Abstract
This study was designed to investigate the effect of polymerized human placenta haemoglobin (PolyPHb) on cardiac dysfunction after severe burns. A total of 60 male Sprague-Dawley rats were randomly divided into 3 groups: Sham, Burn and Burn + PolyPHb groups. Rats were subjected to third-degree burns to 30% of total body surface area and the haemodynamics, cardiac enzyme release and aortic endothelium ultrastructure/function were measured. PolyPHb (0.5 gHb/kg) greatly improved mean arterial pressure, left ventricular developed pressure (LVDP), maximum LVDP increase and decrease rate and reduced left ventricular end-diastolic pressure as compared to the Burn group. The plasma levels of cardiac enzyme including CK-MB and troponin I were also significantly down-regulated in the Burn + PolyPHb group. In addition, PolyPHb treatment markedly restored the endothelium-dependent relaxation impaired by severe burns and pathological changes of endothelium in aorta. Therefore, our data suggest that PolyPHb can limit severe burn-induced myocardial injury, which is associated with protection of aortic endothelium.
Introduction
Severe burns require immediate medical intervention and delayed resuscitation always lead to irreversible shock and cardiac dysfunction [Citation1,Citation2]. It has been well-documented that severe burns reduce myocardial regional blood flow and cause ischemia or hypoxia of the myocardium, which was termed as “shock heart” [Citation3]. Therefore, increasing myocardial oxygen (O2) supply and improving cardiac function are essential to improve the prognosis and survival rate of patients with severe burns.
Polymerized human placenta haemoglobin (PolyPHb) is a novel O2 therapeutic agent, which was initially designed for haemorrhagic shock patients caused by trauma [Citation4]. Our previous studies demonstrated that PolyPHb has potential protective effect against ischemia/reperfusion (I/R) injury in multiple animal models [Citation5–11]. As a promising haemoglobin based oxygen carrier (HBOC), PolyPHb allows transporting more O2 to the hypoxia tissues owing to its higher O2 affinity, lower viscosity and smaller mean diameter than human red blood cells, which would benefit microcirculation perfusion and alleviate myocardial I/R injury. However, whether PolyPHb treatment could affect severe burn-induced myocardial injury or not is still unknown.
Materials and methods
All the experiments were approved by the Institutional Animal Care and Use Committee of Sichuan University, and the animals involved received human care in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85–23, revised 1996). Adult male Sprague-Dawley rats, weighing 250–300 g, were housed at a constant temperature (22 ± 3 °C) on a 12-h light/dark cycle with free access to food and water.
The preparation of PolyPHb
The PolyPHb was prepared as reported previously [Citation4,Citation12]. Briefly, purified and viral inactivated fresh human placenta haemoglobin was modified with bis(3,5-dibromosalicyl) fumarate to achieve optimal O2 affinity. After cross-linkage with glutaraldehyde, the mixture was subject to ultrafiltration and molecular sieve chromatography. The final product had a molecular weight of 64–600 kDa.
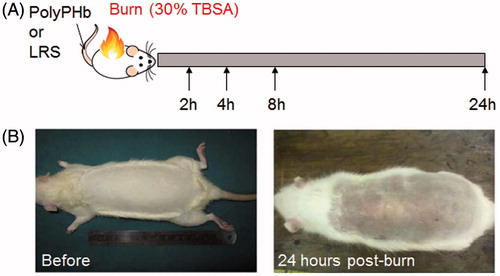
Experimental design and burn procedure
Sixty male Sprague-Dawley rats were randomly divided into three groups: Sham group (n = 20), Burn group (n = 20) and Burn + PolyPHb group (n = 20). Animals were anesthetized with intraperitoneal injection of pentobarbital sodium (50 mg/kg) and a 30% TBSA full-thickness burn injury was produced by exposure to 100 °C water for 10 s (). Sham rats were exposed to water of room temperature. Immediately after burn injury, PolyPHb at a dose of 0.5 gHb/kg or equal amount of lactated Ringer’s solution was infused via caudal vein. For acute resuscitation, lactated Ringer’s solution calculated from the Parkland formula (4 ml/kg/% burn) was intraperitoneally injected immediately (half of the calculated volume) and 8 h after burn injury (the other half). To reduce the potential pain after severe burn, analgesic drug buprenorphine (0.05 mg/kg) was given subcutaneously before rat fully recovered from anaesthesia. All animals were sacrificed 24 h after burn injury.
