Abstract
Quantum dots (QDs) or fluorescent nanocrystals are designed nanoparticles that are promising for several biological and bio-medical applications as well as drug delivery and simultaneous cellular imaging. QD’s have exhibited promising potential primarily in receptor based targeting as a result of their distinctive physicochemical properties. Functionalized QDs (f-QDs) have been developed as effective, safe, nano-sized smart systems to deliver a wide range of bio-actives. Surface modified fluorescent carbon QDs with surface modification have attracted attention as targeting ligand to accomplish cellular targeting with enhanced specificity. Several surface engineered and conjugated fluorescent carbon QDs are presently being explored for the treatment of cancer and the outcome is eagerly awaited.
Introduction
Advances in nanoparticle surface chemistry have paved the way for the development of polymer-encapsulated probes that are highly fluorescent and stable in complex biological milieu [Citation1–4]. Semiconductor nanocrystals, also known as quantum dots (QDs) have emerged as indispensable tool in biomedical research, especially for quantitative, multiplexed and long-term fluorescence imaging as well as simultaneous detection [Citation5–8]. The basic rationale for using QDs may be ascribed to their unique properties that are not generally available for individual molecules or bulk semiconductor solids. QDs ranging specially between 1 and 10 nm glow or fluoresce brightly when excited by a light source such as a laser. These tiny semiconductor particles, generally below 10 nm, have emerged as luminescent nano-probes and carriers for biological applications [Citation9].
Nano shells are tunable plasmonic nanoparticles ∼10–300 nm diameter. As compared to nanoshells, QDs are tunable exitonic nanoparticles ∼1–10 nm diameter [Citation10]. QDs have emerged as a new class of fluorescent probes for bio molecular and cellular imaging due to their unique optical and electronic properties such as size tunable light emission, improved signal brightness, resistance against photo bleaching, and simultaneous excitation of multiple fluorescence colours in comparison with organic dyes and fluorescent proteins. For improving the sensitivity of molecular imaging and quantitative cellular analysis by 1–2 orders of magnitude, these properties have proved to be most promising.
The success of using QDs in biological imaging, sensing and detection has encouraged scientists to further develop this technology for clinical and translational research. The most important emerging applications of QDs appear to be in traceable drug delivery, because it has the potential to elucidate the pharmacokinetics and pharmacodynamics of drug candidates and to provide the design principles for drug carrier engineering.
Quantum dots are currently limited to cell and small animal uses due to its probable long term in vivo toxicity and degradation. Optical imaging is highly sensitive, quantitative, capable of multiplexing and is significantly cheaper as compared to the traditional imaging modalities such as MRI and positron emission tomography that will substantially reduce the cost and shorten the time involved in new drug development. Therefore, for nano-carrier development and optimization, QDs can become an excellent “prototype” from which biocompatible carriers of similar sizes and surface properties could be made for clinical uses. Current applications of QDs in drug delivery are focused on two major areas: using QDs as carriers, and labelling therapeutics or drug carriers with QDs [Citation11–15].
Properties [Citation16]
QDs are about thousand times smaller than width of a hair, and made of tiny bits of metal.
It’s possible to mould QD’s into different shapes and coat with a variety of bio molecules.
QDs possess luminescence property under UV light, with the size of the dots controlling its colour, e.g. 2 nm QDs exhibit bright green luminescence while 5 nm Quantum dots – exhibit red.
Fluorescent quantum dots are usually compounds from group II to VI and III to V, e.g. Ag, Cd, Hg, Ln, P, Pb, Se, Te, Zn, etc.
Size of QD’s is directly related to the λ of light it emits; i.e. smaller the QD’s, shorter the λ it emits.
QDs consist of a broad excitation range.
QD’s have a precise emission λ hence spectra do not overlap in multiple fluorescent emission.
QDs, also called “designer atoms”, possess innumerable optical and electronic properties that make its use as semiconductors.
Advantages [Citation16]
QD’s make it possible to track cell processes for longer periods of time and shed new light on molecular interactions as they are much more resistant to degradation than other optical imaging probes. Real time imaging is the most unique feature of QDs.
QDs are basically nanocrystals, so they provide good contrast for imaging with an electron microscope as scattering increases.
QDs have size-tunable emission (from UV to IR).
QDs show prolonged fluorescence as compared to conventional dyes.
QDs exhibit increased optical activity with innumerable avenues of applications in biotechnology and life sciences.
The extremely small size imparts QDs great versatility by allowing them to be injected into many environments including liquid mixtures, fabrics, and polymer matrices.
