?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.
?Mathematical formulae have been encoded as MathML and are displayed in this HTML version using MathJax in order to improve their display. Uncheck the box to turn MathJax off. This feature requires Javascript. Click on a formula to zoom.Abstract
A scaffold composed of different collagen (COL)/chitosan (CS)/hyaluronic acid sodium (HAS) salt ratios was evaluated by determining porosity, swelling, loss rate in hot water, mechanical property, and cell proliferation to obtain optimum conditions for manufacturing porous scaffolds. Results showed that the optimal ratio of COL/CS/HAS salt porous scaffold was 1:1:0.1. High swelling and loss rate of scaffolds/microspheres (MPs) could lead to high diffusion rate of MPs from the scaffolds, causing an increase in the kartogenin (KGN) release. The porous scaffolds at optimum conditions had a maximum amount of KGN release. Results of in vitro fluorescence staining and cell proliferation suggested that scaffolds/MPs had good biocompatibility and the capability to promote bone marrow stromal cell proliferation, cartilage tissue regeneration, and integration between the repaired and surrounding cartilages. Therefore, this composite could be a promising material for cartilage repair and regeneration, which could be effective in the knee osteoarthritis treatment.
Introduction
The intrinsic properties of articular cartilage, such as avascularity and low cellularity, restrict its spontaneous regeneration [Citation1]. Therefore, various approaches have been tested to improve cartilage healing. In recent years, various scaffolds have been fabricated through chemical and physical cross-linking for cartilage regeneration, including natural or synthetic polymer three-dimensional (3D) porous biomaterials.
The development of tissue engineering (TE) provides a promising therapeutic treatment for cartilage regeneration [Citation2]. Studies on cartilage tissue engineering (CTE) have always focused on scaffolds, growth factors and cells, and considerable progress has been made [Citation3]. Natural or synthetic scaffold has good biocompatibility and can be gradually degraded and absorbed in vivo. 3D biomaterial scaffolds can provide a good environment for cell survival. These scaffolds exchange with gas and remove waste to be used for cells that grow in the scaffold. The seed cells continually grow as the biomaterial gradually degrades to repair the cartilage defect in CTE. As a crucial ingredient of CTE, scaffolds serve as a spatial structure for cell proliferation and differentiation [Citation2].
With the accelerated aging of the population, an increasing number of people are affected by knee osteoarthritis (KOA), which is characterized by the progressive breakdown of articular cartilage and ultimately leads to functional failure of synovial joints. KOA is mediated by several pathogenic mechanisms, including enzymatic degradation of extracellular matrix, deficient new matrix formation, cell death, and hypertrophic differentiation of cartilage cells [Citation4,Citation5]. The only current therapeutic options for KOA are pain management and surgical intervention [Citation6]. However, surgical intervention is expensive and can only be performed in patients with advanced KOA. Intra-articular injection is an effective therapy than drug treatment, such as the injection of glucocorticoid and hyaluronic acid sodium (HAS) salt. However, these anti-inflammatory drugs and analgesics cannot fundamentally solve the problem and improve articular cartilage regeneration. Thus, this method does not provide a long-term curative effect. Kartogenin (KGN) is a nonprotein small molecule inducing chondrogenesis, which was first reported by Johnson et al. [Citation6]. KGN was revealed to significantly promote chondrocyte differentiation of human MSCs in a dose-dependent manner without any toxicity. Most importantly, KGN is a very stable small molecule and thus can be stored and transported at room temperature. KGN is a potential chondrogenesis promoter in CTE compared with protein growth factors. The results show that KGN can promote the healing of cartilage defect. The intra-articular injection can deliver KGN into the articular cavity [Citation7]; however, the amount of drug delivered into the defect area cannot be precisely quantified, and most parts of KGN are absorbed into the circulatory system. Therefore, 3D scaffolds containing microspheres (MPs) for controlled KGN delivery in CTE are urgently needed.
Biodegradable polymers are the main components of scaffolds, including poly (lactic acid) (PLA), poly (caprolactone) (PCL), poly (lactide-co-glycolide) (PLGA), collagen (COL), chitosan (CS) and HAS salt. COL has been extensively used for scaffolds due to its biocompatibility, nontoxicity and biodegradability [Citation8,Citation9]. However, COL is often used in combination with other materials to avoid fast degradation and poor physical performance. CS is a kind of biodegradable material which is widely and easily obtained and has good biocompatibility [Citation10]. In addition, CS can be used as cartilage repair material due to its non-toxicity, non-irritability, biocompatibility, biodegradability and other excellent properties [Citation7]. HAS is the main component of human stromal cells, synovial fluid and other connective tissues. HAS can promote cell proliferation and differentiation, wound healing, osmotic pressure regulation, and aids in water absorption; it also possesses a lubricating property [Citation11,Citation12]. Moreover, HAS possesses an affinity to chondrocytes used in biomaterial science for scaffold preparation [Citation11–14].