Measurement of blood pressure and myocardial function
Under anaesthesia, the right femoral artery was exposed and a polyethylene catheter filled with heparin saline was placed to measure mean arterial pressure (MAP). Another heparin-filled catheter was inserted through the right common carotid artery to the left ventricle for measurement of cardiac function, including left ventricular systolic pressure (LVSP), left ventricular end-diastolic pressure (LVEDP), maximum left ventricular developed pressure increase (+dp/dt) and decrease rate (−dp/dt). All the data were collected after being stable for 5 min with the PowerLab data-acquisition system (AD Instruments Pty Ltd., Bella Vista, , Australia). To avoid adverse influence on cardiovascular system, the catheter in LV was withdrawn after each measurement.
Detection of cardiac enzyme levels
The levels of cardiac enzyme including creatine kinase-MB (CK-MB) and cardiac troponin-I (cTnI) in venous plasma samples were detected by use of an automatic analyser (model AU5400; Olympus Diagnostics, Melville, NY) and cardiac troponin-I ELISA kit (Life Diagnostics, Inc., West Chester, PA).
Evaluation of vascular reactivity of isolated aortic rings
The endothelial function of aorta was measured as reported previously [Citation12]. Briefly, aortic rings (3–4 mm in length) free of fat and connective tissue were mounted between two stainless steel hooks in organ bath chambers (PanLab Systems, Harvard apparatus, Barcelona, Spain). Each chamber contained 10 ml of Krebs–Henseleit (KH) solution (118 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4, 1.77 mM CaCl2, 25 mM NaHCO3, 11.4 mM glucose; pH 7.4, 37 °C) and aerated continuously with 95% O2 and 5% CO2. Special attention was paid during the preparation to avoid damaging endothelium. During 60 min of equilibration period, resting tension of 3.5 g was periodically adjusted and the KH solution was changed every 30 min. The arterial viability was checked by stable and reproducible constriction to the addition of potassium chloride (KCl, 60 mM). After washed and equilibrated for 60 min, these aortic rings were evoked using phenylephrine (10−7 M) to elicit reproducible contractile responses. Acetylcholine (Ach; 1 × 10−8 to 1 × 10−4 M) or sodium nitroprusside (SNP; 1 × 10−10 to 1 × 10−6 M) was then progressively added to induce endothelium-dependent or -independent relaxation, respectively.
Transmission electron microscopy
Twenty-four hours after burn injury, aorta samples were harvested and fixed for 24 h in 3% glutaraldehyde in 0.1 M phosphate buffer saline (pH 7.4), and then fixed with 1% osmium tetroxide for 1 h. After the samples were dehydrated in a series of ethanol and were embedded in Epon 812 (Electron Microscopy Sciences, Hatfield, PA), ultrathin sections were prepared and counterstained with uranyl acetate and lead citrate. The stained sections were examined under a Hitachi H-600IV electron microscope (Hitachi Ltd., Tokyo, Japan) at 75 kV. The pathological changes were assessed in a blinded fashion by two pathologists.
Statistical analysis
All values in the text and figures were expressed as mean ± SD. One-way ANOVA was used to compare the differences among groups followed by Bonferroni’s multiple comparison test as applicable SPSS version 17.0 software (SPSS Inc., Chicago, IL). p values <.05 was considered statistically significant.
Results
PolyPHb improved cardiac function after severe burns
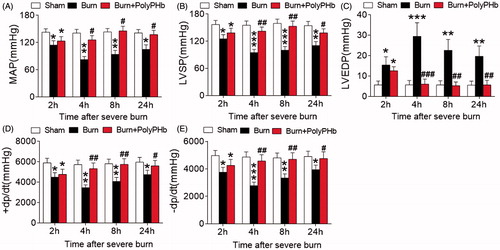
Rats received 30% TBSA full-thickness burn and the haemodynamic parameters were measured at baseline, 2, 4, 8, 24 h after burns. In the Burn group, the MAP and LVSP reflecting LV contractile function were significantly reduced 2 h after burn injury when compared to the Sham group (). This reduction was sustained up to 24 h. Also, the LVEDP was markedly increased and sustained throughout the time points we measured (). Consistently, the ± dp/dt were greatly decreased post-burn (). These data suggest an impairment of both systolic and diastolic function of heart after severe burn injury. Treatment with PolyPHb totally reversed this cardiac dysfunction, as evidenced by significantly increased LVSP and ± dp/dt and markedly suppressed LVEDP.
