Abstract
Purpose: Tumour seriously affects people's quality of life. Colorectal cancer is a refractory tumour in digestive tract tumors. In colorectal cancer, gene expression abnormalities is the main reason for its incidence, we mainly focus on the molecular mechanism of MTAP in the development of colorectal cancer.
Methods: The tumour tissue and its adjacent tissue samples of 50 patients with colorectal cancer were screened from July 2011 to February 2015, and the expression of MTAP was detected. Cell lines that overexpress MTAP and low expression of MTAP were constructed in colorectal cancer cell lines. The cell proliferation, invasion and migration was detected in the cells with different expression levels of MTAP. Immunohistochemistry was used to detect the expression of MTAP in liver metastasis and to investigate its clinical significance. And statistics of clinical significance.
Results: Q-PCR results showed that the expression of MTAP in colorectal cancer cell lines were significantly higher than that normal human colonic myofibroblasts cell line. Cell proliferation test results showed that cell proliferation was accelerated when MTAP was overexpression, cell invasion and migration were simultaneously accelerated. The expression of MTAP in primary liver was positively correlated with metastatic disease in patients with liver metastatic colorectal cancer via EMT.
Conclusions: MTAP accelerates the growth and metastasis of colorectal cancer through EMT.
Introduction
Colorectal cancer (CRC) is the third most malignant cancer worldwide [Citation1]. CRC is among the top 5 leading malignant cancer in terms of both morbidity and mortality in China [Citation2]. Specifically, approximately 20% of patients with CRC already present with distant metastases at the time of initial diagnosis, and only 10–30% of patients with distant metastases are eligible for surgical resection for the treatment of both primary and metastatic tumour lesions [Citation3–5]. The development of colorectal cancer is a complex process involving a range of physiological changes, including apoptosis, proliferation, metastasis and invasion and abnormalities. Clinical manifestations of colorectal cancer, such as anorexia, weight loss, perforation and so on.
Under normal physiological conditions, intracellular acetylation and deacetylation are in a dynamic equilibrium. However, MTAP activity is abnormal in a variety of tumours, it is recruited to bind in a specific promoter region, inhibiting the transcription of a series of genes involved in the genes related with cell proliferation, differentiation, migration, invasion and metastasis [Citation6–8]. 5-Methylthioadenosine phosphorylase (MTAP), a housekeeping gene in normal cell, is a key enzyme in the purine and methionine synthesis remediation pathways. Many studies have confirmed that in a variety of tumours, this gene often occurs selective deletion. The MTAP gene is localized to human chromosome 6, which inhibits the activity of Cyclin–CDK complex or cell proliferating antigen. In 1996, Taunton et al found MTAP, a gene that is most closely related to tumor, which can act on the corresponding histone and regulate gene transcription [Citation9–11]. Another study confirmed that the absence of MTAP can block tumour cell cycle in G1 or G2/M phase, leading to loss of mitotic cells, inhibition of cell growth and the increasing of the cells apoptosis. It is shown that the expression of MTAP is closely related to tumour cell cycle, cell proliferation and apoptosis [Citation12–14]. When the level of MTAP increased, it will prevent the combination of CyclinD1 and CDK4, the cell cycle arrested in the G1 phase, inhibition of cell proliferation. The study also confirmed that MTAP, a cell-cycle negative regulator, is associated with hyperplasia and metastasis of renal carcinoma cells and has a good prognostic value [Citation15,Citation16]. However, there are few studies on the mechanism of MTAP gene in colorectal cancer.
However, the expression of MTAP in colorectal cancer and its mechanism are need for further study, from our hospital in July 2011 – February 2015 Department of Oncology 92 cases of colorectal cancer patients as a research object to explore the MTAP in the Mechanism of action in colorectal. The tissue of 92 patients with colorectal cancer from July 2011 to February 2015 was selected as the study object, and it is expected to explore the mechanism of MTAP in colorectal cancer.
Experimental section
Clinical information
The tumour tissue and paracancerous tissue of 50 patients with colorectal cancer from July 2011 to February 2015 were selected as the study object. All patients had complete clinical data, including general condition, pathologic diagnosis, tumour staging, treatment and survival status and more complete follow-up data. All types of clinical data were well preserved. All studies were approved by the Hospital Ethics Committee.