COL and CS composites have been studied with interesting results [Citation15]. COL and CS mixture with the addition of hyaluronic acid (HA) was obtained as a film [Citation16,Citation17]. Moreover, composites of COL/HA/CS were obtained as 3D porous structures [Citation12,Citation18]. Therefore, we combine the advantages of natural COL, CS, HAS and KGN to fabricate a 3D composite scaffold containing PLGA MPs for controlled KGN delivery. This composite scaffold can be used as a treatment for mild OA and cartilage defect repair because it can promote cartilage differentiation by releasing the loaded active KGN factor. Simultaneously, HAS degradation is enhanced to alleviate and treat OA.
Materials and methods
Materials
Bovine COL was supplied by Bote Biotech, Co., Ltd. (Fuzhou, China). Bovine serum albumin (96%), CS (≥95% degree of deacetylation, 100–200 mPa·s viscosity), poly (vinyl alcohol) (PVA) 1788 low-viscosity, and HAS (95%, Mw 403.31) were purchased from the Shanghai Macklin Biochemical Co., Ltd. (Shanghai, China). PLGA (lactide to glycolide ratio of 75:25, Mw 38–54 kDa) and KGN (≥98%) were obtained from the Aladdin Industrial Corporation (Shanghai, China). CCK-8 Kit was purchased from BestBio (Shanghai, China). N-(3-Dimethylaminopropyl)-N′-ethylcarbodiimide hydrochloride (EDC, ≥99.0%) was purchased from the Sinopharm Chemical Reagent Co., Ltd. (Shanghai, China). N-Hydroxysuccinimide (NHS, ≥98.0%) was purchased from the Tokyo Chemical industry Co., Ltd. (Tokyo, Japan). All other chemical reagents were of analytical grade and obtained from commercial sources.
Optimal ratio analysis of porous scaffolds
Fabrication of porous scaffolds
Porous scaffolds were prepared using COL, CS and HAS solution. COL and CS solutions were first thoroughly mixed on a vortex machine. Then, HAS solution was slowly added to the eddied solution to obtain an ultimate solution [Citation5]. Suspensions in different volumetric ratios were prepared for further experiment ().
Table 1. Design of scaffolds with different volumetric ratios.
The above suspension was poured into a 24-well plate and precooled 2 h at –20 °C and then frozen at –80 °C overnight. Porous scaffolds were obtained by freeze drying the samples until completely dry. The formed relatively strong and stable porous scaffolds were selected for further processing and cell culture. Then, the freeze-dried scaffolds were cross-linked by immersing in 90% (v/v) ethanol solution containing 50 mmol/L EDC and 20 mmol/L NHS. After reaction at room temperature for 8 h, the scaffolds were washed every 30 min at least six times and then freeze-dried again.
According to the COL/HAS ratio shown in , the samples are respectively labelled as COL/CS/HAS, 0.5COL/CS/HAS, 0.1COL/CS/HAS, COL/CS/0.5HAS and COL/CS/0.1HAS.
Test of swelling
The dry samples of the same size weighted W1 were immersed in phosphate-buffer saline (PBS, pH =7.4) at 37 °C for 24 h. The samples were taken out, and the wet samples were placed between two sheets of paper to remove surface water and weighted W0. The scaffold swelling was calculated as follows:
Test of dissolve–loss ratio
The samples (M0) were immersed in distilled water at 37 °C for 24 h. The dried samples in the oven were weighted (M1). The dissolve–loss ratio of the scaffolds was calculated as follows:
Test of porosity
The porosity of scaffolds was calculated through ethanol infiltration method [Citation9,Citation19]. The samples (M0) of equal size were placed in a few equally sized specific gravity bottles filled with ethanol (M1) at room temperature for 5 min. The samples were removed until no gas bubbles existed in the vacuum drying chamber. Then, the specific gravity bottles were rapidly filled with ethanol and reweighted (M2). After immediately removing the sample, the remaining ethanol and the bottles were weighted (M3). The porosity of scaffolds was calculated as follows:
Test of mechanical property
The dry samples were cut into cuboids. The mechanical property of these samples was examined by universal material testing machine (Instron, Wycombe, UK). During the test, the ends of the samples were clamped using a special fixture and were applied to the static tensile load. The beam velocity was 1 mm/min. The maximum tensile stress histogram was drawn with the data obtained.
Cell culture
BMSCs were isolated from the bone marrow of mouse tibias and femurs based on the selective adherence of MSC to plastic surfaces [Citation20]. BMSCs were incubated in DMEM/F-12 supplemented with 10% foetal bovine serum at 37 °C and 5% CO2 in the air. After three generations, the BMSCs were used for experiments.
Cytotoxicity studies
Cell viability and proliferation on scaffolds were assessed by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) staining [Citation21]. The scaffolds were sterilized by γ-rays and put into sterile 24-well plates. A 1 mL BMSC suspension was seeded in the scaffolds at a cell density of 1 × 104 cells/mL and then incubated for 5 h. Scaffolds cultured with BMSCs were transferred to new culture plates to remove non-adherent cells after 8 h. The culture medium was changed once at an interval of two days. BMSCs in scaffolds were incubated for 1, 3, 5 and 7 days. Finally, the medium was removed, and the proliferation was evaluated by the MTT method as described above.