Structure
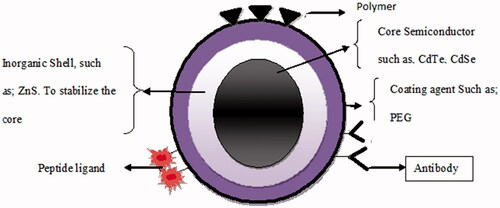
Structurally, QDs consists of a metalloid crystalline core, which, depending on its composition and size, will confer the type of fluorescence it will emit. QDs’ core is composed of materials like cadmium–selenium (CdSe), cadmium–tellurium (CdTe), indium–phosphate (InP) or indium–arsenate (InAs). Zinc sulphide (ZnS) serves as semiconductor shell that stabilizes the core and improves the optical and physical properties, and bioavailability of the material. Additional capping or coatings of biocompatible material or polymer layers, such as POSS-PCU and PEG (polyhedral oligomeric silsesquioxane–polycarbonate urethane, polyethylene glycol), to the QD core– shell promotes water solubility and desired bioactivity (). The surface groups on polymer coatings like –COOH or –NH2 are available for covalent coupling of bioactive molecules. Apart from its distinctive structural and fluorescence properties, QDs also have a direct, relationship between their physical size and the energy of the exciton (electron–hole pairs) and the wavelength of emitted fluorescence. This unique property of size-tunable fluorescence allows QD to have a common excitation profile spanning the ultraviolet (UV), visible, and near-mid infrared regions of the electromagnetic spectrum but different fluorescent emission maxima [Citation17].
Synthesis
The synthesis of CQD’s can be classified by two methods:
METHOD A
METHOD B
A brief account of popular methods of synthesis of CQDs is presented below.
General method of synthesis
The most commonly prepared QD’s by this method have been of cadmium selenium (CdSe). These dots, could be produced by injecting a syringe of the desired organometallic precursors into heated triocytlphosphine oxide (TOPO) that has been vigorously stirred under an inert atmosphere. Interestingly the solution immediately begins to change from colourless to yellow, then orange and red/brown, as the quantum dots increase in size. Heat is removed from the flask when the desired size is reached. Finally, the synthesized quantum dots of different sizes can be identified by placing them under a “black light”. After being excited the quantum dots emit colours depending on their sizes: 514 nm (blue), 544 nm (greenish blue), 559 nm (green), 571(yellowish green), 577 (yellow), 581(yellowish orange) and 610 nm (orange) [Citation16].
Top-down approaches
a) Arc-discharge methods
In this method, the carbonaceous materials (SWCNTs) are fractionated into a number of components that possess size-dependent fluorescent properties and the soot is further oxidized with 3.3M HNO3 for the introduction of carboxyl functional groups, basically for improving the hydrophilicity of the material, the sediment is extracted with NaOH solution (pH 8.4), which produces a stable black suspension that is further separated by gel electrophoresis into SWCNT’s, short tubular carbons and fluorescent C-dots [Citation18].
b) Electrochemical oxidation
MWCNT’s are formed from scrolled graphene layers on carbon papers by chemical vapour deposition, where the nanotubes are used as the working electrode in an electrochemical cell. The cell consists of a Pt wire counter electrode and a Ag/AgClO4 reference electrode with a degassed acetonitrile solution containing 0.1 M tetra butyl ammonium per chlorate (TBA + ClO4−) as the electrolyte. C-dots are also synthesized from the electrochemical oxidation of graphite rod with a Pt mesh counter electrode and Ag/AgCl reference electrode assembly in phosphate buffer solution (pH 7.0) [Citation19].
Bottom-up approaches
a) Laser-ablation methods
C-dots are produced by hot pressing a mixture of graphite powder and cement followed by stepwise baking, curing, and annealing under an argon flow at 900 °CC and 75 KPa. The surface of C-dots is passivated using different polymeric agents such as diamine terminated poly(ethylene glycol), poly(propionyl-ethylenimine-co-ethylenimine), and the highly fluorescent pure C-dots are separated by dialysis against water, followed by centrifugation [Citation20]. A slightly modified technique using 13C powder and more rigorous control is introduced to produce C-dots with high quantum yield of 20% [Citation21]. Yang et al. [Citation22] introduced a single-step procedure wherein a pulsed Nd:YAG laser was used to irradiate graphite or carbon black dispersed in diamine hydrate, diethanolamine, or PEG200N for 2 h, which serve as surface passivating agent.
b) Combustion/thermal/hydrothermal methods
C-dots could be synthesized from the combustion of unscented candles or natural gas burners [Citation23,Citation24]. Water soluble multicolour fluorescent C-dots (<2 nm) are produced from the combustion soot of candles through oxidative acid treatment, which introduced OH and COOH groups to the C-dot surfaces [Citation23]. The resultant particles are purified using polyacrylamide gel electrophoresis (PAGE) fraction.
Another method [Citation4] was reported wherein, surface passivated C-dots were produced using one-step thermal decomposition of low-temperature-melting molecular precursors. Careful selection of the carbon source and surface modifier resulted into better control over the geometry and physical properties of the C-dots. Highly blue luminescent C-dots with PL QY of 31.6–40.6% were prepared by a one-step pyrolytic route from ethylenediamine to tetraacetic acid salts. Another important approach to obtain C-dots was based on chemical oxidation of carbohydrates (glycerol, glycol, glucose, sucrose, citric acid, etc.) [Citation19]. A high-yield synthesis of hydrophilic C-dots by controlled carbonization of sucrose was also exploited. Green luminescent C-dots and non-luminous C-dots were effectively separated, which on functionalization with PEG emitted blue fluorescence. Hydrothermal method to produce C-dots with high quantum yield taking different molecular precursor such as glucose, fructose sucrose, ascorbic acid, etc. has also been exploited [Citation25].
