLGA and van-MBG/PLGA (58.8 ± 4.3 and 56.34 ± 4.6° respectively, p < .05; ). The increase in surface energy is another index for evaluating hydrophilicity. The surface energy of MBG/PLGA and van-MBG/PLGA was higher than that of the PLGA specimen (38.7 ± 4.8, 73.21 ± 3.9 and 71.8 ± 1.8 J/m2 for PLGA, MBG/PLGA, and van-MBG/PLGA, respectively, p < .01).
In vitro degradation
The ion release profiles for various elements, including Si, Ca, and P, were analyzed by ICP-OES (, respectively). No Si ion was released from the PLGA scaffolds after soaking in PBS. The rapid increase in concentration of Si is possibly commensurate with increasing soaking time for the MBG/PLGA scaffolds. The release of Ca and P ions from the MBG/PLGA scaffolds peaked at day 7 but started to decline afterwards.
Figure 4. Results of in vitro degradation of PLGA and BMG/PLGA scaffold. Changes in ion concentration of the immersion extract during scaffolds soaking (a: Si; b: Ca and c: P); Change in pH levels of the immersion extract (d) and weight loss ratio from the scaffolds (e).
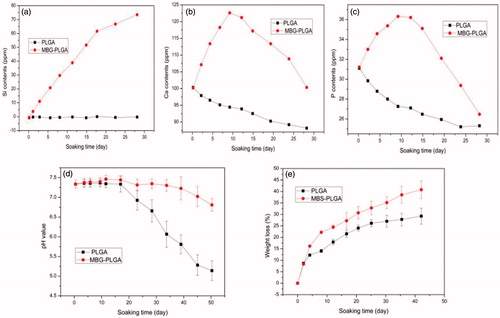
The pH level of the degradation medium decreased in the two scaffolds over time. Compared with MBG/PLGA scaffolds, the pure PLGA scaffolds presented a lower pH level of the medium during degradation (). As shown in , the weight loss ratio of PLGA and MBG/PLGA scaffolds in PBS gradually increased over time. The data show that PLGA scaffolds undergo a more significant weight loss compared with MBG/PLGA scaffolds from day 7 (p < .05). After 42 days of degradation, the total weight loss values for the PLGA and MBG/PLGA scaffolds were 26.5 and 41.2%, respectively. Overall, the MBG/PLGA scaffolds showed higher total weight loss than PLGA scaffolds in vitro.
Vancomycin loading and release behaviour in vitro
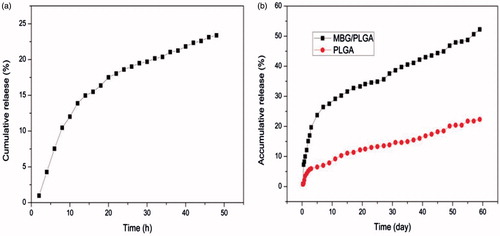
The loading efficiency was calculated as the ratio of loaded vancomycin to the initial amount of vancomycin at different concentrations (0.6, 1.0 and 1.4 g/ml) prior to loading. The vancomycin loading efficiency in the MBGs was calculated as 43.5 ± 1.4 to 48.9 ± 1.9%. shows the vancomycin release profile of the MBGs with a burst release and the cumulative release was approximately 24% after 48 h. As shown in , vancomycin was rapidly released from the two scaffolds within the first 72 h, followed by a slower release with a gentle slope. However, the PLGA scaffolds showed lower initial burst release and only 23% of the loaded vancomycin was released from the scaffold after 58 days. By contrast, after immersion in PBS, the MBG/PLGA scaffolds maintained a release performance that lasted for more than eight weeks with cumulative vancomycin releases of approximately 53%.