Figure 2. PolyPHb attenuated cardiac dysfunction after severe burn. The MAP (A), LVSP (B), LVEDP (C) and ± dp/dt (D and E) of the three groups after severe burn. Values were presented as mean ± SD (n = 15–20 per group). *p < .05, **p < .01, ***p < .001 vs. the Sham group; #p < .05, ##p < .01, ###p < .001 vs. the Burn group. MAP: mean arterial pressure; LVSP: left ventricular systolic pressure; LVEDP: left ventricular end-diastolic pressure;±dp/dt: maximum left ventricular developed pressure increase and decrease rate.
PolyPHb reduced cardiac enzyme release after severe burns
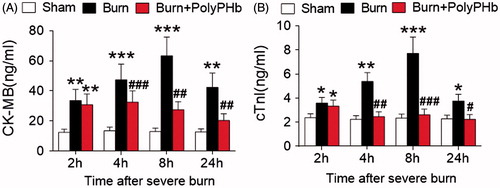
Increased circulating level of cardiac enzyme is a biomarker of cardiac damage. We did not observe any difference in the levels of CK-MB and cTnI among the three groups at baseline (data not shown). As shown in the , the plasma levels of CK-MB and cTnI were significantly increased in the Burn group at 2 h after burn injury (p < .01 and p < .05 vs. the Sham group), which reached peak values at 8 h and kept at high levels up to 24 h. Even though animals received PolyPHb exhibited an elevation of circulating CK-MB and cTnI at 2 h post-burn, the cardiac enzyme levels were normalized during the following period as compared to the Sham group, which provided additional evidence that PolyPHb treatment alleviates severe burn-induced cardiac damage.
Figure 3. PolyPHb limited cardiac enzyme release after severe burn. The release of CK-MB (A) and cTnI (B) of the three groups after severe burn. Values were expressed as mean ± SD (n = 15–20 per group). *p < .05, **p < .01, ***p < .001 vs. the Sham group; #p < .05, ##p < .01, ###p < .001 vs. the Burn group. CK-MB: creatine kinase-MB; cTnI: cardiac troponin-I.
PolyPHb restored endothelium-dependent vasorelaxation impaired by severe burns
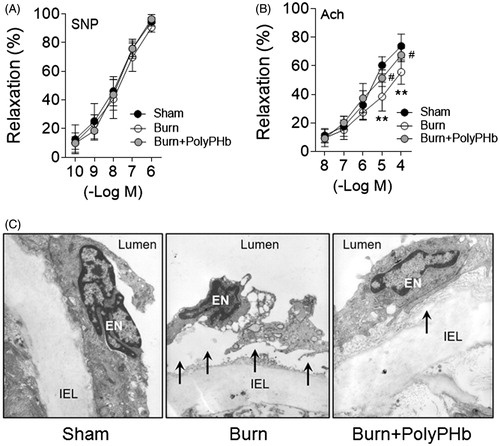
The endothelium is a critical biological barrier for the maintenance of vascular function and cardiac performance [Citation13]. We measured the endothelial function of rat aorta after severe burns by use of an in vitro organ bath system. The aortic rings preconstricted with phenylephrine (10−7 M) were relaxed with the addition of sodium nitroprusside (SNP, 1 × 10−10 to 1 × 10−6 M) or acetylcholine (Ach, 1 × 10−8 to 1 × 10−4 M). The endothelium-independent vasorelaxation induced by SNP did not differ among groups (). However, the maximal endothelium-dependent relaxation induced by Ach (1 × 10−4 M) was much less in the Burn group (55.56 ± 8.54%) than that of the Sham group (73.85 ± 8.21%, p < .01; ). The impaired endothelium-dependent relaxation induced by severe burns was remarkably ameliorated by PolyPHb (67.48 ± 4.67%, p < .05 vs. the Burn group; ). Further experiment demonstrated a pathological phenotype of rat aorta after severe burns characterized by lifting and separation of endothelium from the internal elastic lamina (IEL) (). Also, the aorta from the Burn group exhibited a loss of endothelial cell elongation of cytoplasm and nucleus, which illustrates their contracted phenotype. Consistent with our previous findings, these abnormalities were partially corrected by PolyPHb treatment.