Preparation of PLGA MPs
PLGA MPs were prepared using double emulsion-solvent evaporation method as previously described [Citation22,Citation23]. A 3 mL dichloromethane containing PLGA (3%, w/v) was poured into a 2 mL mixed solution of distilled water and methanol (v/v = 1:1) containing KGN (0.15%, w/v). In addition, the primary emulsion was generated by a high-speed homogenizer (IKA T25 digital, Staufen, Germany) operating at 12,000 rpm for 10 min. Then, the resultant emulsion was added to 50 mL 1% (w/v) PVA solution containing polysorbas 20 (1%, w/v) and homogenized at 12,000 rpm for another 3 min to solidify MPs. After stirring at room temperature for 4 h, MPs were washed five times with distilled water and freeze-dried for collection.
The mean diameters of the MPs were analysed by SEM image using Nano Measurer 1.2 software. The zeta potential was determined by dynamic laser light scattering (Malvern-ZETASIZER nano, Malvern, UK).
Fabrication of scaffolds/MPs
A total of 1 mg MPs dispersed in distilled water was injected into several inner portions of each scaffold and was lyophilized to obtain scaffolds/MPs [Citation9,Citation24]. The samples are respectively labelled COL/CS/HAS/MPs, 0.5COL/CS/HAS/MPs, 0.1COL/CS/HAS/MPs, COL/CS/0.5HAS/MPs and COL/CS/0.1HAS/MPs.
Fourier transform infrared spectrometer (FTIR)
The freeze-dried and pressed composite scaffolds were assessed to identify their chemical structure by using FTIR (Spectrum BX, Perkin Elmer, Waltham, MA).
Scanning electron microscopy (SEM)
The morphology of PLGA MPs containing KGN, porous scaffolds and scaffolds/MPs was characterized by SEM (Philips-FEI XL30 ESEM-TMP, Eindhoven, Netherlands). Other samples were frozen in liquid nitrogen for 2 min, except for MPs. Longitudinal section of the frozen sample was obtained with a scalpel. Then, the samples sprayed with gold were characterized by SEM.
Acridine orange (AO)/ethidium bromide (EB) staining
AO/EB double staining was conducted to detect the cell viability of BMSCs in the extraction solution [Citation25]. BMSC suspension was seeded in the leach liquor of scaffolds/MPs at a cell density of 1 × 104 cells/mL and then respectively incubated for 3, 5 and 7 days. At a predefined time, the medium was removed, and the plates were washed with PBS three times. The leach liquor containing BMSCs was stained by 100 μg/mL AO and 100 μg/mL EB for 10 min and kept away from light. The plates were washed with PBS for another three times. Finally, the pictures were obtained by an inverted microscope.
In accordance with the provisions of GB/T16175-2008 [Citation26], sterile scaffolds and scaffolds/MPs were soaked in DMEM/F-12 medium for 4 h, and the medium was then removed. BMSC suspension was seeded in the scaffolds and scaffolds/MPs at a cell density of 1 × 104 cells/mL and was then incubated for 3, 5 and 7 days. The next steps were the same as above.
Hoechst 33342 staining
Hoechst 33342 staining was exactly the same with AO/EB double staining method on scaffolds and scaffolds/MPs described above.
In vitro release profiles
First, the concentration of KGN gradient curve was drawn. Then, each scaffold/MP was incubated with 2 mL of PBS (pH 7.4) in a shaking bath (100 rpm) at 37 °C for 21 days. At a designated time point, the supernatant was collected, and an equal amount of fresh medium was added to each sample. The amount of proteins in releasing media was determined at 278.4 nm by ultraviolet–visible spectrophotometry.
BMSC proliferation and adhesion on the scaffolds and scaffolds/MPs
Cell proliferation and adhesion on the scaffolds and scaffolds/MPs were assessed by the CCK-8 kit. The scaffolds/MPs were more favourable for cell proliferation and adhesion than scaffolds.
The cells were seeded per sample and incubated for 24 h to evaluate cell adhesion on the samples. Then, the cell-scaffold constructs were washed with PBS three times and immobilized with 2.5% glutaraldehyde (v/v) at 4 °C. After thorough washing with PBS, the samples were dehydrated in a series of ethanol (50%, 75%, 95% and 100%). The freeze-dried gold-sputtered samples under vacuum were observed.
Statistical analysis
All experiment data were expressed as means and standard deviations and statistically analysed by one-way analysis of variance. The level of significance was defined at p < .05.
Results
Optimal ratio of COL/CS/HAS porous scaffolds
The water retention capability of a scaffold is an important property to evaluate the efficacy of biomedical materials. In this study, the swelling rate of all porous scaffolds of different proportions ranges 450–750%, and the scaffolds could maintain their morphological stability after swelling (). HAS is a hydrophilic biopolymer and can form hydrogen bonds with COL and CS. The swelling ratio increased as the proportion of HAS decreased, which increased as COL content increased possibly due to many hydrophilic amino acids in COL [Citation21]. However, the swelling ratio could be improved while the proportion of COL was half that of CS and HAS (p < .05).