Cell attachment, proliferation and morphology
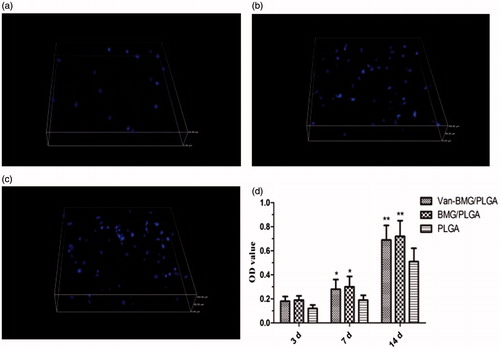
Among all factors that govern cell-biomaterial interactions, cell adhesion is the most crucial. The adherent hBMSCs on these scaffold surfaces were stained with DAPI and then counted, as shown in . After culturing for 3 h, the number of attached cells increased with the addition of MBG. The results demonstrate that MBG/PLGA scaffolds facilitate cell adhesion better than PLGA scaffolds, which are highly hydrophobic and attract low levels of cell adhesion. The cell proliferation rate on specimens after 3, 7 and 14 days was normalized to that of day 1. As determined by MTT assay, cells on the MBG/PLGA and van-MBG/PLGA scaffolds show a significantly higher proliferation rate at different time-points compared with the PLGA scaffolds on days 7 and 14 (). In addition, no significant difference was found between the MBG/PLGA and van-MBG/PLGA groups (p > .05). The results demonstrate that owing to the interconnected porous structure, MBG/PLGA scaffolds have a better ability to promote cell proliferation compared with PLGA scaffolds, even those loaded with vancomycin.
Figure 6. Attachment of the human mesenchymal stem cells on the different surfaces of the scaffolds. (a: PLGA; b:MBG/PLGA and c: Van-MBG/PLGA). (d) The proliferative tendency of the human mesenchymal stem cells at days 3, 7 and 14. *p < .05 compared with PLGA group; **p < .01 compared with PLGA group.
shows cells spreading on the surface of PLGA, MBG/PLGA and van-MBG/PLGA. On the first day, the hBMSCs exhibited either a polygonal or fusiform-shaped morphology. The cells on the MBG/PLGA scaffolds appeared clustered and confluent at day 4, whereas cells on the PLGA scaffolds showed fewer filopodia extensions and intercellular connections.
Figure 7. Cytoskeletal morphology and spreading of the human mesenchymal stem cells on the different scaffolds after four days (a: PLGA; b: MBG/PLGA and c: Van-MBG/PLGA). Representative images of cells stained with fluorescein isothiocyanate-phalloidin for actin filaments and nuclei counterstained with 4′,6′-diamidino-2-phenylindole.
Differentiation of hBMSCs on the surface of scaffolds
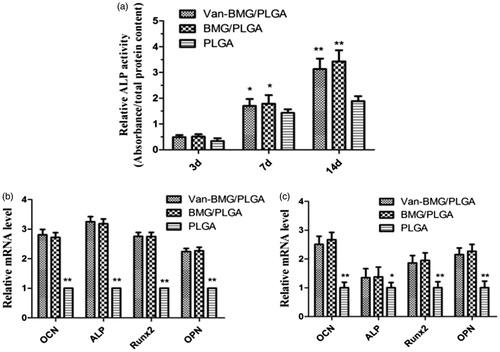
As shown in , our results showed that the ALP activity of hBMSCs on the surface of MBG/PLGA and van-MBG/PLGA was significantly higher compared with the activity on cells cultured on the surface of PLGA at 7 and 14 days. RT-PCR assays were performed to assess the osteogenic response of hBMSCs on the PLGA, MBG/PLGA and van-MBG/PLGA scaffolds. Compared with the PLGA scaffolds, MBG/PLGA scaffolds with/without vancomycin promoted the gene expression levels of ALP, BMP-2, Runx2 and OCN at 7 and 14 days (). The results showed that the presence of MBG promoted the osteogenic differentiation of hBMSCs on scaffolds. No significant difference was observed between the two MBG/PLGA scaffolds with/without vancomycin (p > .05), suggesting that the osteoblast differentiation of hBMSCs on the van-MBG/PLGA scaffolds is not suppressed due to drug cytotoxicity.
Antibacterial efficacy of vancomycin-loaded scaffolds
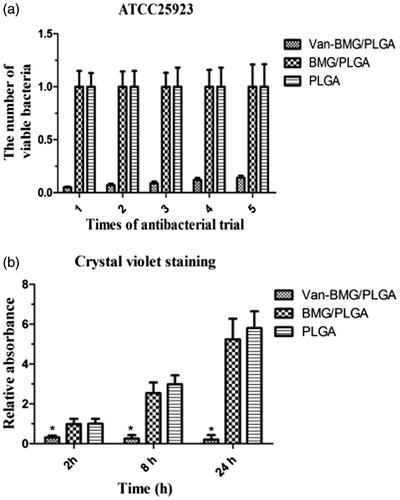
The number of adherent S. aureus bacteria was quantitatively evaluated by the spread plate method. As expected, the van-MBGs/PLGA supported the fewest viable bacteria compared with either MBG/PLGA or PLGA alone after 24 h of culture (p < 0.001). This finding indicates that van-MBGs/PLGA exhibits strong antimicrobial activity against S. aureus (ATCC25923). Moreover, the antibacterial effects did not obviously deteriorate even after six cycles of bacterial exposure, suggesting that the vancomycin-loaded sample possesses stable antibacterial activity ().