Figure 4. PolyPHb ameliorated endothelial damage caused by severe burn. (A) The SNP-induced endothelium-independent relaxation and Ach-induced endothelium-dependent relaxation of aorta. Values are presented as mean ± SD (n = 5 per group). **p < .01 vs. the Sham group; #p < .05 vs. the Burn group. (B) Representative transmission electron microscopy images of the ultrastructure of the aortic endothelium (n = 3). (C) Original magnification ×10,000. Black arrows indicate endothelial cell lifting and separation from the IEL. SNP: sodium nitroprusside; Ach: acetylcholine; IEL indicates internal elastic lamina.
Discussion
This study provided distinct evidence that treatment with 0.5 gHb/kg PolyPHb improves the blood pressure and cardiac function after severe burns. Further study demonstrated that this beneficial effect is associated with the amelioration of endothelial dysfunction and structural abnormality caused by severe burns.
It has been reported that body fluid loss following severe burns may lead to ischemia and hypoxia of organs [Citation14]. Meanwhile, the inflammatory response after burns may induce changes of structures and functions of endothelial cells and neutrophils of blood vessel, which cause disturbance of microcirculation and myocardial damage [Citation3,Citation14]. Myocardial damage may exacerbate the hypovolemic shock after burn injury and eventually lead to multiple organ dysfunction syndrome (MODS). In this study, the cardiac dysfunction and drop of blood pressure were observed as early as 2 h after burns, which were companied with an elevation of cardiac enzyme release. Even though PolyPHb treatment showed little influence at the early stage, it did improve haemodynamics and inhibit enzyme release from 4 h after burn injury, exhibiting a promising protective effect.
As a novel HBOC, PolyPHb was designed by Chengmin Yang’s group in China [Citation4]. PolyPHb can provide a better perfusion for microcirculation due to its high O2 affinity, low viscosity and small mean diameter. Our group has demonstrated that PolyPHb offers beneficial effects on multiple organs under stress, including heart [Citation6,Citation8], liver [Citation11], lung [Citation15] and kidney [Citation16]. This study expands the potential therapeutic applications of PolyPHb on heart damage caused by severe burns. Our finding was also supported by previous report that diaspirin cross-linked haemoglobin, another HBOC product, could improve haemodynamic parameters and survival rate in a rat model of burn injury [Citation17].
There are likely to be several factors that contribute to cardiac changes after burn injury. Systemic inflammation-caused direct damage to myocytes and endothelial dysfunction have been widely observed after trauma and could contribute to long-term cardiovascular dysfunction [Citation18,Citation19]. Our data clearly indicate that PolyPHb attenuated the impairment of endothelium-dependent vasorelaxation. High level of nitrite oxide (NO) was found in burn patients and has been implicated in the development of the unmanageable inflammatory response [Citation20,Citation21]. Although the precise mechanism for the endothelium protection of PolyPHb is still unknown, it is possible that it scavenged excessive NO and restored nitroso-redox imbalance, which has been reported in our previous study that PolyPHb suppressed NO-mediated myocardial apoptosis and restored nitroso-redox balance [Citation6].
In conclusion, our data suggested that PolyPHb can attenuate cardiac damage in a rat model of severe burn injury, which is associated with the improvement of endothelial function of aorta.
Disclosure statement
The authors report no conflicts of interest.
Funding
This study was supported by grants from the National Nature Science Foundation of China (81471042, 81770815 and 81100180), the 2013 Research Fund for Outstanding Young Scholars of Sichuan University (to Tao Li), the National High Technology Research and Development Program of China (863 Program, 2012AA021903), the Science and Technology Support Program of Sichuan Province (2013SZ0059) and the Special Foundation for Technology Innovation Program of Sichuan Province (2013ZZ0006).