Figure 1. Swelling (A), dissolve–loss tests (B), porosity (C), mechanical property (D) and cell proliferation (E) of different proportions of composite scaffolds. The values are the means and standard deviations (n = 3, *p < .05, **p < .01, ***p < .001).
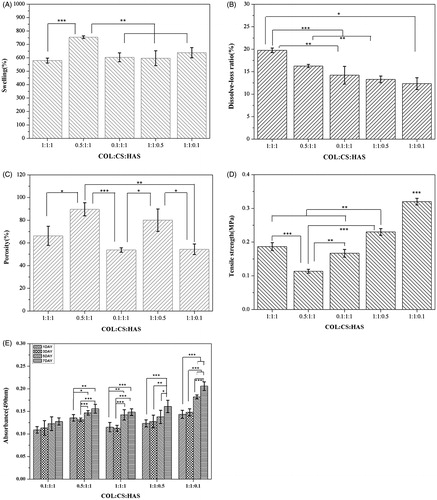
shows the dissolve–loss ratio of porous scaffolds in different proportions. The dissolve–loss ratio increased as the proportion of COL increased possibly due to many hydrophilic amino acids in COL. Moreover, the dissolve–loss ratio of porous scaffolds decreased as the proportion of HAS decreased possibly due to the production of more hydrogen bonds.
shows the porosity of scaffolds in different proportions. COL/CS/HAS, 0.1COL/CS/HAS and COL/CS/0.1HAS ranged from 50 to 70%. 0.5COL/CS/HAS and COL/CS/0.5HAS ranged from 80 to 90%. The interconnected porous structure can transport nutrients and metabolic waste; thus, this structure is beneficial for adhesion, growth, and proliferation of seed cells in scaffolds.
From , the tensile strength could be worse while the proportion of COL was half that of CS and HAS. The tensile strength increased as the proportion of HAS increased because HAS improves the elasticity of the sample [Citation12]. The increase in tensile strength was beneficial to the structural and morphological stabilities of the composite scaffolds compared with the pure COL and HAS. COL/CS/0.1HAS showed extremely significant difference (p < .05).
Cell proliferation, adhesion, differentiation, and other functions can be affected by the nature of the scaffolds. Thus, the properties of scaffolds have a considerable effect on their biocompatibility. shows that BMSCs can colonize the scaffolds. The number of BMSCs gradually increased when the incubation time of cells increased from 1 to 7 days, which demonstrated that the cells proliferated well on scaffolds throughout the entire culture process. Moreover, the absorbance (490 nm) of the COL/CS/0.1HAS was higher than others, which considerably increased after three days (p < .05). The results suggested that the COL/CS/0.1HAS had a more favourable capability to promote cell proliferation than the other scaffolds.
In summary, the optimal porous scaffolds are COL/CS/0.1HAS.
Characterization of MPs
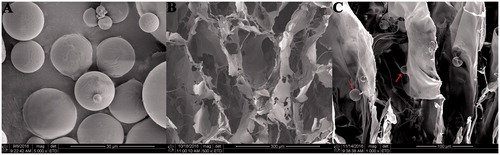
The PLGA MPs loaded with KGN were spherical with smooth external surfaces and displayed different sizes (). All MPs showed that the patterned surface was good for cell adhesion [Citation27]. The mean diameter increased from 1.86 to 121.2 μm with the decrease of the homogenized speed of multiple emulsions due to the decrease in shear force [Citation9,Citation28]. Normally, the drug loading content would increase with the size increase because the drug diffusion of MPs with large surface area-to-volume ratio would be augmented during the solidification procedure [Citation9,Citation29]. The loading content of KGN was measured at 278.4 nm by UV spectrophotometry and was calculated for the standard curve. The encapsulation efficiency and drug loading content of PLGA MPs were (20.67 ± 0.33)% and (0.98 ± 0.21)%, respectively. The zeta potentials of PLGA MPs ranged –1.13 mV to –1.73 mV in distilled water. The MPs have a negative surface charge due to the ionization of carboxyl groups of PLGA and the adsorption of anions from the PVA [Citation30]. From the zeta potentials, the MPs are unstable and easy to agglomerate, but they have no effect on the KGN release.
SEM of porous scaffolds and scaffolds incorporated with MPs
shows the morphology of COL/CS/0.1HAS scaffolds and scaffolds/MPs. The scaffolds exhibited an interconnected porous structure, and the pore size ranged 100–150 μm, which met the standards of scaffolds. shows that MPs were attached to the surface of the scaffolds, and the incorporation of MPs had no effect on the porous structure of scaffolds. In addition, the rough surface of the scaffold was good for cell adhesion.