Figure 9. Evaluation of bacterial adhesion and biofilm formation. (a) The number of viable bacterial colonies after bacteria dissociation from the various scaffolds using the spread plate method. (b) Absorption of crystal violet was used to estimate the biofilm formation by ATCC35984 on the scaffolds. *p < .01 compared with van-BMG/PLGA group.
Biofilm inhibition of vancomycin-loaded scaffolds
The biofilm inhibition activity of scaffolds in vitro was analyzed by CV staining. As the culturing duration increased from 2–24 h, CV staining intensity on both unloaded samples markedly increased. By contrast, van-MBG/PLGA scaffolds showed significantly lower intensity than unloaded scaffolds, demonstrating that vancomycin loading leads to good anti-biofilm effects ().
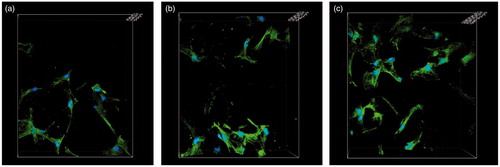
Biofilm formation on the surfaces of the scaffolds in vitro was observed using CLSM. A three-dimensional view of CLSM is shown in . After 24 h of culture, the van-MBG/PLGA scaffold showed considerably less green fluorescence and more red fluorescence than the PLGA and MBG/PLGA scaffolds. This finding was in accordance with the CV staining results. The two unloaded groups contained a significantly greater amount of living adherent bacteria compared with the van-MBG/PLGA group. By contrast, only a few dead bacteria were observed on van-MBG/PLGA, indicating that the vancomycin-loaded samples possess superior antibacterial activity.
Discussion
Novel scaffolds with good osseointegration and antibacterial capability are of great considerable importance in the field of bone tissue engineering. MBG with their extensive surface areas and high porosity, providing excellent drug-loading capabilities, promoting the response of osteoblasts and resulting in enhanced osseointegration, have been extensively studied as bone implant materials [Citation12,Citation19]. However, MBGs are not optimal for scaffolds because of their high brittleness and low fracture toughness and do not meet the mechanical strength requirements of clinical bone repair, especially in mesoporous form [Citation28,Citation33]. Synthetic bioresorbable polymers are known for their high modifiability because these polymers can be easily fabricated into complex structures [Citation6,Citation15,Citation34,Citation35]. However, their performance in cell attachment, proliferation and osteoconductivity is poor because of the lack of specific cell-recognition signals [Citation36]. These problems can be solved by incorporating MBG particles into biodegradable PLGA matrix materials to combine the properties of osteoconductivity of the inorganic material with the flexibility of the polymers. In this study, freezing-drying method was used to fabricate a novel porous composite scaffold loaded with vancomycin. The present study of in vitro degradation behavior and water contact angles confirmed that the MBG-incorporated PLGA scaffold could possess controllable degradability, stabilize the pH of the surrounding environment and improve the hydrophilicity of the scaffolds. More importantly, porosity and well-interconnected pore channels are the main features of the combined scaffolds produced by this approach; these features enable bone ingrowth and sustained antibiotic release.