Cell lines
Human colorectal cell lines (SW480, HT-29 and LS-174 T) and normal human colonic myofibroblasts cell line (CCD-18co) were purchased from the American Type Culture Collection (ATCC, Manassas, VA) and maintained in Dulbecco’s modified Eagle’s medium (DMEM, Hyclone, GE Health, Logan, UT, USA) supplemented with 10% foetal bovine serum (FBS; Hyclone, GE Health, Logan, UT, USA), 2.0 mM L-glutamine, 100 U/mL penicillin and 100 μg/mL streptomycin, and incubated at 37 °C in a humidified incubator supplemented with 5% CO2 and 95% air.
Q-PCR
The Q-PCR test is carried out according to the following three steps: (1) Trizol method to extract RNA, the A260/280 value should be between 1.8–2.0; (2) reverse transcription, Reverse transcription was performed using the SuperScript VILO cDNA Synthesis Kit (Thermo Fisher Scientific, USA) according to the instructions; (3) PCR, PCR using Master Mix premix and primers (Superarray, USA). Quantitative PCR was performed in a system at 25 μL. The Primer sequences are:
MTAP: F- TACGGUCAUGUCCAAAGUTAT; R- ATUGUUGGACAUGACCGGAAC
GAPDH: F- GCCCTGAGGGCCCGAACTGTTACT; R- CAGACGCACGGCTTTGACCTTCTT.
Stable transfection of overexpression and low expression of MTAP in SW480 cell lines
Cells were transfected with high-expressed and low-expressed MTAP plasmids (Guangzhou Ruibo, China). 48 h later, RNA was extracted for detection. Overexpressed and low expressed MTAP plasmids and MTAP sequences were synthesized in Guangzhou Ruibo Biotechnology Company, and sequence of the control group was unintentionally replaced.
Western blotting
Tissue protein was extracted using Pittiurin Protein Kit. The extracted proteins were separated by SDS-PAGE electrophoresis. The protein on the gel after electrophoresis was transferred to the PVDF membrane (EMS Millipore, Mendota Heights, MN, USA), followed by blocking the unadsorbed protein region with skimmed milk. The PVDF membrane with the protein band was sequentially labeled with the specific antibody and Alexa Fluor-conjugated secondary antibodies. The Odyssey infrared imaging system (Li-Cor, Lincoln, NE, USA) was used for detection.
MTT cell assay
Cells were seeded in 96-well plates at density of 2 × 103 cells/well. At each time point, cells were stained with 100 μL sterile MTT dye (0.5 mg/mL, Sigma, Santa clara, CA, USA) for 4 h at 37 °C, followed by removal of the culture medium and addition of 100 μL of dimethyl sulphoxide (Sigma, Santa clara, CA, USA). The absorbance was measured at 570 nm, with 655 nm as the reference wavelength. Each experiment was performed in triplicates.
Migration and invasion assays
A 24-well plate containing 8-mm-pore size chamber inserts (Corning, Corning, NY, USA) was used to evaluate the migration and invasion of tumour cells. For the migration assay, 10 × 104 cells were seeded in the upper chamber. For the invasion assay, the membrane was coated with Matrigel (BD Biosciences, San Jose, CA, USA) to form a matrix barrier, and then, 20 × 104 cells were placed in the upper chamber. In each lower chamber, 600 ml of DMEM medium with 10% FBS was added. Cells were incubated at 37 °C and allowed to migrate for 36 h or invade for 48 h. After incubation, the cells that had migrated through the pore were fixed with 4% paraformaldehyde and stained with 0.1% crystal violet. Then, the cells were counted and photographed under an IX71 inverted microscope (Olympus, Tokyo, Japan).
Immunohistochemistry
The tissues were fixed in formalin and paraffin-embedded. Tissue sections were placed in 0.01 M sodium citrate buffer (pH 6.0) to about 95 °C and heated for 10 to 15 min. Then, digested with 0.1% trypsin and 0.4% pepsin for 5 to 30 min. Dewaxed sections after incubation with specific antibody and secondary antibodies and observed under fluorescence microscopy (Leica, Germany).
Statistical analyses
The data were analyded by SPSS18.0 statistical software package (SPSS Inc., Chicago, IL, USA).. The statistical method was analysed by t-test, Pearson correlation analysis and χ2 test. p < .05 showed a statistically significant difference.