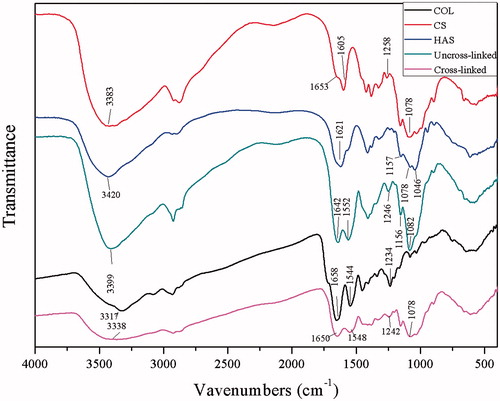
FTIR spectroscopy of the composite
The FTIR spectra of COL and CS had common characteristic peaks of amide A (3317/3383 cm−1), amide I (1658/1653 cm−1), amide II (1544/1605 cm−1) and amide III (1234/1258 cm−1) bonds. In addition, the peak of C–O–C (1078 cm−1) from CS was found (). Moreover, HAS appeared as a new peak at 1157 cm−1 in IR spectra. The FTIR spectra of COL/CS/0.1HAS depicted characteristic absorption bands at 1650 cm−1, 1548 cm−1 and 1242 cm−1 from amide I, II and III bonds, respectively. A peak at 1078 cm−1 is typical for C–O–C moiety in polysaccharides. After the addition of HAS, a new peak at 1156 cm−1 appeared from the glucosidic ring vibration of HAS [Citation31]. Amide A has shifted from 3420 to 3317 cm−1. Amide I has shifted from 1658 cm−1 to 1652 cm−1, and amide II has shifted from 1605 cm−1 to 1544 cm−1. Usually, the shift of bands in the FTIR spectra is due to interactions between polymers or biopolymers by hydrogen bonds or electrostatic interactions [Citation12]. Thus, those hydrogen bonds or electrostatic interactions were also important to enhance the mechanical property of the composite scaffolds. The cross-linked materials did not appear to be a new peak compared with the uncross-linked. Most of the molecular chains can be β-pleated sheet after the cross-linking process. The cross-linking agent only plays the role of intermediate agents, and it will be cleared out in the late stage.
Biological properties
AO/EB double staining
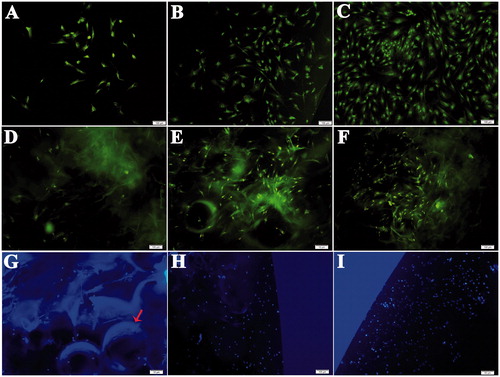
BMSC proliferation in the leach liquor of sterile scaffolds/MPs and sterile scaffolds/MPs was determined through AO/EB double staining. These cells were cultured for 3, 5 and 7 days to demonstrate the biocompatibility of the porous scaffolds and scaffolds/MPs. Viable cells were stained only by AO and exhibited a bright green intact structure. Apoptotic cells were stained by AO and EB and appeared red orange with condensed chromatin. Staining results of BMSCs in the leach liquor of scaffolds/MPs () and in scaffolds/MPs () further revealed that BMSCs were bright green, and the integrity of the cell nuclei was maintained. The cells were also reproduced as culture duration was prolonged. This finding was consistent with cell proliferation results obtained through MTT assay.
Figure 4. BMSCs were stained with AO/EB and Hoechst 33342. BMSCs in the leach liquor of scaffolds/MPs were stained with AO/EB cultured at (A) 3 days, (B) 5 days and (C) 7 days. BMSCs in scaffolds/MPs were stained with AO/EB and cultured at (D) 3 days, (E) 5 days and (F) 7 days. BMSCs in scaffolds/MPs were stained with Hoechst 33342 and cultured at (G) 3 days, (H) 5 days and (I) 7 days. The arrow indicates PLGA MPs (bar =100 μm).
Hoechst 33342 staining
The normal nucleus was complete, the fluorescent staining was uniform, and the nuclei of apoptotic cells were strongly stained with Hoechst 33342 staining [Citation32]. As shown in , when cells in scaffolds/MPs were stained, the fluorescence of BMSCs was relatively weak due to the insufficient transparency of the composite; however, the nucleus was spherical, and the fluorescence was uniform. The cells not only showed a good 3D state but the number of cells also significantly increased with uniform distribution. The results showed that the composite loaded with KGN had good biocompatibility and was favourable for BMSC growth.
Cell adhesion and proliferation on scaffolds/MPs
shows BMSC proliferation on the scaffolds with or without MPs. The number of BMSCs gradually increased when the incubation time of cells increased from 1 to 7 days, which demonstrated that the cells proliferated well throughout the entire culture period on scaffolds. The absorbance was nearly equal because the cells need an adaptation period within three days compared with the 1 and 3 days. Moreover, the absorbance of scaffolds with MPs was higher than the scaffolds without MPs at the same incubation time. BMSC proliferation considerably increased after three days. The results suggested that the scaffolds/MPs had a more favourable capability to promote cell proliferation than the scaffolds without MPs. Therefore, KGN-loaded MPs played an important role in proliferation.