The porosity of the scaffolds is not the only decisive factor affecting bone regeneration. Pore size and interconnectivity between the pores are other crucial morphological properties of a porous scaffold [Citation37]. Previous studies have proven that optimal interconnection between the pores and a pore size of 150 to 500 μm are essential for cell infiltration, diffusion of nutrients and wastes, vascularization and bone ingrowth [Citation38]. MSCs are key cell sources for tissue repair and bone regeneration and their cellular response, including attachment, spreading, proliferation and their differentiation state on the surface of scaffolds, is essential to osseointegration [Citation39]. The SEM results indicated that MBG is uniformly dispersed in the PLGA scaffolds and that the MBG-modified scaffolds possess an interconnected porous architecture with pore sizes ranging from 200 to 400 μm. Even after vancomycin loading, the MBG/PLGA scaffolds showed a similar morphology of pure MBG, which may be due to the improved hydrophilicity resulting from MBG addition. The combination of MBG into the PLGA matrix markedly improved the attachment and proliferation of hBMSCs compared with PLGA alone because of the increase in specific surface area. Moreover, analysis of ALP activity in vitro showed that MBG/PLGA scaffolds exhibit better enhancement of proliferation and improved phenotype of osteoblasts compared with PLGA alone. Notably, the mRNA expression levels of osteogenesis-related gene on MBG/PLGA scaffolds were significantly enhanced compared with those on the PLGA scaffolds; this finding suggests that incorporation of MBG into the PLGA scaffolds further promotes the differentiation of cells beyond that of PLGA alone. Cell viability and osteogenic differentiation were not suppressed after vancomycin loading. A previous study by Yan et al. also demonstrated that MBG improves the bioactivity of scaffolds and stimulates the deposition of calcium phosphates [Citation40]. In addition, mesoporous silica contains silicon and silicon dioxide, which are osteoinductive materials and catalysts for bone formation [Citation28]. The high specific surface area, large pore volume and mesoporous structure of MBG/PLGA scaffolds ultimately strengthens the bone-matrix interface. Our results revealed that the degradation rate of MBG-containing scaffolds was evidently faster than that of PLGA scaffolds. The MBG incorporation into the PLGA scaffolds could favor the change in pH and ion release. More importantly, this approach is beneficial for matching the rate of scaffold degradation to that of new bone formation.
Chronic osteomyelitis and infected bone defects are common complications in severe open fractures, compromising bone healing and threatening loss of limb [Citation41]. However, parenteral antibiotic therapy has had limited success, failing to reach and maintain effective concentrations at sites of infections because of the damaged vascularity and necrotic bone tissue [Citation17,Citation18]. In addition, serious systemic toxicity that damages internal organs should be a concern [Citation42]. Consequently, over the past several years numerous investigators have attempted to find alternatives to systemic antibiotic administration [Citation19,Citation43]. Bone-graft substitute materials without antibacterial activity are vulnerable to bacterial adhesion and biofilm formation, eventually leading to treatment failure due to infection [Citation17,Citation43]. The successful treatment of chronic osteomyelitis and infected bone defects must both eliminate bacteria and boost bone regeneration.
Vancomycin is a wide-spectrum antibiotic that is used to treat bone infection, which is most often caused by S. aureus in orthopedic situations [Citation18,Citation25]. After the initial colonization, S. aureus can produce a multilayered biofilm, which is difficult to eradicate using antibiotics [Citation17,Citation18]. In our current study, the hydrophilicity of vancomycin means that the drug can be easily stored within the mesoporous channels of MBG with a high pore volume. After fabrication of the MBG/PLGA composite scaffolds, the PLGA matrix may avoid direct contact between vancomycin-loaded MBGs and the release medium. Our results show that drug-loaded scaffolds produce an initial fast release followed by a relatively slow release, which effectively inhibits bacterial adhesion and biofilm formation, as confirmed qualitatively and quantitatively by CV staining and CLSM, respectively. In the initial phase, antibiotic release was mostly attributed to the outer surface of MBG/PLGA composites, which is controlled by osmotic pressure. This outcome is beneficial in cases of acute infection, as it instantly leads to locally-high doses of antibiotics. With the gradual degradation of the PLGA matrix, the loaded vancomycin further within the composites would be released into the medium. Thus, vancomycin-loaded scaffolds prolong the effective drug release for up to eight weeks and the cumulative release reaches more than 50%. Diffusion of vancomycin loaded into mesoporous channels into the medium requires a longer penetration time, which partially explains how the controlled release is sustained. The fast initial release combined with the sustained release of vancomycin markedly enhances the antibacterial ability of the composite scaffolds by killing planktonic bacteria during the short-term and inhibiting bacterial colonization in the long-term. The action facilitates treatment of infection inside the osseous defect. In this work, bacterial adhesion and biofilm formation, which are critical to bone healing in infected condition, were inhibited after implantation of vancomycin-laden scaffolds.
Conclusion
In the present study, we propose a novel bone tissue-engineering scaffold, generated by combining osteoinductive and biodegradable composite biomaterials by freeze drying and using this we prepared a PLGA scaffold with vancomycin-loaded MBG. Our results confirm that the incorporation of MBG exerts a beneficial impact on the interconnected porous structure and vancomycin loading onto the scaffold does not compromise the microporous architecture or the osteogenic potential. The evenly-distributed mesoporous channels create an ideal network which to load antibiotics and also provides a sustained release mechanism. The presence of vancomycin-loaded MBG enhances simultaneous antimicrobial effects and in vitro bioactivity. In consequence, the inorganic-organic composite scaffolds are potential candidates for both bone repair and treatment of peri-implant infection because of their combined advantageous properties of stimulating bone ingrowth and preventing biofilm formation. Future studies will focus on evaluating the ability of these biomaterials to prevent infection, their biocompatibility and release kinetics in vivo.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Lee EJ, Kasper FK, Mikos AG. Biomaterials for tissue engineering. Ann Biomed Eng. 2014;42:323–337.