Interestingly C-dots was synthesized by hydrothermal treatment of cheap and readily available orange juice and due to high photo stability and low toxicity these C-dots are demonstrated as excellent probes in cellular imaging. Production of bi functional florescent C-dots is also reported by hydrothermal treatment of soya milk, which exhibited good electrocatalytic activity towards oxygen reduction [Citation26].
c) Microwave/ultrasonic synthesis [Citation27]
A microwave pyrolysis approach was reported for the synthesis of C-dots with electrochemiluminescence properties by combining PEG200 and a saccharide (e.g. glucose and fructose) in water to form a transparent solution, followed by heating in a 500 W microwave oven for 2–10 min. The size and photoluminescence properties of these C-dots are related to the duration of the microwave heating. Later C-dots, that exhibited colourful PL covering the entire visible-to-NIR spectral range from glucose or active carbon was synthesized by using an ultrasonic treatment method. Further, water-soluble fluorescent C-dots from active carbon by a one-step hydrogen peroxide-assisted ultrasonic treatment were also prepared. These C-dots exhibited up-conversion fluorescent properties and emitted bright and colourful photoluminescence covering the entire visible-to-near infrared spectral range. Recently [Citation28] photo catalytic active fluorescent N-doped C-dots (NCDs) was synthesised via one-pot ultrasonic synthesis, which was used in the photo degradation of methyl orange under visible light.
Chemical method
Electrochemical route [Citation29]
tZnO quantum dots were prepared at room temperature, using an electrochemical route. An electrochemical bath comprised of a mixture of acetonitrile and tetrahydrofuran, in the ratio of 4:1, along with the capping agent. The capping agent tetraoctylammoniumbromide (TOAB) also acted as the electrolyte. Zinc served as a sacrificing anode while platinum served as the cathode. The ZnO QDs thus obtained were in powder form, which were re-dispersed in acetonitrile for further characterization.
Microwave synthesis [Citation30]
a) Rapid microwave-assisted synthesis
Citric acid (4.2 g) and ethylenediamine were dissolved in ultrapure water. Thirty mL of the solution was then transferred to a sealed digestion vessel and placed into the microwave digestion furnace. The microwave digestion system was equipped with controllable temperature units within variation of ±1 °C at the set temperature. The system can be operated at 2450 MHz frequency, and work at 0–1000 W power. When set at 600 W power, the reaction temperature was rapidly elevated to 200 °C. The synthesis process could be accomplished in 5 min. The CD samples were naturally cooled to lower than 80 °C. The product, which was brown-yellow and transparent, was subjected to dialysis (500-Da cut off) in order to obtain the CDs. The CD samples were diluted for optical characterization.
b) Synthesis of CdTe QDs
The microwave based synthesis procedure was similar to modifications made to the microwaving procedure. The procedure yielded QDs in the 2–6 nm size range.
Carbonization [Citation31]
C-dots were prepared by controlled carbonization of sucrose modified method wherein sucrose was dissolved in deionised water and concentrated sulphuric acid was added drop wise into the solution with continuous vigorous stirring. The yellowish brown coloured reaction mixture was neutralized with sufficient amount of NaOH. The dark brown colour reaction mixture was isolated by centrifugation and further purified by dialysis against Milli-Qwater under constant stirring condition. In order to concentrate the amount of C-dots the solution was dried in a rotary vacuum evaporator and frozen dried for the quantification of C-dots.
Hydrothermal synthetic method
Single step hydrothermal synthetic method [Citation32]
In single step hydrothermal synthetic method, aqueous solution of 1% Good’s buffer (HEPES, BES or MES) was taken in a Teflon-lined stainless steel autoclave and placed at 160 °C for 8 h. After cooling down to room temperature, a light yellow coloured solution was collected, which was centrifuged, supernatant was collected and dialysed against deionised water to remove the remained unreacted molecules.
Hydrothermal oxidation method [Citation33]
In this method citric acid was dissolved in N,N-dimethylformamide (DMF) and o-phenylenediamine was added to achieve molar ratio of 2:1 (carboxyl:amino). The mixed solution was heated hydrothermally in a stainless steel autoclave at 20 °C for 5 h. The obtained brown solution was evaporated at 80 °C under reduced pressure to remove DMF the oily brown product was dissolved in dichloromethane and the water soluble part was extracted with deionised water. Sephadex G-25 gel chromatographic column was then used for purification and the fluorescent fractions were collected, lyophilized and stored at room temperature.
Hydrothermal carbonization chitosan method [Citation34]
In this procedure, amino-functionalized fluorescent carbon nanoparticles were synthesized by dissolving chitosan in acetic acid solutions and then the mixture was sealed into a Teflon equipped stainless steel autoclave, which was then placed in a muffle furnace followed by hydrothermal treatment at 180 °C for 12 h. After the reaction, the autoclave was allowed to cool down naturally. The obtained dark brown solution was centrifuged to remove the less-fluorescent deposit. The upper brown solution having an average size of 5 nm exhibited strong blue luminescence under excitation at 365 nm. The deposited nanoparticles had larger diameters in the range of 30–40 nm and exhibited very weak fluorescence after ultrasonic disperse in water. Pure upper brown luminescent carbon nanoparticles obtained by freeze dried had a yield of 7.8%.