Figure 5. Cell proliferation (A) in the scaffolds and scaffolds/MPs was assessed by the CCK-8 kit. Cell adhesion (B,C) on the scaffolds/MPs was assessed by SEM. The right arrow indicates MPs, and the left arrow indicates BMSC. The values are the means and standard deviations (n = 6, **p < .01, ***p < .001).
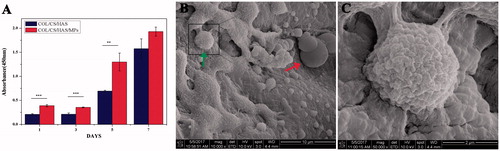
The cell attachment capability of scaffolds/MPs was evaluated by SEM (). BMSCs with rough morphology were attached and tiled on the surface and within the scaffolds/MPs. The results revealed that the scaffolds/MPs could promote cell adhesion, which could be beneficial for tissue integration after in vivo implantation.
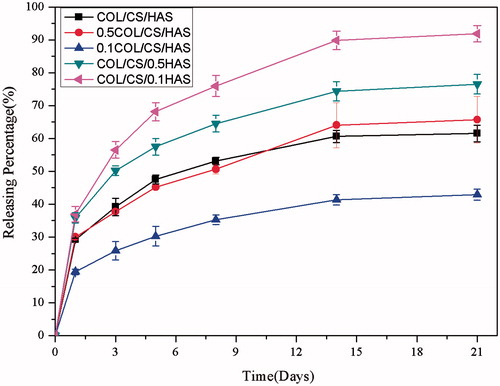
In vitro controlled-release properties of KGN
The release of KGN presented a triphasic release manner, which comprised a typical burst release within the first 12 h, followed by a period of rapid release rates from days 3 to 14, and finally sustained and controlled release after these days. Ordinarily, drug release from scaffolds/MPs is mainly caused by swelling, degradation and diffusion [Citation24]. The burst release in the initial stage could be attributed to scaffold swelling and KGN diffusion. The MPs distributed on the interior surface of scaffold diffused through the exoteric microporous structure due to the rapid swelling of scaffold, and the KGN on the surface of MPs quickly diffused due to the high drug concentration gradient [Citation33]. After three days, the degradation of scaffold had markedly increased, leading to the increase of MP diffusion from the scaffold and the release of KGN. Once degradation of scaffolds and diffusion of MPs from scaffolds were completed, the release kinetics was mainly controlled by the degradation of MPs, resulting in the sustained KGN release [Citation34]. Moreover, the burst release from scaffold/MPs was from 20% to 36% (). The cumulative release of KGN from the scaffold/MPs was evidently lower than that from the MPs alone with the same size, implying that the scaffolds/MPs had a double-sustained effect on protein release [Citation35]. Compared with the swelling and degradation results of scaffolds/MPs, the release profiles of KGN were found to be nearly in accordance with the mass loss of scaffolds [Citation36,Citation37]. In this study, the release rate of KGN from scaffolds/MPs increased with the decrease of HAS due to the decreased degradation of the scaffolds. Generally, a high degradation rate and swelling ratio of scaffold could lead to a high diffusion rate of the MPs from scaffold, resulting in an increase in the drug release from the MPs. In addition, the decrease of KGN release rate from the scaffolds/MPs was observed when the size of MPs increased [Citation9]. Therefore, the optimal ratio of COL to HAS in the scaffolds/MPs and the release rate of proteins could be adjusted by the size of MPs based on the mechanical properties, porosity, swelling, degradation and release profiles. The controlled and sustained release of proteins from scaffolds/MPs was observed without severe initial burst over 21 days, implying the potential to be used as controlled KGN release system in CTE.
Discussion
In this study, a COL/CS/0.1HAS/MP scaffold with excellent biocompatibility was successfully developed as a carrier of KGN to treat early- and middle-staged OA by considering three steps. (1) The optimal ratio of COL/CS/HAS composite was 1:1:0.1 based on the properties of COL/CS/HAS scaffold. (2) The results of in vitro biocompatibility and AO/EB, as well as Hoechst 33342 fluorescence staining of BMSCs, revealed that the scaffolds with optimal ratio could significantly enhance cell proliferation. Scaffolds/MPs obviously enhanced BMSC proliferation. (3) The KGN-controlled release could reach 94%. Therefore, the composite could effectively promote the formation of normally functional cartilage, and KOA would be effectively treated.