- Li X, Yang Y, Fan Y, et al. Biocomposites reinforced by fibers or tubes as scaffolds for tissue engineering or regenerative medicine. J Biomed Mater Res. 2014;102:1580–1594.
- Okamoto M, John B. Synthetic biopolymer nanocomposites for tissue engineering scaffolds. Prog Polym Sci. 2013;38:1487–1503.
- Levingstone TJ, Matsiko A, Dickson GR, et al. A biomimetic multi-layered collagen-based scaffold for osteochondral repair. Acta Biomater. 2014;10:1996–2004.
- Zhang J, Zhao S, Zhu Y, et al. Three-dimensional printing of strontium-containing mesoporous bioactive glass scaffolds for bone regeneration. Acta Biomater. 2014;10:2269–2281.
- Gentile P, Chiono V, Carmagnola I, et al. An overview of poly(lactic-co-glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int J Mol Sci. 2014;15:3640–3659.
- Sridhar R, Sundarrajan S, Venugopal JR, et al. Electrospun inorganic and polymer composite nanofibers for biomedical applications. J Biomater Sci Polym Ed. 2013;24:365–385.
- Zhang Q, Lu H, Kawazoe N, et al. Pore size effect of collagen scaffolds on cartilage regeneration. Acta Biomater. 2014;10:2005–2013.
- Zhao S, Zhang J, Zhu M, et al. Three-dimensional printed strontium-containing mesoporous bioactive glass scaffolds for repairing rat critical-sized calvarial defects. Acta Biomater. 2015;12:270–280.
- Cao W, Hench LL. Bioactive materials. Ceram Int. 1996;22:493–507.
- Li Y, Liu YZ, Long T, et al. Mesoporous bioactive glass as a drug delivery system: fabrication, bactericidal properties and biocompatibility. J Mater Sci: Mater Med. 2013;24:1951–1961.
- Wu T, Cheng N, Xu C, et al. The effect of mesoporous bioglass on osteogenesis and adipogenesis of osteoporotic BMSCs. J Biomed Mater Res. 2016;104:3004–3014.
- Zeng D, Zhang X, Wang X, et al. The osteoimmunomodulatory properties of MBG scaffold coated with amino functional groups. Artif Cells Nanomed Biotechnol. Forthcoming. [cited 2017 Aug 30]. doi:10.1080/21691401.2017.1369428
- Qi X, Pei P, Zhu M, et al. Three dimensional printing of calcium sulfate and mesoporous bioactive glass scaffolds for improving bone regeneration in vitro and in vivo. Sci Rep. 2017;7:42556.
- Li X, Wang X, Zhang L, et al. MBG/PLGA composite microspheres with prolonged drug release. J Biomed Mater Res Part B Appl Biomater. 2009;89:148–154.
- Zhu M, Zhang L, He Q, et al. Mesoporous bioactive glass-coated poly(l-lactic acid) scaffolds: a sustained antibioticdrug release system for bone repairing. J Mater Chem. 2011;21:1064–1072.
- Cook GE, Markel DC, Ren W, et al. Infection in Orthopaedics. J Orthop Trauma. 2015;29 Suppl 12:S19–S23.
- Xie Z, Liu X, Jia W, et al. Treatment of osteomyelitis and repair of bone defect by degradable bioactive borate glass releasing vancomycin. J Control Release. 2009;139:118–126.
- Soundrapandian C, Datta S, Kundu B, et al. Porous bioactive glass scaffolds for local drug delivery in osteomyelitis: development and in vitro characterization. AAPS PharmSciTech. 2010;11:1675–1683.
- Kluin OS, van der Mei HC, Busscher HJ, et al. Biodegradable vs non-biodegradable antibiotic delivery devices in the treatment of osteomyelitis. Expert Opin Drug Deliv. 2013;10:341–351.
- ter Boo GJ, Grijpma DW, Moriarty TF, et al. Antimicrobial delivery systems for local infection prophylaxis in orthopedic- and trauma surgery. Biomaterials. 2015;52:113–125.