Results
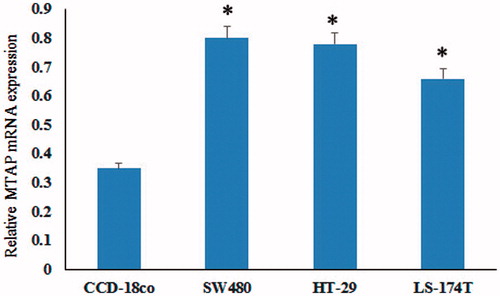
MTAP mRNA was upregulated in colorectal cancer cell lines
The mRNA expression of MTAP in colorectal cancer cell lines SW480, HT29, LS-174 T and colonic myofibroblasts cell (CCD-18co) were detected by Q-PCR. The results showed that MTAP was highly expressed in colorectal cancer cell lines compared to the normal cells (). These results indicated that MTAP mRNA was upregulated in colorectal cancer cell lines.
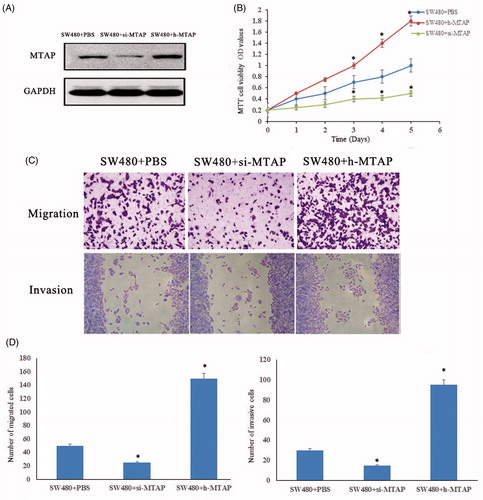
MTAP accelerates cell proliferation, invasion and migration
MTAP is highly expressed in colorectal cancer, and the malignant proliferation of tumor cells is one of the causes of difficult to cure tumours. Therefore, the MTAP high expression and low expression cells were successfully constructed to investigate the proliferation and migration (). As showed in , the proliferation ability of MTAP high expressed was significant increased compared to the control group, and the proliferation ability of MTAP low expressed was significant decreased compared to the control group. Transwell assay confirmed that after 48 h the cell invasion ability of MTAP low-expressed cell was reduced, while in the MTAP high-expressed cell was significantly enhanced. It is well known that cell migration indicates the movement of cells. The migration ability of MTAP low-expressed cells was absolutely stopped after 48 h, but the migration ability of MTAP overexpressed cells was significantly increased ().
Figure 2. MTAP accelerated tumour cell proliferation and migration. (A) Successful construction of MTAP high expressed and low expressed in SW40 cells; (B) the proliferation ability of MTAP-high-expressed and low-expressed cells were detected by MTT assay; (C and D) the migration and invasive ability of MTAP high-expressed and low-expressed cells were detected. *indicated p < .05.
MTAP protein overexpression in colorectal cancer tissues
In order to further explore the expression of MTAP in colorectal cancer tissues, the expression of MTAP protein in 50 cases of colorectal cancer tissues was detected by immunohistochemistry, and the adjacent tissues were used as controls. The positive expression of MTAP in colorectal cancer tissues was higher than that in the adjacent control group, as showed in .
High expression of MTAP accelerated the hepatic metastasis of colorectal cancer
Tumour development and metastasis were inseparable, especially in the late stage of the tumor. In present study, the relationship between the MTAP and metastasis of colorectal cancer was being explored. Immunohistochemical study was performed on liver metastatic tissue of 23 colorectal cancer patients. As showed in , the expression of MTAP in the in situ tumour tissue was significantly weaker than that in the liver metastatic tissue. The statistical results shown in , the expression rate of MTAP was statistically different between in situ carcinoma and metastatic foci. Furthermore, the IHC results showed that the expression of the epithelial marker E-cadherin was decreased, whereas the expression levels of the mesenchymal markers vimentin, fibronectin, and slug were increased in liver metastasis specimens compared to primary tumour tissues (). The above results demonstrated that MTAP promoted CRC metastasis via EMT.
Figure 3. High protein expression of MTAP in colorectal cancer. The expression of MTAP was significantly increased in colorectal cancer tissues compared to paracancerous tissues by IHC. *indicated p < .05.