References
- Evers LH, Bhavsar D, Mailänder P. The biology of burn injury. Exp Dermatol. 2010;19:777–783.
- Jeschke MG, Gauglitz GG, Kulp GA, et al. Long-term persistance of the pathophysiologic response to severe burn injury. PLoS One. 2011;6:e21245.
- Huang Y, Li Z, Yang Z. Roles of ischemia and hypoxia and the molecular pathogenesis of post-burn cardiac shock. Burns. 2003;29:828–833.
- Li T, Yu R, Zhang H-H, et al. A method for purification and viral inactivation of human placenta hemoglobin. Artif Cells Blood Substit Immobil Biotechnol. 2006;34:175–188.
- Li T, Jiang Y, Zhang Z, et al. Effect of polymerized human placenta hemoglobin on hemodynamic parameter and cardiac function in a rat hemorrhagic shock model. Artif Cells Blood Substit Immobil Biotechnol. 2012;40:256–260.
- Li T, Li J, Liu J, et al. Polymerized placenta hemoglobin attenuates ischemia/reperfusion injury and restores the nitroso-redox balance in isolated rat heart. Free Radic Biol Med. 2009a;46:397–405.
- Li T, Liu J, Yang Q, et al. Polymerized placenta hemoglobin improves cardiac functional recovery and reduces infarction size of isolated rat heart. Artif Cells Blood Substit Immobil Biotechnol. 2009;37:48–52.
- Li T, Zhang P, Liu J, et al. Protective effects of hemoglobin-based oxygen carrier given to isolated heart during ischemia via attenuation of mitochondrial oxidative damage. Free Radic Biol Med. 2010;48:1079–1089.
- Li T, Zhou R, Xiang X, et al. Polymerized human placenta hemoglobin given before ischemia protects rat heart from ischemia reperfusion injury. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:392–397.
- Wu W, Li T, Liu J, et al. Pretreatment before ischemia induction with polymerized human placenta hemoglobin (PolyPHb) attenuates ischemia/reperfusion injury-induced myocardial apoptosis. Artif Cells Blood Substit Immobil Biotechnol. 2011;39:3–6.
- You Z, Li Q, Li B, et al. Isovolemic hemodilution with glutaraldehyde-polymerized human placenta hemoglobin (PolyPHb) attenuated rat liver ischemia/reperfusion injury. Artif Cells Nanomed Biotechnol. 2013;42:83–87.
- Li T, Zhou R, Yao Y, et al. Angiotensin-converting enzyme inhibitor captopril reverses the adverse cardiovascular effects of polymerized hemoglobin. Antioxid Redox Signal. 2014;21:2095–2108.
- Deanfield JE, Halcox JP, Rabelink TJ. Endothelial function and dysfunction: testing and clinical relevance. Circulation. 2007;115:1285–1295.
- Nielson CB, Duethman NC, Howard JM, et al. Burns: pathophysiology of systemic complications and current management. J Burn Care Res. 2017;38:e469–e481.
- Li T, Zhang Z, Wu W, et al. Resuscitation with polymerized human placenta hemoglobin attenuated hemorrhagic shock-induced lung injury. Artif Cells Nanomed Biotechnol. 2013;41:27–31.
- Li T, Zhang Z, Liao D, et al. The effect of polymerized placenta hemoglobin on renal ischemia/reperfusion injury. Artif Cells Blood Substit Immobil Biotechnol. 2012b;40:396–399.
- Soltero RG, Hansbrough JF. The effects of diaspirin cross-linked hemoglobin on hemodynamics, metabolic acidosis and survival in burned rats. J Trauma. 1999;46:286–291.
- Brocq ML, Leslie SJ, Milliken P, et al. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1631–1674.
- Frantz S, Nahrendorf M. Cardiac macrophages and their role in ischaemic heart disease. Cardiovasc Res. 2014;102:240–248.
- Preiser JC, Reper P, Vlasselaer D, et al. Nitric oxide production is increased in patients after burn injury. J Trauma. 1996;40:368–371.
- Rawlingson A. Nitric oxide, inflammation and acute burn injury. Burns. 2003;29:631–640.