Inducing cartilage regeneration is a difficult process [Citation38,Citation39]. In this study, we combined the advantages of natural COL, CS and HAS to fabricate a 3D composite scaffold containing KGN-loaded PLGA MPs for controlled KGN delivery. COL/CS/HAS composite scaffolds induced BMSC proliferation, and KGN simultaneously promoted BMSC differentiation. Interestingly, the number of cells in COL/CS/0.1HAS composite scaffolds was the highest among all ratios after in vitro biocompatibility test was performed for seven days. This result may be attributed to COL/CS/0.1HAS composite scaffolds with appropriate pore structure, which can optimally promote cell adherence and proliferation. AO/EB and Hoechst 33342 fluorescence staining results revealed that KGN enhanced cellular growth behaviour, and nearly no apoptotic cells were present. Therefore, this composite scaffold can be used for mild OA treatment and cartilage defect repair because it promotes cartilage differentiation by releasing the active KGN factor. Simultaneously, HAS degradation is enhanced to alleviate and treat OA. Ultimately, the proposed treatment can be applied in cartilage repair engineering.
Conclusions
We embedded KGN-incorporated PLGA MPs in COL/CS/HAS porous scaffolds to fabricate an excellent biomaterial for cartilage repair and ultimately treat OA. The optimal ratio of COL/CS/HAS porous scaffolds (COL/CS/0.1HAS) was determined according to the porosity, swelling, loss rate in hot water, mechanical property, and MTT. SEM images of scaffolds/MPs revealed a porous microstructure with an interpenetrating network. BMSC proliferation assay, AO/EB and Hoechst 33342 fluorescence staining showed that the composite did not cause cytotoxicity. COL/CS/0.1HAS scaffolds had the maximum amount of KGN release, and KGN could be controlled release. Fluorescence staining and BMSC proliferation showed that the proposed scaffolds could be used to repair articular cartilage defects and induce articular cartilage regeneration. Importantly, KGN-COL/CS/0.1HAS scaffolds enhanced BMSC proliferation more obviously compared with COL/CS/0.1HAS scaffolds. Therefore, the prepared KGN-COL/CS/HAS porous scaffolds may be applied to repair cartilage defects, which may be effective in KOA treatment.
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 81501576) and the Natural Science Foundation of Fujian Province (2015J01341, 2017J01482).
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Li X, Ding J, Wang J, et al. Biomimetic biphasic scaffolds for osteochondral defect repair. Regen Biomater. 2015;2:221–228.
- Li X, Ding J, Zhang Z, et al. Kartogenin-incorporated thermogel supports stem cells for significant cartilage regeneration. ACS Appl Mater Interfaces. 2016;8:5148–5159.
- Mardones R, Jofre CM, Minguell JJ. Cell therapy and tissue engineering approaches for cartilage repair and/or regeneration. IJSC. 2015;8:48–53.
- Ode ADG, Glaeser JD, Matziolis G, et al. Toward biomimetic materials in bone regeneration: functional behavior of mesenchymal stem cells on a broad spectrum of extracellular matrix components. J Biomed Mater Res. 2010;95:1114–1124.
- Mathews S, Bhonde R, Gupta PK, et al. Novel biomimetic tripolymer scaffolds consisting of chitosan, collagen type 1, and hyaluronic acid for bone marrow-derived human mesenchymal stem cells-based bone tissue engineering. J Biomed Mater Res B Appl Biomater. 2014;102:1825–1834.
- Johnson K, Zhu S, Tremblay MS, et al. A stem cell based approach to cartilage repair. Science. 2012;336:717–721.
- Kang ML, Ko JY, Kim JE, et al. Intra-articular delivery of kartogenin-conjugated chitosan nano/microparticles for cartilage regeneration. Biomaterials. 2014;35:9984–9994.
- Huang S, Fu X. Naturally derived materials-based cell and drug delivery systems in skin regeneration. J Control Release. 2010;142:149–159.
- Cao H, Chen MM, Liu Y, et al. Fish collagen-based scaffold containing PLGA microspheres for controlled growth factor delivery in skin tissue engineering. Colloids Surf B Biointerfaces. 2015;136:1098–1106.
- Liu BJ, Ma LN, Su J, et al. Biocompatibility assessment of porous chitosan-Nafion and chitosan-PTFE composites in vivo. J Biomed Mater Res A. 2014;102:2055–2060.
- Khvatova GI, Semeikin AV. Molecular-biological problems of drug design and mechanism of drug action. Pharm Chem J. 2011;44:651–653.
- Sionkowska A, Kaczmarek B, Lewandowska K, et al. 3D composites based on the blends of chitosan and collagen with the addition of hyaluronic acid. Int J Biol Macromol. 2016;89:442–448.
- Freed LE, Marquis JC, Langer R, et al. Composition of cell–polymer cartilage implants. Biotechnol Bioeng. 1994;43:605–614.
- Kawasaki K, Ochi M, Uchio Y, et al. Hyaluronic acid enhances proliferation and chondroitin sulfate synthesis in cultured chondrocytes embedded in collagen gels. J Cell Physiol. 1999;179:142–148.
- Sionkowska A, Kaczmarek B, Lewandowska K. Modification of collagen and chitosan mixtures by the addition of tannic acid. J Mol Liquids. 2014;199:318–323.