Solvothermal synthesis [Citation35]
In a typical synthesis of CQDs, PEG-200 was mixed with NaOH solution, the mixture was shaken well to produce a homogeneous solution that was allowed to sonicate in a sonication bath for 1 h. A distinct yellow coloured solution was formed, which was kept undisturbed for overnight to yield CQDs with a characteristic yellowish brown colour.
A low temperature “green” solvothermal route was also reported for the synthesis of CQDs wherein PEG-200 was mixed with concentrated sodium hydroxide solution to produce a homogeneous solution, which was then subjected to solvothermal heating at 160 °C for 24 h to produce CQDs.
Chemical method (at basic pH) [Citation36]
In chemical method 0.1 M of zinc acetate and 0.2 mM of manganese acetate solution were taken and mixed with 0.2 M DEA water. All three solutions were mixed with vigorous stirring at 600 rpm followed by addition of 14 mmol oleic acid with vigorous stirring at 800 rpm (20 °C) for30 min (adjusted to basic pH 6.2). After 30 min of nucleation the solution was refluxed and continuously stirred for 2 h. The unreacted molecules were filtered and re-dispersed in ethanol and isopropanol followed by centrifugation at 1600 rpm for30 min. Mn+2 ions were removed from QDs surface by heating the solution in DEA at 150 °C for 30 min. Obtained nanocrystals were precipitated with ethanol and again centrifuged for10 min at 1600 rpm and finally dispersed in water.
One-pot synthesis of highly monodispersed QD’s [Citation37]
In an inert atmosphere glove-box, 5.49 mmol of tetraoctyl ammonium bromide (TOAB) was dissolved in 100 ml anhydrous toluene, 0.2 ml (2.07 mmol) CCl4 was added to the solution and stirred for 30 min. CQDs were formed by the dropwise addition of 4 ml of 1 M lithium aluminium hydride in THF over a period of 5 min. The solution was then left to stir for 30 min. The excess reducing agent was quenched with the addition of 30 ml of methanol, upon which the dispersion became transparent. Chemically passivated CQDs were formed by modifying the carbon–hydrogen bonds at the surface via the addition of 200 μL of a0.1 M H2PtCl6 in isopropyl alcohol as a catalyst, followed by 3 ml of 1-dodecene. After stirring for 30 min, the CQDs were removed from the glove box and the organic solvent was removed by rotary evaporation. The resulting dry powder was then re-dispersed in 50 ml hexane and sonicated for 30 min. The solution was then filtered twice using both filter paper and PVDF membrane to remove the surfactant, after which it was washed with 100 ml of deionised water (three times). Alkyl-terminated CQDs remained in the hexane phase.
l-Cysteine capped CdSeQDs [Citation38]
l-Cysteine capped CdSeQDs were prepared wherein equal proposed amounts of NaBH4 and Se powder were placed in a 100 ml round-bottom flask and 10 mL of oxygen free dH2O was added to the mixture. The reaction was allowed to be completed at 40 °C for 30 min (reaction 1). NaBH4 is able to reduce the elemental selenium to Se2−, which provides selenide ions incorporated in the structure of CdSe nanocrystals. In another 100 ml roundbottom, 20 ml of CdCl2 (0.02 M) and l-cysteine (0.0012 M) were added and pH of the solution was adjusted at 11. pH was adjusted by Sodium hydroxide (1 M) where upon the colour of solution became milky, which was cleared following the adjustment of pH at 11 and dissolution of l-cys. Afterwards, the solution was de-aerated under the flow of argon for 30 min. In the next step, 6 ml of the reaction (1) was added into the solution to obtain the Se/Cd molar ratio of 0.75. Finally, the flask was sealed tightly and the solution was refluxed under the flow of argon for 60 min. The l-cys capped CdSeQDs were obtained through ethanol precipitation (1:1 v/v) by centrifugation at 3000g for 5 min. The QDs were then washed (3×) with absolute ethanol and examined later.
Applications [Citation16]
As another type of inorganic nano-fluorescent probe, QD’s have showed outstanding advantages in the long time, multi-colour fluorescence imaging and detection. QD’s have wide range of applications from diagnosis to treatment of cancers. Moreover, the surface modification property of QD’s assures increased drug-delivery efficiency. Some of its major applications are discussed below.
Pharmaceutical field
In the field of diagnosis, magnetic resonance imaging is that the most developed application of metallic particles. A very new generation of biosensors based on the optical properties of colloidal gold and fluorescent nanocrystals, referred to as quantum dots seems to be extremely rewardable in diagnosis and medical imaging. As far as therapeutic applications are concerned, the potentialities of metal nanoparticles to assist fulfilling the necessity of targeted drug delivery appears worth exploring. The detection of active ingredient with fluorescence will unveil unexplored rewarding avenues [Citation39].
Paclitaxel loaded chitosan nanoparticles and quantum dots were prepared with the particle size of 153.6 nm and 302.8 nm, respectively. The activities nanocarrier systems were determined using 7–12-dimethylbenz[a]antracene (DMBA)- induced breast cancer model in six-week-old female, non-pregnant, Wistar albino rats. Paclitaxel loaded nanocarrier systems were administered intraperitoneally to tumour bearing rats and their tumour volumes were measured. At the end of the experiment rats were sacrificed and their tissue sections were analysed. Both nanocarrier systems (chitosan nanoparticles or quantum dots) successfully reduced the tumours size [Citation40].