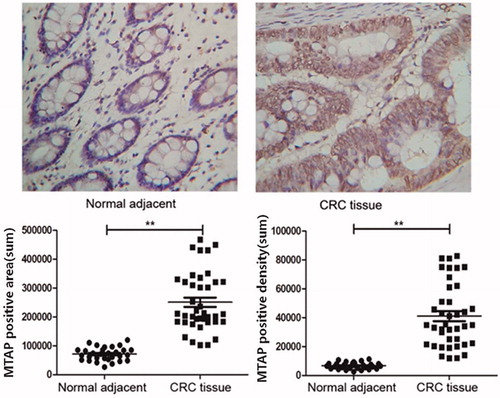
Figure 4. High expression of MTAP accelerated the liver metastasis of colorectal cancer. IHC examinations indicated increased expression levels of MTAP and the mesenchymal markers fibronectin, vimentin and slug and decreased expression of epithelial marker E-cadherin in the clinical liver metastasis specimens compared to the primary tumor (×200). *indicated p < .05.
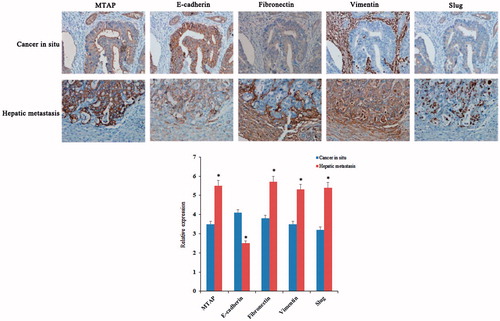
Table 1. Statistics of MTAP in 23 cases of liver metastatic colorectal cancer patients.
Discussion
A large number of studies have confirmed that abnormal MTAP function is closely related to the development of various tumours. Studies have shown that by immunohistochemical detection of MTAP in 170 cases of surgical resection of primary liver cancer tissue and adjacent normal tissue expression, found that liver cancer tissue MTAP expression was significantly higher than the adjacent normal tissue and tumour staging related. By analysing the expression of MTAP in 170 cases of primary hepatocellular carcinoma and adjacent normal tissues, it was found that the expression of MTAP in hepatocellular carcinoma was significantly higher than that in adjacent normal tissues, and was closely related to tumour stage [Citation17]. And it was confirmed that MTAP expression in renal cell carcinoma and fibroblastoma cells was higher than that in liver tissue and fibroblasts [Citation18]. It was also confirmed that MTAP inhibitors can inhibit the growth of liver cancer, lung cancer, cervical cancer, prostate cancer, breast cancer and a series of malignant tumour cells, and induce tumour cell apoptosis [Citation19]. These findings fully suggest that abnormal expression of MTAP is closely related to the development of malignant tumours, but its role in colorectal cancer has not been studied [Citation20].
Herein, the expression of MTAP in colorectal cancer was detected by IHC. The results showed that expression of MTAP in tumour tissue was significantly higher than that in adjacent tissues of colorectal cancer patients, and it was consistent in colorectal cancer cell lines. The infinite proliferation of tumour cells leads to difficult tumour healing, while accelerated cell cycle rapid tumour cell proliferation. The results showed that the cell proliferation was significantly affected by MTAP, and rate was significantly increased in SW480 when MTAP was high expressed, and MTAP was also regulated cell invasion and cell migration.
EMT refers to the biological phenomenon by which epithelial cells lose their epithelial characteristics and obtain a mesenchymal phenotype, and this process is related to embryonic development, tissue repair, fibrosis, tumour invasion and metastasis. During EMT, the expression of the epithelial marker E-cadherin is lost, whereas the expression levels of mesenchymal markers, including vimentin, fibronectin and slug, increase. Moreover, EMT has been found to be associated with CRC budding growth (tumour buds), angiogenesis, cancer stem cell activity, tumor invasion and metastasis [Citation21–23].
The liver metastasis of colorectal cancer is more likely to occur in patients with advanced disease. In present study, the level of MTAP in colorectal cancer patients with liver metastases was higher than that in primary tumour. Further clinical metastatic tumour specimens confirmed that MTAP promoted CRC metastasis via EMT. Interestingly, E-cadherin expression was restored in metastatic CRC liver specimens, which may be associated with the mesenchymal-epithelial transition (MET) of tumour cells after liver metastases. Thus, subsequent studies are needed to further elucidate the mechanisms by which MTAP promotes EMT in CRC.
In conclusion, our results revealed that MTAP overexpression correlated with proliferation, invasion and migration in CRC cell lines and is related to EMT, and a follow-up, in-depth study is ongoing.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386.
- Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. CA Cancer J Clin. 2016;66:115–132.