- Wu Y, Hu Y, Cai J, et al. Coagulation property of hyaluronic acid-collagen/chitosan complex film. J Mater Sci Mater Med. 2008;19:3621–3629.
- Yamada S, Yamamoto K, Ikeda T, et al. Potency of fish collagen as a scaffold for regenerative medicine. BioMed Res Int. 2014;2014:302932.
- Yan J, Li X, Liu L, et al. Potential use of collagen-chitosan-hyaluronan tri-copolymer scaffold for cartilage tissue engineering. Artif Cells Blood Subst Biotechnol. 2006;34:27–39.
- Safandowska M, Pietrucha K. Effect of fish collagen modification on its thermal and rheological properties. Int J Biol Macromol. 2013;53:32–37.
- Li J, Zhao Z, Liu J, et al. MEK/ERK and p38 MAPK regulate chondrogenesis of rat bone marrow mesenchymal stem cells through delicate interaction with TGF-beta1/Smads pathway. Cell Prolif. 2010;43:333–343.
- Wang J, Yang Q, Cheng N, et al. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater Sci Eng C Mater Biol Appl. 2016;61:705–711.
- Song K, Liu Y, Macedo HM, et al. Fabrication and evaluation of a sustained-release chitosan-based scaffold embedded with PLGA microspheres. Mater Sci Eng: C. 2013;33:1506–1513.
- Hafezi Ghahestani Z, Alebooye Langroodi F, Mokhtarzadeh A, et al. Evaluation of anti-cancer activity of PLGA nanoparticles containing crocetin. Artif Cells Nanomed Biotechnol. 2017;45:955–960.
- Liu HF, Fan HB, Cui YL, et al. Effects of the controlled-released basic fibroblast growth factor from chitosan-gelatin microspheres on human fibroblasts cultured on a chitosan-gelatin scaffold. Biomacromolecules. 2007;8:1446–1455.
- Wang ML, Lu CH, Xu QY, et al. Four new citrinin derivatives from a marine-derived Penicillium sp fungal strain. Molecules. 2013;18:5723–5735.
- International Organization for Standardization. Biological evaluation of medical devices - Part 5: Tests for in vitro cytotoxicity (ISO 10993-5). Geneva: International Organization for Standardization; 2009.
- Kim T, Yoon J, Lee D, et al. Gas foamed open porous biodegradable polymeric microspheres. Biomaterials. 2006;27:152–159.
- Kuo CF, Tsao N, Chou HH, et al. Release of FITC-BSA from poly(l-lactic acid) microspheres analysis using flow cytometry. Colloids Surf B Biointerfaces. 2012;89:271–276.
- Wu J, Kong T, Yeung KW, et al. Fabrication and characterization of monodisperse PLGA-alginate core–shell microspheres with monodisperse size and homogeneous shells for controlled drug release. Acta Biomater. 2013;9:7410–7419.
- Andreas K, Zehbe R, Kazubek M, et al. Biodegradable insulin-loaded PLGA microspheres fabricated by three different emulsification techniques: investigation for cartilage tissue engineering. Acta Biomater. 2011;7:1485–1495.
- Sionkowska A, Wisniewski M, Skopinska J, et al. Thermal and mechanical properties of UV irradiated collagen/chitosan thin films. Polym Degrad Stab. 2006;91:3026–3032.
- Sawicki W, Moskalewski S. Hoechst 33342 staining coupled with conventional histological technique. Stain Technol. 1989;64:191–196.
- Kim TH, Lee H, Park TG. Pegylated recombinant human epidermal growth factor (rhEGF) for sustained release from biodegradable PLGA microspheres. Biomaterials. 2002;23:2311–2317.
- Mollica F, Biondi M, Muzzi S, et al. Mathematical modelling of the evolution of protein distribution within single PLGA microspheres: prediction of local concentration profiles and release kinetics. J Mater Sci: Mater Med. 2008;19:1587–1593.
- Chen MM, Huang YQ, Cao H, et al. Collagen/chitosan film containing biotinylated glycol chitosan nanoparticles for localized drug delivery. Colloids Surf B-Biointerfaces. 2015;128:339–346.
- Niu XF, Feng QL, Wang MB, et al. Porous nano-HA/collagen/PLLA scaffold containing chitosan microspheres for controlled delivery of synthetic peptide derived from BMP-2. J Control Release. 2009;134:111–117.
- Ungaro F, Biondi M, d'Angelo I, et al. Microsphere-integrated collagen scaffolds for tissue engineering: effect of microsphere formulation and scaffold properties on protein release kinetics. J Control Release. 2006;113:128–136.
- Mondal S, Haridas N, Letha SS, et al. Development of injectable high molecular weight hyaluronic acid hydrogels for cartilage regeneration. J Macromol Sci A. 2016;53:507–514.
- Deng T, Huang S, Zhou S, et al. Cartilage regeneration using a novel gelatin–chondroitin–hyaluronan hybrid scaffold containing bFGF-impregnated microspheres. J Microencapsul. 2007;24:163–174.