Drug delivery system methods
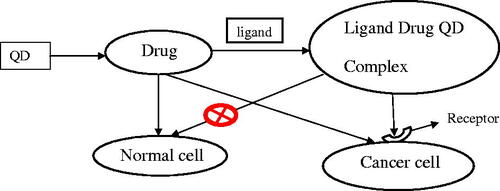
The development of multifunctional nanoparticle probes based on semiconductor or QDs for cancer targeting and imaging in living animals has been reported. The structural design involved encapsulating luminescent QDs with an ABC triblock copolymer and binding this amphiphilic polymer to tumour-targeting ligands and drug-delivery functionalities. In vivo targeting studies on human prostate cancer growing in nude mice showed that the QD probes accumulated at tumours both by the enhanced permeability and retention of tumour sites and by antibody binding to cancer-specific cell surface biomarkers. Sensitive and multicolour fluorescence imaging of cancer cells under in vivo conditions was achieved by using both subcutaneous injection of QD-tagged cancer cells and systemic injection of multifunctional QD probes. New possibilities for ultra-sensitive and multiplexed imaging of molecular targets in vivo has been raised by significant results obtained upon integrating a whole body macro-illumination system with wavelength resolved spectral imaging for efficient background removal and precise delineation of weak spectral signatures [Citation41] ().
Diagnosis drug delivery and drug screening [Citation16]
The use of quantum dots for the investigation of intracellular delivery of nanocrystals offers new possibilities in order to study sub-cellular processes due to its small, uniform size and the unique optical properties. Recent progress in the surface chemistry of QDs has definitely expanded their use in biological applications, decreased their cytotoxicity and rendered QDs a powerful tool for the investigation of distinct cellular processes like uptake, receptor trafficking and intracellular delivery. Further, the unique optical property of QDs has a far-reaching application on the screening of drug in the near future. Different emission colours of QDs were embedded into polymer micro beads at various controlled concentration ratios of QDs, thereby forming microspheres with distinguished spectral characteristic and luminescence characteristic and they can be used for identifying and differentiating biological molecules [Citation16].
The use of nanocrystals and quantum dots of heavy metals (gadolinium, silver, gold, copper, cadmium, etc.) as photonic agents (e.g. photodynamic therapy), chemical markers, or visualization aids (e.g. contrast agents) for diagnostic methods such as magnetic resonance imaging, single-photon emission computed tomography, and positron emission tomography is now commonplace [Citation42].
As diagnostics in clinical applications
One of the most important applications of QD’s is for cancer diagnosis as its luminescent and stable conjugates make it possible to visualize cancer cells in living animals. When combined with florescence microscopy it enables to follow cells at high resolution in living animals. Her2 carbohydrate encapsulated QDs with detectable luminescent properties are useful for imaging of cancer.
Viral diagnosis is yet another area of QDs application. Rapid and sensitive diagnosis of respiratory syncytial virus (RSV) is important for infection control and evolution of antiviral drugs. RSV could be detected rapidly and sensitively by antibody conjugated nanoparticles that is also helpful in estimating relative levels of surface protein expression. Dual colour QD’s or fluorescence energy transfer nanobeads can be concurrently excited with a single light source and detect the presence of particles of the RSV in a matter of hours [Citation43].
Bimodal molecular imaging
Quantum dots with a water-soluble and paramagnetic micellular coating synthesized as a molecular imaging probe for both fluorescence microscopy and MRI may be of great use for the detection of (tumour) angiogenesis [Citation44].
Evaluating multiple biomarkers
A remarkable feature of QDs is that they are available in a variety of colours, allowing uniquely coloured QD for each biomarker being assayed. The fluorescence emitted by the QD is then analysed by multiplexed imaging and computer aided analysis that provides quantitative results for each biomarker [Citation41].
In vivo imaging with quantum dots
Non-targeted near infrared emitting quantum dot core T2-MP EviTags were tested in tumour bearing mice and were found to be capable of generating a reasonable signal to noise image when compared to the control. Further, the bio distribution pattern as confirmed from the optical image showed favourable clearance of the non-targeted T2-MP EviTags through lymphatics, kidneys and bladder. The development of T2-MPEviTags as non-invasive optical molecular imaging probes will have a great impact on the early detection, diagnosis and treatment monitoring of cancer [Citation39]. shows the in-vivo imaging using QDs.
Figure 3. Live cell imaging Alivisatos et al. [Citation48].
![Figure 3. Live cell imaging Alivisatos et al. [Citation48].](/cms/asset/377f16ad-7ffa-4202-9d8b-518e2e9d7d38/ianb_a_1411932_f0003_c.jpg)
For brain tumour diagnosis
One report evaluated the feasibility and specificity of using QDs labelled antibodies for rapid visualization of epidermal growth factor receptor (EGFR)expression in human brain tumour cells and in surgical frozen section slides of glioma tissue. The study demonstrated that QD-labelled antibodies can provide a quick and accurate method for characterizing the presence/absence of a specific predictive biomarker. This can also be used to measure the ability of particles of different sizes to access the tumour and simultaneously image and differentiate tumour vessels from both the peri vascular cells and the matrix [Citation45].