- Kleespies A, Fuessl KE, Seeliger H, et al. Determinants of morbidity and survival after elective non-curative resection of stage IV colon and rectal cancer. Int J Colorectal Dis. 2009;24:1097–1109.
- Platell C, Ng S, O’Bichere A, et al. Changing management and survival in patients with stage IV colorectal cancer. Dis Colon Rectum. 2011;54:214–219.
- Karoui M, Roudot-Thoraval F, Mesli F, et al. Primary colectomy in patients with stage IV colon cancer and unresectable distant metastases improves overall survival: results of a multicentric study. Dis Colon Rectum. 2011;54:930–938.
- Jemal A, Siegel R, Xu J, et al. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300.
- Filipowicz W, Bhattacharyya SN, Sonenberg N. Mechanisms of posttranscriptional regulation by microRNAs: are the answers in sight? Nat Rev Genet. 2008;9:102–114.
- Bertino JR, Waud WR, Parker WB, et al. Targeting tumors that lack methylthioadenosine phosphorylase(MTAP) activity. Cancer Biol Ther. 2011;11:627–632.
- Munshi PN, Lubin M, Bertino JR. 6-Thioguanine: a drug with unrealized potential for cancer therapy. Oncologist. 2014;19:760–765.
- Kadariya Y, Yin B, Tang B, et al. Mice heterozygous for germ-line mutations in methylthioadenosine phosphorylase (MTAP) die prematurely of T-cell lymphoma. Cancer Res. 2009;69:5961–5969.
- Efferth T, Miyachi H, Drexler HG, et al. Methylthioadenosine phosphorylase as target for chemoselective treatment of T-cell acute lymphoblastic leukemic cells. Blood Cells Mol Dis. 2002;28:47–56.
- Izbicka E, Diaz A, Streeper R, et al. Distinct mechanistic activity profle of pralatrexate in comparison to other antifolates in in vitro and in vivo models of human cancers. Cancer Chemother Pharmacol. 2009;64:993–999.
- Tang B, Testa JR, Kruger WD. Increasing the therapeutic index of 5-fluorouracil and 6-thioguanine by targeting loss of MTAP in tumor cells. Cancer Biol Ther. 2012;13:1082–1090.
- Tedeschi PM, Kathari YK, Farooqi IN, et al. Leucovorin rescue allows effective high-dose pralatrexate treatment and an increase in therapeutic index in mesothelioma xenografts. Cancer Chemother Pharmacol. 2014;4:93–99.
- Asselin BL, Devidas M, Wang C, et al. Effectiveness of high-dose methotrexate in T-cell lymphoblastic leukemia and advanced-stage lymphoblastic lymphoma: a randomized study by the Children's Oncology Group (POG 9404)). Blood. 2011;118:874–883.
- Ren MY, Wu XY. The research progress of toll-like receptors. Curr Immunol. 2006;26:340–342.
- Leal MF, Lima EM, Silva PN, et al. Promoter hypermethylation of CDH1, FHIT, MTAP and PLAGL1 in gastric adenocarcinoma in individuals from Northern Brazil. WJG. 2007;13:2568–2574.
- Chow WA, Bedell V, Gaytan P, et al. Methylthioadenosine phosphorylase gene deletions are frequently detected by fluorescence in situ hybridization in conventional chondrosarcomas. Cancer Genet Cytogenet. 2006;166:95–100.
- Hellerbrand C, Mühlbauer M, Wallner S, et al. Promoter hypermethylation is causing functional relevant downregulation of methylthioadenosine phosphorylase (MTAP) expression in hepatocellular carcinoma. Carcinogenesis. 2006;27:64–72.
- Watanabe F, Takao M, Inoue K, et al. Immunohistochemical diagnosis of methylthioadenosine phosphorylase (MTAP) deficiency in non-small cell lung carcinoma. Lung Cancer. 2009;63:39–44.
- Gurzu S, Silveanu C, Fetyko A, et al. Systematic review of the old and new concepts in the epithelial-mesenchymal transition of colorectal cancer. World J Gastroenterol. 2016;22:6764–6775.
- Wang EL, Qian ZR, Nakasono M, et al. High expression of Toll-like receptor 4/myeloid differentiation factor 88 signals correlate with poor prognosis in colorectal cancer. Br J Cancer. 2010;102:908–915.
- Manfredi S, Lepage C, Hatem C, et al. Epidemiology and management of liver metastases from colorectal cancer. Ann Surg. 2006; 244:254–259.