- García-Quintanilla M, Pulido MR, Carretero-Ledesma M, et al. Vaccines for antibiotic-resistant bacteria: possibility or pipe dream? Trends Pharmacol Sci. 2016;37:143–152.
- Kohira N, West J, Ito A, et al. In vitro antimicrobial activity of a siderophore cephalosporin, S-649266, against Enterobacteriaceae clinical isolates, including carbapenem-resistant strains. Antimicrob Agents Chemother. 2015;60:729–734.
- Wellington EM, Boxall AB, Cross P, et al. The role of the natural environment in the emergence of antibiotic resistance in gram-negative bacteria. Lancet Infect Dis. 2013;13:155–165.
- Loc-Carrillo C, Wang C, Canden A, et al. Local intramedullary delivery of vancomycin can prevent the development of long bone Staphylococcus aureus infection. PLoS One. 2016;11:e0160187.
- Luo S, Jiang T, Yang Y, et al. Combination therapy with vancomycin-loaded calcium sulfate and vancomycin-loaded PMMA in the treatment of chronic osteomyelitis. BMC Musculoskelet Disord. 2016;17:502.
- Yang H, Hao Y, Liu Q, et al. Preparation and in vitro study of hydrochloric norvancomycin encapsulated poly microspheres for potential use in osteomyelitis. Artif Cells Nanomed Biotechol. 2017;45:1326–1330.
- Ma J, Lin H, Li X, et al. Hierarchical porous bioactive glasses/PLGA-magnetic SBA-15 for dual-drug release. Mater Sci Eng C Mater Biol Appl. 2014;39:21–28.
- Garg S, Thakur S, Gupta A, et al. Antibacterial and anticancerous drug loading kinetics for (10-x)CuO-xZnO-20CaO-60SiO2-10P2O5 (2 ≤ x ≤ 8) mesoporous bioactive glasses. J Mater Sci Mater Med. 2017;28:11.
- Gu J, Wang T, Fan G, et al. Biocompatibility of artificial bone based on vancomycin loaded mesoporous silica nanoparticles and calcium sulfate composites. J Mater Sci: Mater Med. 2016;27:64.
- Wan M, Zhang J, Wang Q, et al. In situ growth of mesoporous silica with drugs on titanium surface and its biomedical applications. ACS Appl Mater Interfaces. 2017;9:18609–18618.
- Wu T, Hua X, He Z, et al. The bactericidal and biocompatible characteristics of reinforced calcium phosphate cements. Biomed Mater. 2012;7:045003.
- Montazerian M, Dutra Zanotto E. History and trends of bioactive glass-ceramics. J Biomed Mater Res A. 2016;104:1231–1249.
- Amirthalingam M, Kasinathan N, Amuthan A, et al. Bioactive PLGA-curcumin microparticle-embedded chitosan scaffold: in vitro and in vivo evaluation. Artif Cells Nanomed Biotechnol. 2017;45:233–241.
- Kasinathan N, Amirthalingam M, Reddy ND, et al. Polycaprolactone-based in situ implant containing curcumin-PLGA nanoparticles prepared using the multivariate technique. Artif Cells Nanomed Biotechnol. 2016;44:1520–1528.
- Choi JS, Kim HS, Yoo HS. Electrospinning strategies of drug-incorporated nanofibrous mats for wound recovery. Drug Deliv Transl Res. 2015;5:137–145.
- Hollister SJ. Porous scaffold design for tissue engineering. Nat Mater. 2005;4:518–524.
- Hutmacher DW. Scaffolds in tissue engineering bone and cartilage. Biomaterials. 2000;21:2529–2543.
- Polymeri A, Giannobile WV, Kaigler D. Bone marrow stromal stem cells in tissue engineering and regenerative medicine. Horm Metab Res. 2016;48:700–713.
- Yan X, Huang X, Yu C, et al. The in-vitro bioactivity of mesoporous bioactive glasses. Biomaterials. 2006;27:3396–3403.
- Tinubu J, Scalea TM. Management of fractures in a geriatric surgical patient. Surg Clin North Am. 2015;95:115–128.
- Yi HG, Choi YJ, Kang KS, et al. A 3D-printed local drug delivery patch for pancreatic cancer growth suppression. J Control Release. 2016;238:231–241.
- Mantripragada VP, Jayasuriya AC. Effect of dual delivery of antibiotics (vancomycin and cefazolin) and BMP-7 from chitosan microparticles on Staphylococcus epidermidis and pre-osteoblasts in vitro. Mater Sci Eng C Mater Biol Appl. 2016;67:409–417.