Different nanoparticles are compared for their respective optical properties that is shown in .
Table 1. Summary of optical properties of different nanosystems in diagnostics [Citation5].
Limitations of QDs
Quantum dots offer several advantages over organic dyes, but QDs have their own slew of problems that must be addressed. When placed into live cells QDS exhibit aggregation which can interfere with cell function. Also a dilemma exists in trying to get quantum dots inside cells without killing the cells in the delivering process. Although quantum dots are in the nanometre range, biconjugation with different molecules will increase the size of the dots making delivery into cells more difficult. By far the toxicity of quantum dots to cells is a major issue.
Quantum dots toxicity
The usefulness of quantum dots is derived from their peak emission frequency’s extreme sensitivity – quantum mechanical in nature – to both the dot’s size and composition. QDs have been pointed out as possible replacements for organic dyes in the imaging of biological systems, due to their excellent fluorescent properties, good chemical stability, broad excitation ranges and high photo bleaching thresholds. However, the main drawback of QDs is their toxicity and therefore their implementation is complicated. If this toxicity problem could be addressed, QDs may one day be safely utilized in many areas. For example, cadmium telluride (CdTe, which is toxic) QD based nanocomposites can be used as fluorescent probes for biological imaging, they can also be utilized to monitor targeted drug delivery and for controlled modification of structural and functional properties of intracellular components. Thus, gelatine is being used during the production of CdTe QDs thereby reducing the toxicity of the particles. This approach could as well be useful for the development of other nanoparticle composites with reduced toxicity [Citation46].
Barriers to in vivo application
Firstly, QD complexes, including their capping materials may be immunogenic, which could result in both dangerous immune reactions in subjects, and could also make the QDs ineffective as a result of antibody binding. Secondly, the heavy metals embraced in the core, and the materials used for capping (e.g. MPA) may be toxic to the host. Thirdly, the size of QD complexes prevents renal excretion, making clearance from the blood stream unlikely. This will result in final uptake and concentration in the liver, which is particularly sensitive to cadmium toxicity [Citation47].
Detecting cell death
Quantum dot combined with a novel carrier of the magnetic resonance imaging (MRI) agent gadolinium, has been developed as nanoparticle that can spot apoptosis using both MRI and fluorescence imaging. Tests in animals showed that this nanoparticle can provide anatomical information using MRI and cellular level information using fluorescence imaging. Imaging programmed cell death in the body could provide an early explanation that an antitumor therapy is actually killing cancer cells. MRI experiments showed that the nanoparticle produced an imaging signal that was approximately 40 times stronger than that produced by the gadolinium carrier alone. Sequential imaging experiments could be able to detect injury-induced apoptosis in mice [Citation47].
Conclusions
Although the research of QD nano-carriers for drugs has witnessed some developments, yet the application of QD nano-carriers for drug delivery is still at infancy. Application of QD nano-carriers for drugs in biology is a burgeoning new field having enormous therapeutic potential. Hence with the continuous development of the technique of QD nano carriers for drugs, new development opportunities for drug screening, disease screening, and gene sequencing, plurality of biomedical research will automatically follow.
As powerful imaging probes, QDs have already played an important role in fundamental biology and in vitro disease diagnostics and prognostics. Their unique structural and surface properties, such as their tunable and uniform size, flexible drug linking and doping mechanisms, large surface-to-volume ratio and wide spectrum of surface reactive groups have enabled a new vista of research to be unveiled, targeted and traceable drug delivery. However, high-quality QDs (visible and near infrared dots with a narrow emission profile and high quantum yield) are mainly made with heavy metals whose long-term toxicity are largely unknown at the current time. Despite this limitation, QDs have been applied to cells and small animals as drug carriers, serving as an outstanding discovery tool for drug screening and validation, and as prototype materials for drug carrier engineering. If high-quality QDs can be prepared from relatively non-toxic compounds (e.g. silicon and carbon), or if the toxic components can be inertly protected from exposure and subsequently cleared from the body, then the clinical relevance of QDs could be foreseeable. One primary challenge of drug delivery is maintaining a therapeutically optimum concentration of the drug in the targeted tissue while preventing toxicity realizing the Magic Bullet Concept of Paul Ehrlich. This issue has not been addressed thus far. Ideally, the engineered QDs would be able to stabilize therapeutic compounds, increase their plasma circulation time while reducing the concentration of free drug to minimize unwanted side effects, and to release the drug with a well-controlled profile.
In addition, the therapeutic compounds may be covalently linked to the QD surface via cleavable chemical bonds, so that the bio conjugates are initially large enough to avoid renal filtration, and subsequently, after the ligands are cleaved, small enough to be cleared out of the body.
These intelligent, multifunctional, low- or non-toxic nanomachines are only a few possible achievements for the future. With advances being made in the identification of new targeting ligands, the development of specialized nanoparticles and the discovery of elegant conjugation techniques, the QD-based bio nanotechnology will be constantly expanding its list of amazing applications. A large number of high-quality and high powered trials specifically addressing issues like the ineffectiveness of QD’s, toxicity of QD’s, inverse effect on renal excretion will need to be reviewed before QDs can be counted on for human use, and such a process is likely to be extended.
Future perspective
Designing and synthesis of luminescent hydrophilic QDs will pave the way for new research works as well as help in the selective and specific labelling of cells and bio molecules. Moreover, the interference effect of quantum dots with normal physiology is to be studied and quantified. Size and imaging propensity of QDs could be further expedited in drug delivery. However establishing QDs toxicity (or ruling it out) explorations remains the most desirable as well as most decisive challenge. Differential imaging based on colour variations in QDs due to their size is yet another avenue worth exploring in the effective management of cancers. Synthesis of QDs using less toxic materials is likely to be beneficial in many ways. Synthesized QDs aimed at specific targets as well as simultaneous real-time imaging may provide a new approach in revolutionizing management of cancers. It may be concluded that functionalized QDs may have a great future in pharmaceutical, medical as well as other diverse fields.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Briguglio I, Piras S, Corona P, et al. Benzotriazole: an overview on its versatile biological behavior. Eur J Med Chem. 2015; 97:612–648.
- Cukierman E, Khan DR. The benefits and challenges associated with the use of drug delivery systems in cancer therapy. Biochem Pharmacol. 2010;80:762–770.
- de Bono JS, Ashworth A. Translating cancer research into targeted therapeutics. Nature. 2010;467:543–549.
- Cho K, Wang X, Nie S, et al. Therapeutic nanoparticles for drug delivery in cancer. Clin Cancer Res. 2008;14:1310–1316.
- Michalet X, Pinaud FF, Bentolila LA, et al. Quantum dots for live cells, in vivo imaging, and diagnostics. Science. 2005;307:538
- Boles MA, Ling D, Hyeon T, et al. The surface science of nanocrystals. Nat Mater. 2016;15:141–153.
- Mudshinge SR, Deore AB, Patil S, Bhalgat CM. Nanoparticles: emerging carriers for drug delivery. Saudi Pharm J. 2011; 19:129–141.
- Liu J, Lau SK, Varma VA, et al. Multiplexed detection and characterization of rare tumor cells in Hodgkin’s lymphoma with multicolor quantum dots. Anal Chem. 2010;82:6237–6243.
- Peer D, Karp JM, Hong S, et al. Nanocarriers as an emerging platform for cancer therapy. Nature Nanotechnol. 2007;2:751–760.
- Murray CB, Norris DJ, Bawendi MG. Synthesis and characterization of nearly monodisperse CdE (E = sulfur, selenium, tellurium) semiconductor nanocrystallites. J Am Chem Soc. 1993;115:8706–8715.
- Wu X, Liu H, Liu J, et al. Immunofluorescent labeling of cancer marker Her2 and other cellular targets with semiconductor quantum dots. Nat Biotechnol. 2003;21:41–46.
- Han H-S, Niemeyer E, Huang Y, et al. Quantum dot/antibody conjugates for in vivo cytometric imaging in mice. Proc Natl Acad Sci USA. 2015;112:1350–1355.
- Zipfel WR, Williams RM, Webb WW. Nonlinear magic: multiphoton microscopy in the biosciences. Nat Biotechnol. 2003;21: 1369–1377.
- Valley CC, Arndt-Jovin DJ, Karedla N, et al. Enhanced dimerization drives ligand-independent activity of mutant epidermal growth factor receptor in lung cancer. Mol Biol Cell. 2015;26:4087–4099.
- Soo Choi H, Liu W, Misra P, et al. Renal clearance of quantum dots. Nat Biotechnol. 2007;25:1165–1170.
- Shekhar Dey N, Bhanoji Rao ME. Quantum dot: novel carrier for drug delivery. Int J Res Pharm Biomed Sci. 2011;2:448–456.
- Rizvi SB, Yildirimer L, Ghaderi S, et al. A novel POSS-coated quantum dot for biological application. Int J Nanomed. 2012; 7:3915–3927.
- Zhang Z, Hao J, Zhang J, et al. Protein as the source for synthesizing fluorescent carbon dots by a one-pot hydrothermal route. RSC Adv. 2012;2:8599–8601.
- Chen G, Wu S, Hui L, et al. Assembling carbon quantum dots to a layered carbon for high-density supercapacitor electrodes. Scientific Rep. 2016;6:19028.
- Sun YP, Wang X, Lu F, et al. Doped carbon nanoparticles as a new platform for highly photoluminescent dots. J Phys Chem C. 2008;112:18295–18298.
- Yang ST, Wang X, Wang H, et al. Carbon dots as nontoxic and high-performance fluorescence imaging agents. J Phys Chem C. 2009;113:18110–18114.
- Wang X, Qu K, Xu B, et al. Microwave assisted one-step green synthesis of cell-permeable multicolor photoluminescent carbon dots without surface passivation reagents. J Mater Chem. 2011; 21:2445–2450.
- Sun Y-P, Wang X, Lu F, et al. Doped carbon nanoparticles as a new platform for highly photoluminescent dots. J Phys Chem C, Nanomater Interfaces. 2008;112:18295–18298.
- Ray SC, Saha A, Jana NR, et al. Fluorescent carbon nanoparticles: synthesis, characterization, and bioimaging application. J Phys Chem C. 2009;113:18546–18551.
- Wei W, Xu C, Wu L, et al. Non-enzymatic-browning-reaction: a versatile route for production of nitrogen-doped carbon dots with tunable multicolor luminescent display. Scientific Rep. 2014; 4:3564.
- Sahu S, Behera B, Maiti TK, et al. Simple one-step synthesis of highly luminescent carbon dots from orange juice: application as excellent bio-imaging agents. Chem Commun. 2012;48:8835–8837.
- Yang Z, Li Z, Xu M, et al. Controllable synthesis of fluorescent carbon dots and their detection application as nanoprobes. Nano-Micro Lett. 2013;5:247–259.
- Misra RD. Quantum dots for tumor-targeted drug delivery and cell imaging. Nanomedicine (London, England). 2008;3:271–274.
- Bahnemann DW, Kormann C, Hoffmann MR. Preparation and characterization of quantum size zinc oxide: a detailed spectroscopic study. J Phys Chem. 1987;91:3789–3798.
- He H, Wang X, Feng Z, et al. Rapid microwave-assisted synthesis of ultra-bright fluorescent carbon dots for live cell staining, cell-specific targeting and in vivo imaging. J Mater Chem B. 2015;3:4786–4789.
- Lavkush Bhaisare M, Pandey S, Shahnawaz Khan M, et al. Fluorophotometric determination of critical micelle concentration (CMC) of ionic and non-ionic surfactants with carbon dots via stokes shift. Talanta. 2015;132:572–578.
- Samantara AK, Maji S, Ghosh A, et al. Good's buffer derived highly emissive carbon quantum dots: excellent biocompatible anticancer drug carrier. J Mater Chem B. 2016;4:2412–2420.
- Wang B, Wang S, Wang Y, et al. Highly fluorescent carbon dots for visible sensing of doxorubicin release based on efficient nanosurface energy transfer. Biotechnol Lett. 2016;38:191–201.
- Yang Y, Cui J, Zheng M, et al. One-step synthesis of amino-functionalized fluorescent carbon nanoparticles by hydrothermal carbonization of chitosan. Chem Commun (Cambridge, England). 2012;48:380–382.
- Mitra S, Chandra S, Pathan SH, et al. Room temperature and solvothermal green synthesis of self passivated carbon quantum dots. RSC Adv. 2013;3:3189–3193.
- Bajwa N, Kumar Mehra N, Jain K, et al. Targeted anticancer drug delivery through anthracycline antibiotic bearing functionalized quantum dots. Artificial Cells, Nanomed Biotechnol. 2016; 44:1774–1782.
- Linehan K, Doyle H. Efficient one-pot synthesis of highly monodisperse carbon quantum dots. RSC Adv. 2014;4:18–21.
- Johari-Ahar M, Barar J, Alizadeh AM, et al. Methotrexate-conjugated quantum dots: synthesis, characterisation and cytotoxicity in drug resistant cancer cells. J Drug Target. 2016;24:120–133.
- Sonvico F, Dubernet C, Colombo P, et al. Metallic colloid nanotechnology, applications in diagnosis and therapeutics. Curr Pharm Des. 2005;11:2095–2105.
- Demir GM, Ilhan M, Akkol EK, et al. Effect of paclitaxel loaded chitosan nanoparticles and quantum dots on breast cancer. Proceedings. 2017;1;1074.
- Kumar S, Boehm J, Lee JC. p38 MAP kinases: key signalling molecules as therapeutic targets for inflammatory diseases. Nat Rev Drug Discov. 2003;2:717–726.
- Dichello GA, Sarker DK. Chapter 27 – encapsulation of lethal, functional, and therapeutic medicinal nanoparticles and quantum dots for the improved diagnosis and treatment of infection A2 – Ficai, Anton. In: Grumezescu AM, editor. Nanostructures for antimicrobial therapy. Amsterdam (The Netherlands): Elsevier; 2017. p. 597–622.
- Zhong X, Feng Y, Knoll W, et al. Alloyed ZnxCd1-xS nanocrystals with highly narrow luminescence spectral width. J Am Chem Soc. 2003;125:13559–13563.
- Mulder WJM, Koole R, Brandwijk RJ, et al. Quantum dots with a paramagnetic coating as a bimodal molecular imaging probe. Nano Lett. 2006 2006;6:1–6.
- Stroh M, Zimmer JP, Duda DG, et al. Quantum dots spectrally distinguish multiple species within the tumor milieu in vivo. Nat Med. 2005;11:678–682.
- Schulte PA, Salamanca-Buentello F. Ethical and scientific issues of nanotechnology in the workplace. Environ Health Perspect. 2007;115:5–12.
- Akerman ME, Chan WC, Laakkonen P, et al. Nanocrystal targeting in vivo. Proc Natl Acad Sci USA. 2002;99:12617–12621.
- Parak WJ, Boudreau R, Le Gros M, et al. Cell motility and metastatic potential studies based on quantum dot imaging of phagokinetic tracks. Adv Mater. 2002;14:882–885.